Kinetics of a Reaction-Diffusion Mtb/SARS-CoV-2 Coinfection Model with Immunity
Abstract
1. Introduction
2. A Reaction–Diffusion Mtb/SARS-CoV-2 Coinfection Model
3. Basic Properties
- (i)
- The uninfected equilibrium always exists;
- (ii)
- The Mtb immune-free equilibrium is defined when ;
- (iii)
- The COVID-19 immune-free equilibrium is defined if ;
- (iv)
- The Mtb equilibrium with immunity exists if ;
- (v)
- The COVID-19 equilibrium with immunity exists if ;
- (vi)
- The Mtb/SARS-CoV-2 coinfection equilibrium exists if , , , , and .
- (i)
- The uninfected equilibrium , where . Thus, always exists.
- (ii)
- The Mtb immune-free equilibrium . The components are given as follows:where . We note that is positive, whilst , , and are positive when . Hence, exists if . The parameter appoints the onset of Mtb infection with inactive CTLs.
- (iii)
- The COVID-19 immune-free equilibrium . Its coordinates are written as follows:where . Thus, , whilst and when . Accordingly, is defined when . The threshold locates the start of COVID-19 infection, where the CTL immunity is inactive.
- (iv)
- The Mtb equilibrium with immunity , wherewhere . We note that , , and are always positive, while if . This implies that exists if . The threshold sets the activation of CTLs versus Mtb-infected EPCs.
- (v)
- The COVID-19 equilibrium with immunity . The components are given aswhere . We see that , while if . Hence, is defined when . Here, the threshold defines the stimulation status of CTL immunity versus SARS-CoV-2-infected EPCs.
- (vi)
- The Mtb/SARS-CoV-2 coinfection equilibrium . The components are defined aswhere . We see that , , and are positive if , while and are positive if , and if . In addition, we need the two conditions and . Hence, exists when the above conditions are met.
4. Global Properties
5. Numerical Simulations
- (i)
- We choose , , and . This gives and . This indicates that is GS (Figure 1), which comes to an agreement with Theorem 2. This case simulates the condition of an individual with no Mtb and SARS-CoV-2 infections.
- (ii)
- We select , , and to obtain , , and . The result agrees with Theorem 3 that the equilibrium is GS (Figure 2). In this situation, the patient has Mtb monoinfection and the CTL immunity is inefficient.
- (iii)
- We take , , and . We obtain , , and . These conditions implicate the global stability of , which harmonizes with Theorem 4 (Figure 3). In this case, the patient has SARS-CoV-2 monoinfection in the absence of CTLs.
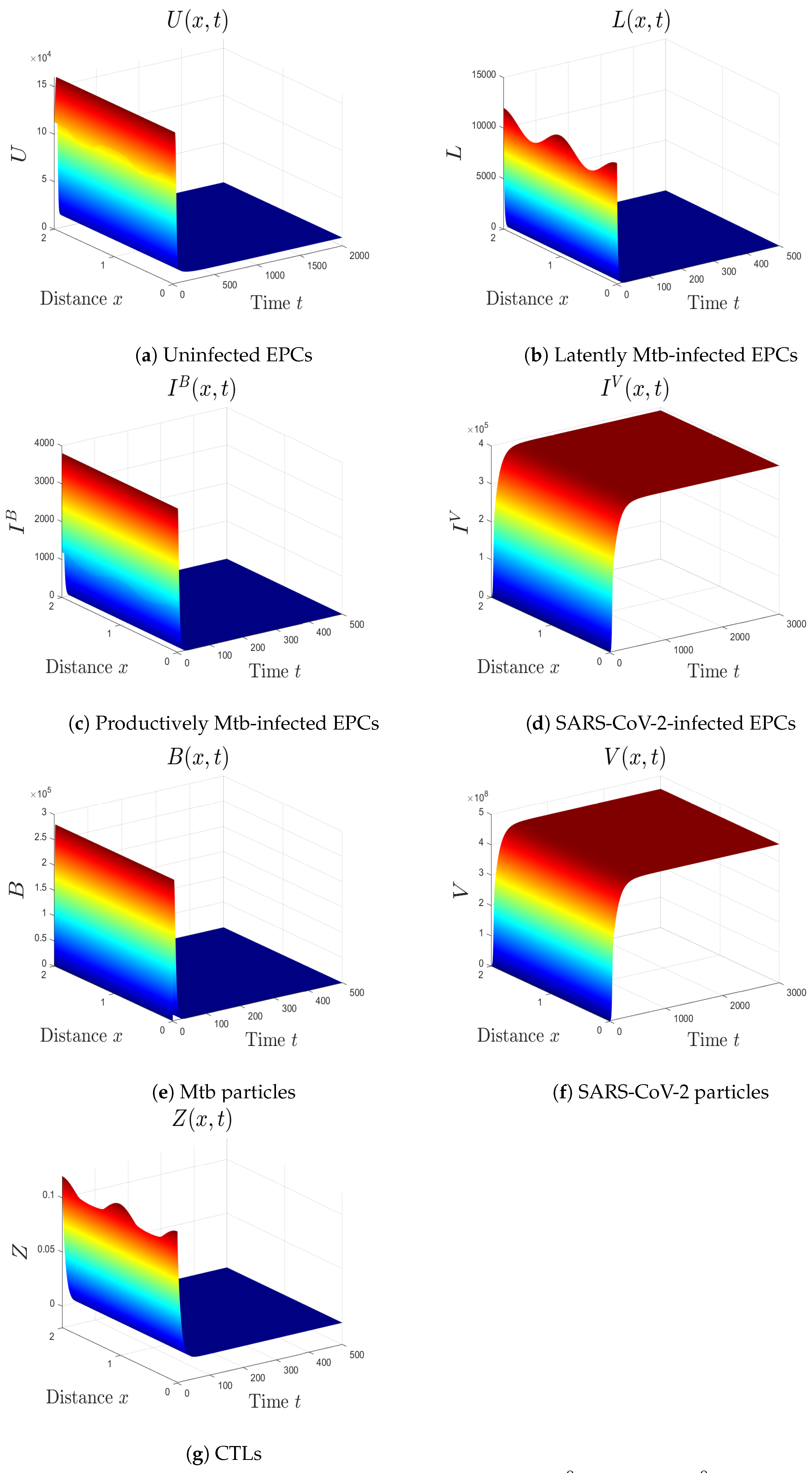
- (iv)
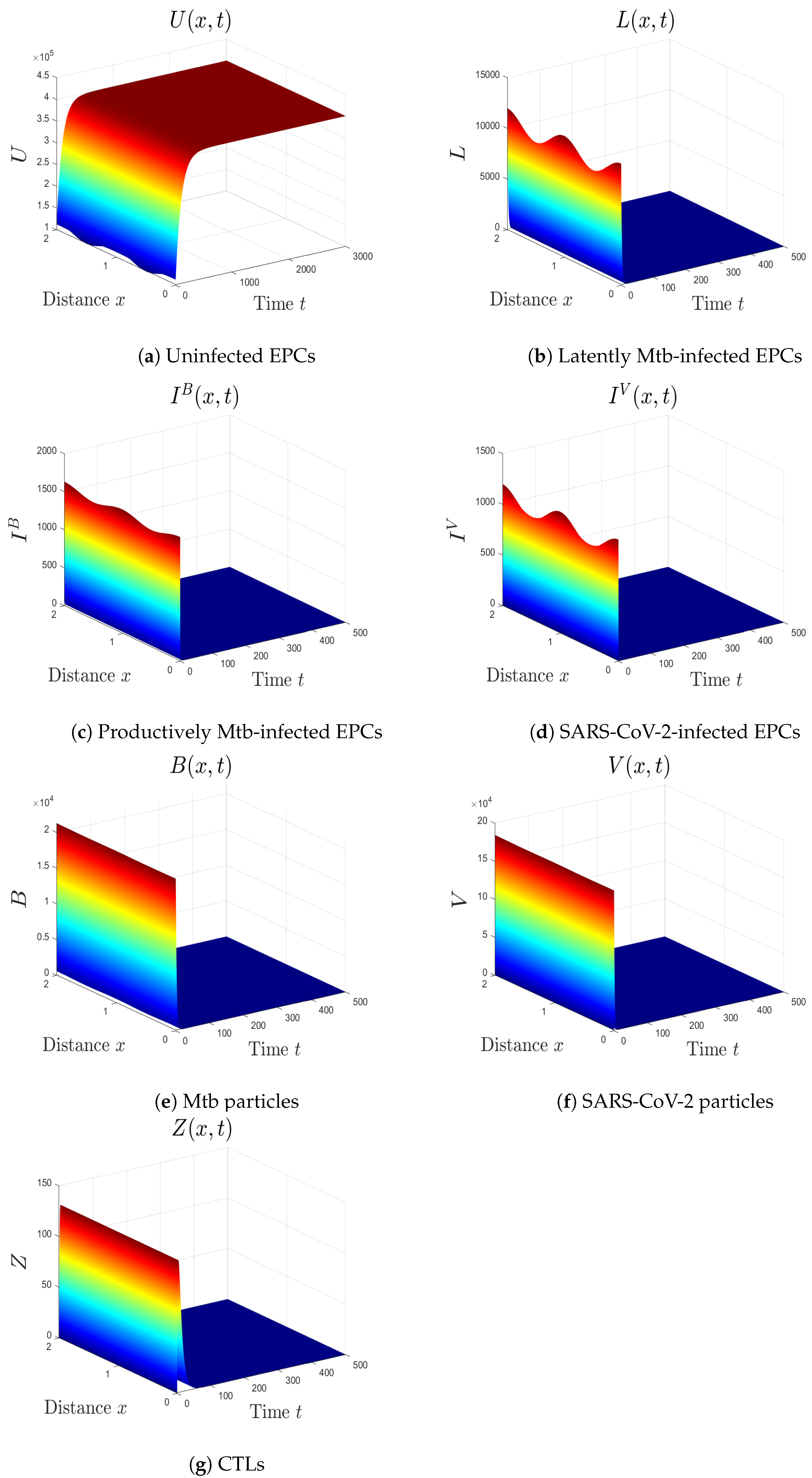
- We choose , , and . This gives and . This implies that the equilibrium is GS (Figure 4), which comes to an agreement with Theorem 5. Here, the CTL immunity is turned on to exterminate the Mtb infection. Consequently, the densities of Mtb-infected cells and Mtb particles decrease, whilst the density of healthy cells increases.
- (v)
- We consider , , and . Thus, we obtain and . In favor of Theorem 6, is GS (Figure 5). This case mimics the condition of a COVID-19 patient with active CTLs which work on removing SARS-CoV-2-infected cells.
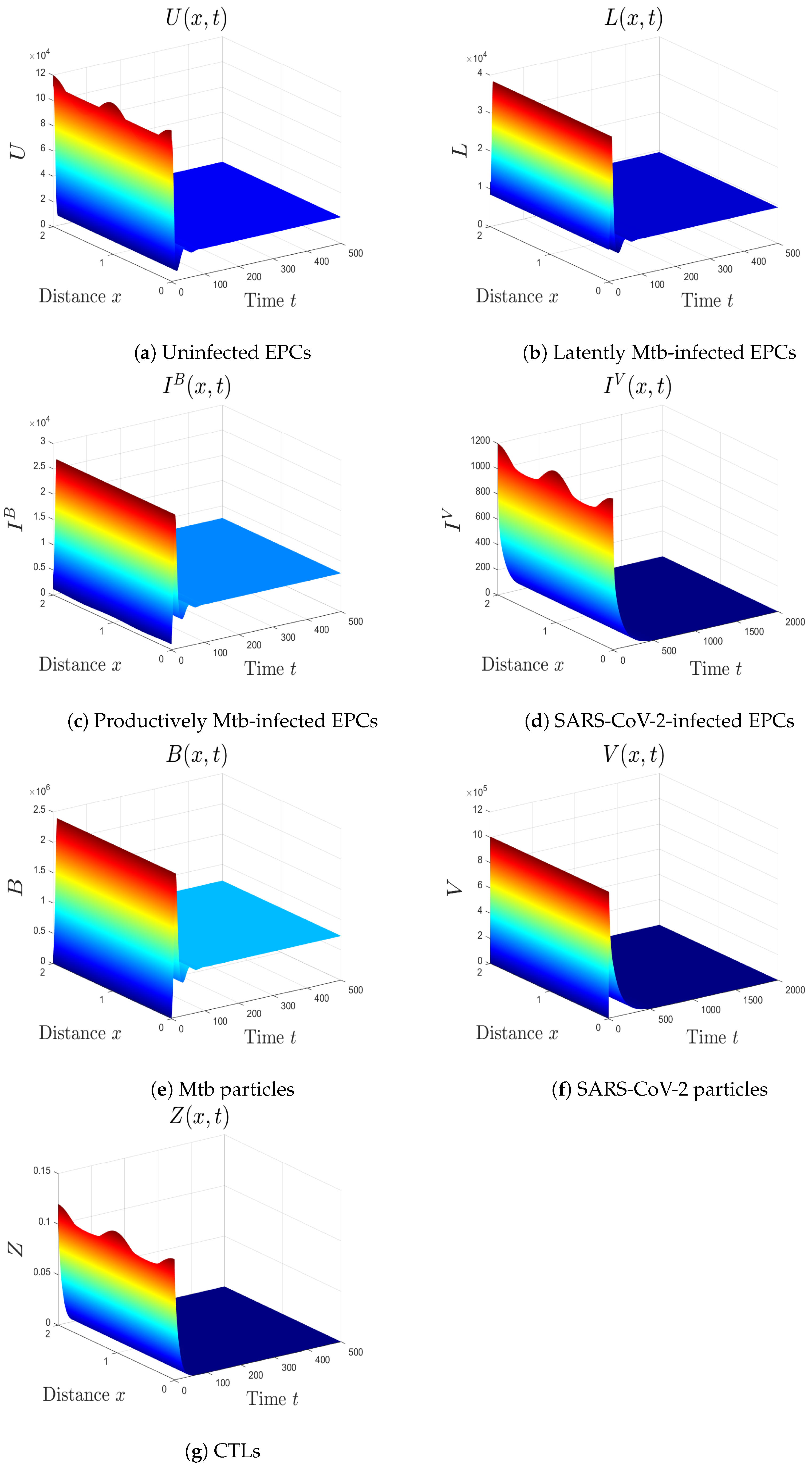
- (vi)
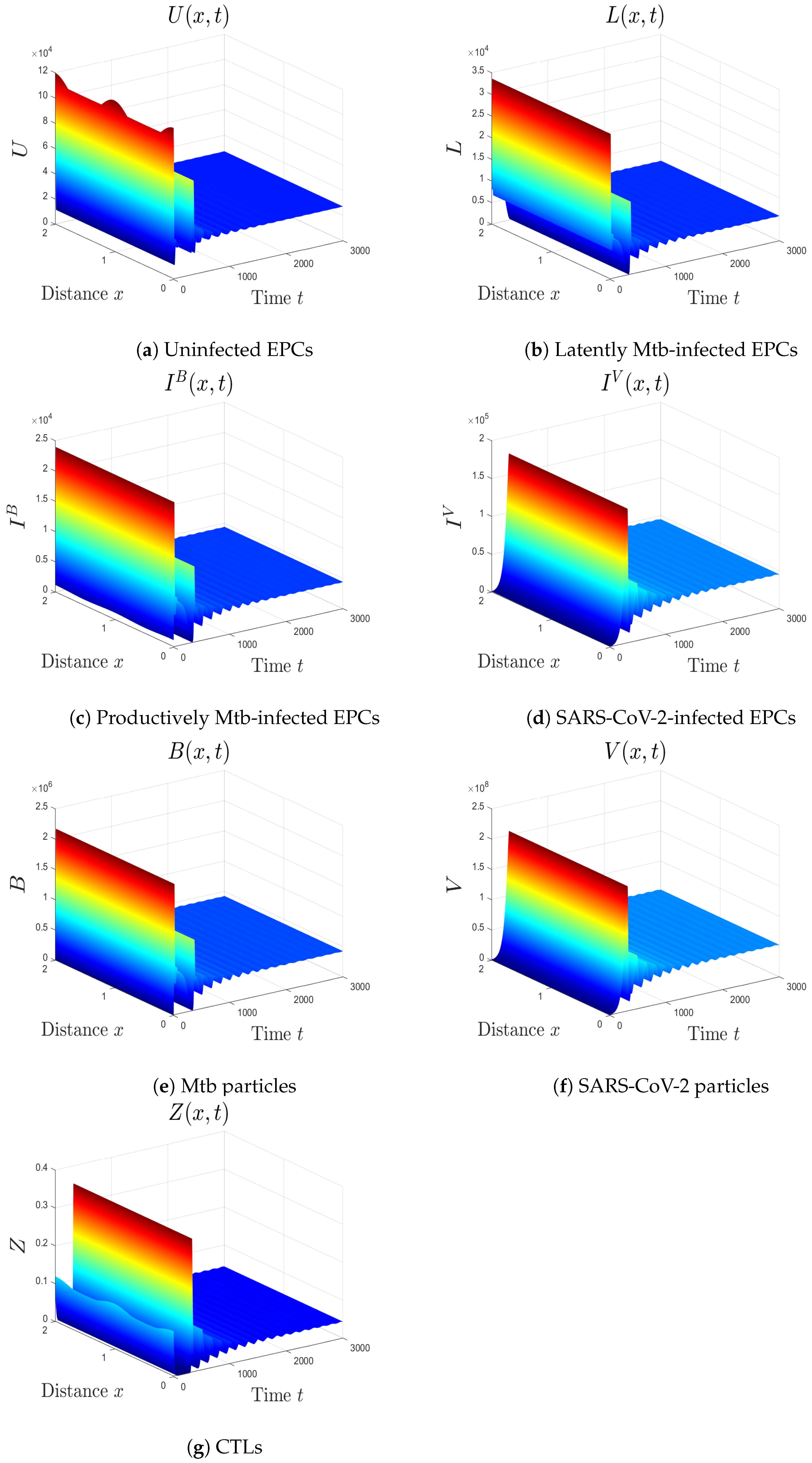
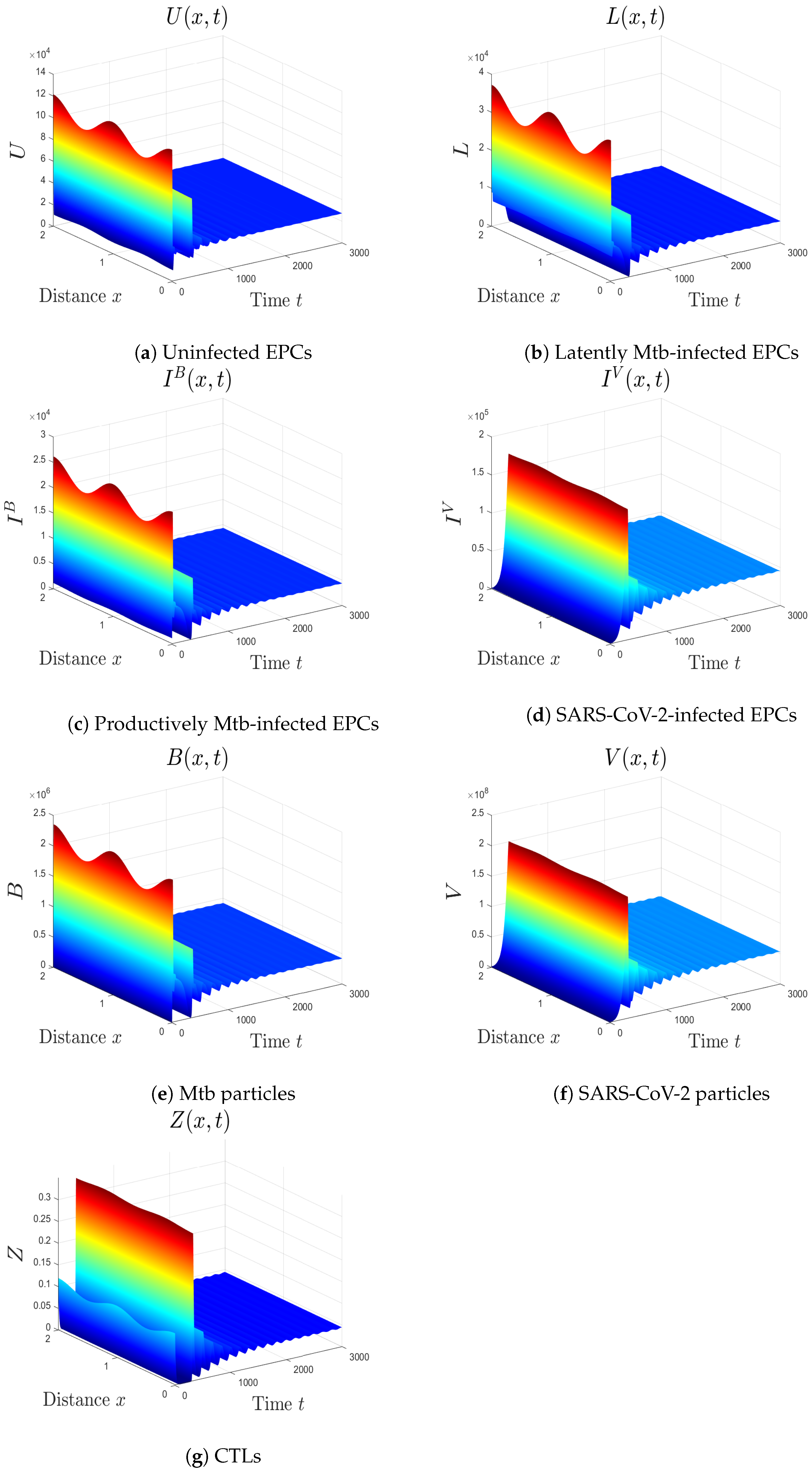
- We choose , , and . These values give , , , and . This implicates the global stability of , , which is compatible with Theorem 7 (Figure 6). In this situation, the person has SARS-CoV-2/Mtb coinfection with robust CTL immunity.
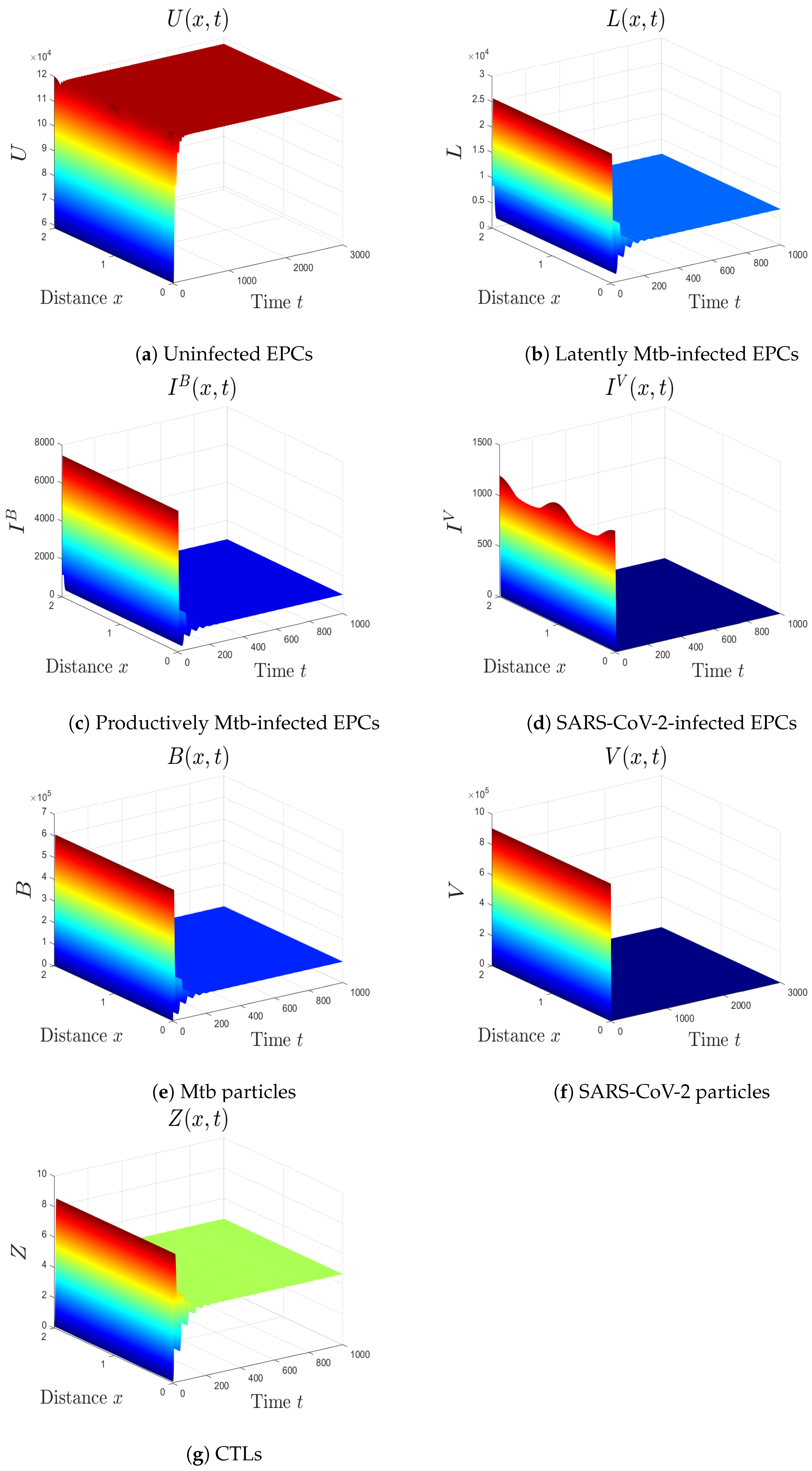
5.1. The Movement from the Monoinfection to the Coinfection State
5.2. The Impact of the Diffusion Coefficients On Coinfection
6. Conclusions and Future Works
- (i)
- The uninfected equilibrium constantly exists. It is GS if and . This equilibrium imitates the status of a healthy individual with negative SARS-CoV-2 and Mtb tests.
- (ii)
- The Mtb immune-free equilibrium is marked if , while it is GS if and . The patient here suffers from Mtb monoinfection, where the CTL immunity has not yet been activated.
- (iii)
- The COVID-19 immune-free equilibrium occurs when . It is GS if and . Here, the patient has SARS-CoV-2 monoinfection with inefficient CTLs.
- (iv)
- The Mtb equilibrium with immunity exists if , while it is GS if . In this condition, the CTL immunity is stimulated to eliminate Mtb infection.
- (v)
- The COVID-19 equilibrium with immunity exists if , and it is GS if . This simulates the case of an individual with COVID-19 infection and active CTL immunity.
- (vi)
- The Mtb/SARS-CoV-2 coinfection equilibrium exists and it is GS if , , , , and . Here, the patient with a single infection becomes infected with both SARS-CoV-2 and Mtb.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronavirus Disease (COVID-19), Weekly Epidemiological Update (12 October 2022), World Health Organization (WHO). 2022. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---1-march-2023 (accessed on 1 January 2023).
- Song, W.; Zhao, J.; Zhang, Q.; Liu, S.; Zhu, X.; An, Q.Q.; Xu, T.T.; Li, S.J.; Liu, J.Y.; Tao, N.N.; et al. COVID-19 and Tuberculosis coinfection: An overview of case reports/case series and meta-analysis. Front. Med. 2021, 8, 657006. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Shah, Z.; Yasmeen, N.; Baloch, Z.; Xia, X. Pathogenesis of SARS-CoV-2 and Mycobacterium tuberculosis coinfection. Front. Immunol. 2022, 13, 909011. [Google Scholar] [CrossRef] [PubMed]
- Luke, E.; Swafford, K.; Shirazi, G.; Venketaraman, V. TB and COVID-19: An exploration of the characteristics and resulting complications of co-infection. Front. Biosci. 2022, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Gatechompol, S.; Avihingsanon, A.; Putcharoen, O.; Ruxrungtham, K.; Kuritzkes, D.R. COVID-19 and HIV infection co-pandemics and their impact: A review of the literature. AIDS Res. Ther. 2021, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Shariq, M.; Sheikh, J.; Quadir, N.; Sharma, N.; Hasnain, S.; Ehtesham, N. COVID-19 and tuberculosis: The double whammy of respiratory pathogens. Eur. Respir. Rev. 2022, 31, 210264. [Google Scholar] [CrossRef]
- Tapela, K.; Olwal, C.O.; Quaye, O. Parallels in the pathogenesis of SARS-CoV-2 and M. tuberculosis: A synergistic or antagonistic alliance? Future Microbiol. 2020, 15, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Petrone, L.; Petruccioli, E.; Vanini, V.; Cuzzi, G.; Gualano, G.; Vittozzi, P.; Nicastri, E.; Maffongelli, G.; Grifoni, A.; Sette, A.; et al. Coinfection of tuberculosis and COVID-19 limits the ability to in vitro respond to SARS-CoV-2. Int. J. Infect. Dis. 2021, 113, S82–S87. [Google Scholar] [CrossRef]
- Liang, K. Mathematical model of infection kinetics and its analysis for COVID-19, SARS and MERS. Infect. Genet. Evol. 2020, 82, 104306. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.V. Mathematical modelling on diffusion and control of COVID–19. Infect. Dis. Model. 2020, 5, 588–597. [Google Scholar] [CrossRef]
- Ivorra, B.; Ferrández, M.R.; Vela-Pérez, M.; Ramos, A.M. Mathematical modeling of the spread of the coronavirus disease 2019 (COVID-19) taking into account the undetected infections. The case of China. Commun. Nonlinear Sci. Numer. Simul. 2020, 88, 105303. [Google Scholar] [CrossRef]
- Yang, C.; Wang, J. A mathematical model for the novel coronavirus epidemic in Wuhan, China. Math. Biosci. Eng. 2020, 17, 2708–2724. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.V.; Prakash, J. Mathematical modelling on phase based transmissibility of Coronavirus. Infect. Dis. Model. 2020, 5, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, K.; Hasanzadeh, N.; Parastesh, F.; Hamarash, I.I.; Jafari, S.; Hussain, I. A fractional-order model for the novel coronavirus (COVID-19) outbreak. Nonlinear Dyn. 2020, 101, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.M.; Rui, J.; Wang, Q.P.; Zhao, Z.Y.; Cui, J.A.; Yin, L. A mathematical model for simulating the phase-based transmissibility of a novel coronavirus. Infect. Dis. Poverty 2020, 9, 24. [Google Scholar] [CrossRef]
- Almocera, A.S.; Quiroz, G.; Hernandez-Vargas, E.A. Stability analysis in COVID-19 within-host model with immune response. Commun. Nonlinear Sci. Numer. 2020, 95, 105584. [Google Scholar] [CrossRef]
- Hernandez-Vargas, E.A.; Velasco-Hernandez, J.X. In-host mathematical modeling of COVID-19 in humans. Annu. Control 2020, 50, 448–456. [Google Scholar] [CrossRef]
- Li, C.; Xu, J.; Liu, J.; Zhou, Y. The within-host viral kinetics of SARS-CoV-2. Math. Biosci. Eng. 2020, 17, 2853–2861. [Google Scholar] [CrossRef]
- Blower, S.M.; Mclean, A.R.; Porco, T.C.; Small, P.M.; Hopewell, P.C.; Sanchez, M.A.; Moss, A.R. The intrinsic transmission dynamics of tuberculosis epidemics. Nat. Med. 1995, 1, 815–821. [Google Scholar] [CrossRef]
- Castillo-Chavez, C.; Feng, Z. To treat or not to treat: The case of tuberculosis. J. Math. Biol. 1997, 35, 629–656. [Google Scholar] [CrossRef]
- Feng, Z.; Castillo-Chavez, C.; Capurro, A. A model for tuberculosis with exogenous reinfection. Theor. Popul. Biol. 2000, 57, 235–247. [Google Scholar] [CrossRef]
- Castillo-Chavez, C.; Song, B. Dynamical models of tuberculosis and their applications. Math. Biosci. Eng. 2004, 1, 361–404. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wu, J.; Heffernan, J. A simple in-host model for Mycobacterium tuberculosis that captures all infection outcomes. Math. Popul. Stud. 2017, 24, 37–63. [Google Scholar] [CrossRef]
- He, D.; Wang, Q.; Lo, W. Mathematical analysis of macrophage-bacteria interaction in tuberculosis infection. Discret. Contin. Dyn. Syst. Ser. B 2018, 23, 3387–3413. [Google Scholar] [CrossRef]
- Yao, M.; Zhang, Y.; Wang, W. Bifurcation analysis for an in-host Mycobacterium tuberculosis model. Discret. Contin. Dyn. Syst. Ser. B 2021, 26, 2299–2322. [Google Scholar] [CrossRef]
- Zhang, W. Analysis of an in-host tuberculosis model for disease control. Appl. Math. Lett. 2020, 99, 105983. [Google Scholar] [CrossRef]
- Ibargüen-Mondragón, E.; Esteva, L.; Burbano-Rosero, E. Mathematical model for the growth of Mycobacterium tuberculosis in the granuloma. Math. Biosci. Eng. 2018, 15, 407–428. [Google Scholar] [PubMed]
- Pinky, L.; Dobrovolny, H.M. SARS-CoV-2 coinfections: Could influenza and the common cold be beneficial? J. Med. Virol. 2020, 92, 2623–2630. [Google Scholar] [CrossRef]
- Agha, A.D.A.; Elaiw, A.M. Global dynamics of SARS-CoV-2/malaria model with antibody immune response. Math. Biosci. Eng. 2022, 19, 8380–8410. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Agha, A.D.A.; Azoz, S.A.; Ramadan, E. Global analysis of within-host SARS-CoV-2/HIV coinfection model with latency. Eur. Phys. J. Plus 2022, 137, 174. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Agha, A.D.A. Global dynamics of SARS-CoV-2/cancer model with immune responses. Appl. Math. Comput. 2021, 408, 126364. [Google Scholar] [CrossRef]
- Mekonen, K.; Balcha, S.; Obsu, L.; Hassen, A. Mathematical modeling and analysis of TB and COVID-19 coinfection. J. Appl. Math. 2022, 2022, 2449710. [Google Scholar] [CrossRef]
- Bandekar, S.; Ghosh, M. A co-infection model on TB—COVID-19 with optimal control and sensitivity analysis. Math. Comput. Simul. 2022, 200, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, Y.; Nagappa, B.; Sharma, N.; Basu, S.; Chopra, K. COVID-19 and tuberculosis: A mathematical model based forecasting in Delhi, India. Indian J. Tuberc. 2020, 67, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Elaiw, A.M.; Agha, A.D.A. Analysis of the in-host dynamics of tuberculosis and SARS-CoV-2 coinfection. Mathematics 2023, 11, 1104. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Z. Dynamics of a diffusive HBV model with delayed Beddington-DeAngelis response. Nonlinear Anal. Real World Appl. 2014, 15, 118–139. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, Y. Stability of a CD4+ T cell viral infection model with diffusion. Int. J. Biomath. 2018, 11, 1–16. [Google Scholar] [CrossRef]
- Protter, M.H.; Weinberger, H.F. Maximum Principles in Differential Equations; Prentic Hall: Englewood Cliffs, NJ, USA, 1967. [Google Scholar]
- Henry, D. Geometric Theory of Semilinear Parabolic Equations; Springer: New York, NY, USA, 1993. [Google Scholar]
- Korobeinikov, A. Global properties of basic virus dynamics models. Bull. Math. Biol. 2004, 66, 879–883. [Google Scholar] [CrossRef]
- Roy, P.; Roy, A.; Khailov, E.; Basir, F.A.; Grigorieva, E. A model of the optimal immunotherapy of psoriasis by introducing IL-10 and IL-22 inhibitor. J. Biol. Syst. 2020, 28, 609–639. [Google Scholar] [CrossRef]
- Cao, X.; Roy, A.; Basir, F.A.; Roy, P. Global dynamics of HIV infection with two disease transmission routes—A mathematical model. Commun. Math. Biol. Neurosci. 2020, 2020, 8. [Google Scholar]
- Khalil, H.K. Nonlinear Systems; Prentice-Hall: Hoboken, NJ, USA, 1996. [Google Scholar]
- Sumi, T.; Harada, K. Immune response to SARS-CoV-2 in severe disease and long COVID-19. iScience 2022, 25, 104723. [Google Scholar] [CrossRef]
- Ain, Q.T.; Chu, Y. On fractal fractional hepatitis B epidemic model with modified vaccination effects. Fractals 2022, 1–18. [Google Scholar] [CrossRef]
- Ain, Q.T.; Anjum, N.; Din, A.; Zeb, A.; Djilali, S.; Khan, Z. On the analysis of Caputo fractional order dynamics of Middle East Lungs Coronavirus (MERS-CoV) model. Alex. Eng. J. 2022, 61, 5123–5131. [Google Scholar]
- Elaiw, A.M.; Agha, A.D.A. Global stability of a reaction-diffusion malaria/COVID-19 coinfection dynamics model. Mathematics 2022, 10, 4390. [Google Scholar] [CrossRef]
- Bellomo, N.; Burini, D.; Outada, N. Multiscale models of Covid-19 with mutations and variants. Netw. Heterog. Media 2022, 17, 293–310. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algarni, A.; Al Agha, A.D.; Fayomi, A.; Al Garalleh, H. Kinetics of a Reaction-Diffusion Mtb/SARS-CoV-2 Coinfection Model with Immunity. Mathematics 2023, 11, 1715. https://doi.org/10.3390/math11071715
Algarni A, Al Agha AD, Fayomi A, Al Garalleh H. Kinetics of a Reaction-Diffusion Mtb/SARS-CoV-2 Coinfection Model with Immunity. Mathematics. 2023; 11(7):1715. https://doi.org/10.3390/math11071715
Chicago/Turabian StyleAlgarni, Ali, Afnan D. Al Agha, Aisha Fayomi, and Hakim Al Garalleh. 2023. "Kinetics of a Reaction-Diffusion Mtb/SARS-CoV-2 Coinfection Model with Immunity" Mathematics 11, no. 7: 1715. https://doi.org/10.3390/math11071715
APA StyleAlgarni, A., Al Agha, A. D., Fayomi, A., & Al Garalleh, H. (2023). Kinetics of a Reaction-Diffusion Mtb/SARS-CoV-2 Coinfection Model with Immunity. Mathematics, 11(7), 1715. https://doi.org/10.3390/math11071715






