Abstract
We derive a mathematical model that describes the competition of two populations in a chemostat in the presence of a virus. We suppose that only one population is affected by the virus. We also suppose that the substrate is continuously added to the bioreactor. We obtain a model taking the form of an “SI” epidemic model using general increasing growth rates of bacteria on the substrate and a general increasing incidence rate for the viral infection. The stability of the steady states was carried out. The system can have multiple steady states with which we can determine the necessary and sufficient conditions for both existence and local stability. We exclude the possibility of periodic orbits and we prove the uniform persistence of both species. Finally, we give some numerical simulations that validate the obtained results.
MSC:
34C60; 34C23; 92D25; 93D30; 93D20; 34C37; 34C55; 34C15
1. Introduction
A bioreactor (Figure 1) is a tank (a lake) in which microorganisms multiply (yeasts, bacteria, fungi, algae, etc.) which consume substrates or feed on other organisms to develop, and which use precursors and activators to produce biomass, synthesize metabolites, or even bioconvert molecules of interest (e.g., depollution). Thanks to the bioreactor, it is possible to control the culture conditions (temperature, pH, aeration, etc.) and, therefore, to collect relatively reliable experimental data for monitoring bacterial growth and/or the chemical reaction of interest. If we consider a competition between two species for an essential substrate, a classical postulate, known as the competitive exclusion principle, suggests that, at most, one species can survive and the other species disappear. This principle has been frequently demonstrated mathematically and validated experimentally (see, for example, [1,2,3,4,5]). Several works [6,7,8,9,10,11,12,13,14] have tried to explain coexistence of bacterial competitors using several approaches. Increasingly interested in aquatic environments, researchers are discovering that the organisms colonized by viruses are much more varied than the bacterial species anticipated [15]. The shape and size of some viruses are also surprising. Finally, we are beginning to measure the impact of viral diversity on the living world. Through various mechanisms, such as the destruction of a dominant species to the benefit of rarer species [16] or the transfer of viral genes to the host, viruses (bacteriophage) maintain the biodiversity of aquatic ecosystems and facilitate genetic mixing [17]. Several works [17,18] confirm that viruses have a significant role in aquatic bacterial diversity. Therefore, the role of viruses in aquatic ecosystems cannot be neglected and should be taken into account when modeling bacterial competition in an aquatic ecosystem.
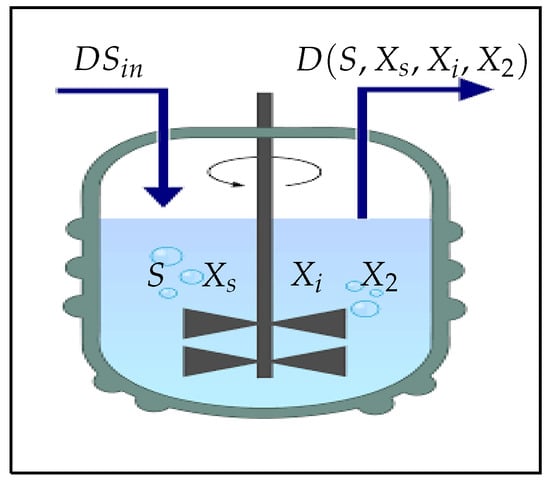
Figure 1.
A chemostat is a well-stirred bioreactor [19] where a limiting substrate () is continuously added to a liquid culture containing two competitors () in the presence of a virus affecting only the first competitor.
Note that a viral infection can be modeled with an epidemiological model, using either a deterministic, delayed, or stochastic approach [20,21,22,23,24]. One of the basic models for the spread of a disease was proposed in [25], dividing the population into three compartments, namely the infected population compartment (I), the susceptible population compartment (S), and the recovered population compartment (R), known as the “SIR” model. An extension of the “SIR” models is given by the “SEIR” (Susceptible, Exposed, Infected, Recovered) ones [26,27,28,29]. The “SEIR” epidemic models were extended to “SVEIR” models (Susceptible, Vaccinated, Exposed, Infected, Recovered), taking into account the proportion of immigrants who have been vaccinated [30,31,32,33]. Most of these works investigated the proposed models by giving the basic reproduction number and the local and global stability of the steady states using local linearisation and Lyapunov theory.
An important question has been asked in [34]: does the presence of a virus induce the stable coexistence of bacterial competitors in an aquatic-like system?
The response was given by proposing and analyzing a mathematical model of exploitative competition in a continuous reactor containing a virus [34]. The authors assume that only the species which have the best affinity with the substrate are affected by the virus. They proved under certain conditions that the coexistence of competitor bacteria is possible. Mestivier et al. [35] and Weitz et al. [36] proposed some mathematical models where the virus dynamics are given explicitly. It is shown that the coexistence between two competitor bacteria is possible in the presence of a virulent virus. Similarly, in [37], the authors considered a mathematical model where the virus behaviour is given explicitly and they give some conditions satisfying the coexistence of all competitors.
In this paper, we propose a generalized model of the one given in [34] by considering general increasing growth rates of bacteria on the substrate and a general increasing incidence rate for the viral infection. We introduce the model in Section 2 and we give some general results. In Section 3, we discuss the case where there is no viral infection where the competitive exclusion principle is valid. In Section 4, we reduce the system to a three-dimensional one which facilitates the mathematical analysis. We discuss the local analysis in Section 4.1, we prove that there is no periodic orbits on the faces in Section 4.2, and then we conclude on the persistence in Section 4.3 and the uniform persistence in Section 4.4. Then, we return in Section 5 to the main model where we discuss the uniform persistence. Then, we give some numerical simulations in Section 6. Finally, we summarize the main results and discuss certain implications in Section 7.
2. Modeling Bacterial Competition in the Presence of a Virus
Consider a bio-reactor in which the bacterial competition of two species in the presence of a virus that affects only species 1 was studied (see Figure 1 and Figure 2). Therefore, species 1 is present in two compartments, susceptibles () and infectives or bacteriophage (); however, species 2 is present in a single form (). We know that a virus requires a host to replicate; that is why we do not explicitly model the virus dynamics and we assume that the virus spreads when an infected species takes contact with a susceptible one, which is the case of the classical epidemic models (“SI”, “SIS”, “SIR”, “SIRS”, “SEIR”, “SEIRS”, “SVEIR”). The limiting substrate (S) was added instantaneously to the reactor with a flow rate D and a concentration . The culture liquid, containing the substrate, species 1 (either infected or not), and species 2, is continuously mixed and removed at the same flow rate, D. Note that the viral infection concerns only species 1. We neglected all natural mortality rates compared to the dilution rate.
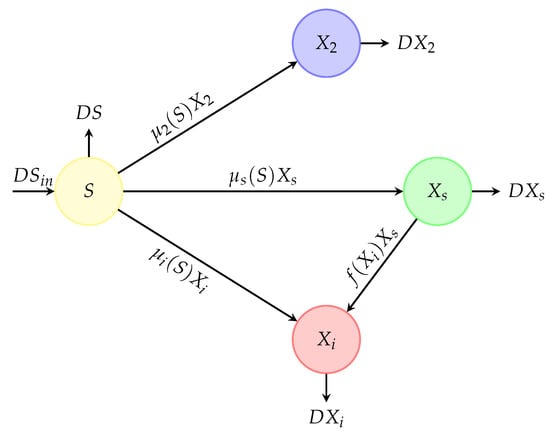
Figure 2.
Competition diagram of the competition of the two species in the presence of a virus inside a bioreactor. Compartments , and are described by circles and transition rates between compartments are described by arrows and labels.
We proposed a mathematical model describing the competition of two species for a single non-reproducing growth-limiting substrate in a continuous reactor that it is well-stirred in the presence of a virus that affects only species 1 (species 2 is not susceptible to the virus attack, Figure 2). The mathematical model takes the form of an “SI” epidemic model where the main goal is to find under what conditions the coexistence of all species is possible. This model is a generalization of the model proposed in [34] by considering generalized growth rates for all species and also a generalized incidence rate for the viral infection. The model is given by the following fourth-dimensional system of ordinary differential equations:
Here, S denotes the concentration of the resource with , whereas , and stand for the concentrations of susceptible species 1, infected species 1, and species 2, respectively, with initial conditions satisfying and . Note that D and describe the dilution rate and the substrate input concentration, respectively, and are assumed to be constant and positive. and denote the yield coefficients, commonly referred to as the substrate-to-species-1 (either infected or not) and substrate-to-species-2 yields, respectively. The significance of the variables and parameters is shown in Table 1.

Table 1.
Variables and parameters meaning of system (1).
By making the following change of variable, we obtain a more simplified model. Let , and . Then, the model takes the form:
Let us define some operating parameters as follows: , , and . All the mentioned parameters are positive. Through the paper, we will consider the most important case by using the following assumption:
Assumption 1.
The growth rates , , and are increasing, non-negative, functions, such that . Furthermore, for all and .
Let us define the values and as the solutions of and , respectively.
Remark 1.
- 1.
- Assumption 1 expresses that species 1 has the best affinity with the substrate and then it wins the competition in the absence of the infection. Once the infection is present, Assumption 1 expresses that the non-infected species 1 () still has the best affinity with the substrate; however, infected species 1 () has a growth rate () smaller than both growth rates ( and ) of the non-infected species 1 and species 2.
- 2.
- Monod functions (or Holling’s functions type II) are candidate functions that can express growth rates (Figure 3):where , and are Monod constants. , , and are positive constants. All constants can be chosen such that the functions , , and satisfy Assumption 1. For example, we can take and .
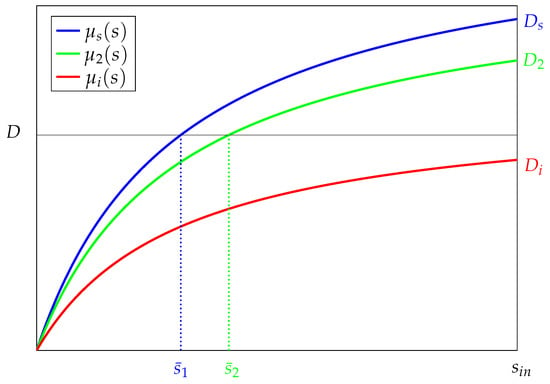 Figure 3. Typical growth rates and where and .
Figure 3. Typical growth rates and where and .
The model (2) of the chemostat is a dynamical system defined on the non-negative cone, for which we recall some fundamental properties (see, for instance, [38]).
Proposition 1.
System (2) satisfies
Proof.
- The invariance of is confirmed by the following points: , , , and .Consider the variable . By adding all equations of model (2), we deduce that:and, therefore, we obtain:with . Since all compartments of the sum are non-negative, we can conclude on the boundedness of the solution.
- It can be deduced from the relation (3).
□
Lemma 1.
and exist and are unique and satisfy .
Proof.
The function is continuous and increasing, such that and ; therefore, exists and is unique. The function is continuous and increasing, such that and ; therefore, exists and is unique. Since for all then . □
Assumption 2.
The incidence rate μ is an increasing, non-negative, concave function, such that . Furthermore, μ satisfies:
and
Remark 2.
Monod (or Holding type II) function is a candidate function that can express the incidence rate , where k is the Monod constant. is the maximum incidence rate. Note that μ satisfies Assumption 2.
The incidence rate satisfies the following lemma.
Lemma 2.
The incidence rate μ satisfies .
Proof.
Let , and the function . Since ( is an increasing function) and ( is a concave function), then and . Therefore, . Similarly, let ; then, once is a concave function. Thus, and . □
Let us define the basic reproduction number for system (2) using the next-generation operator approach proposed in [39] and deduced from the third equation (infected compartment) of system (2) and, therefore, given by:
Here, describes the mean number of infective produced in a chemostat by introducing a single infective into a totally susceptible population inside the reactor. describes the average time that an infective individual passes inside the chemostat as an infective.
For the rest of the paper, we consider the most important case where .
Assumption 3.
or, equivalently, .
Let us recall the classical ‘chemostat’ model in the absence of the virus.
3. Virus-Free Subsystem
Consider the following three-dimensional system which is the virus-free subsystem:
This model is the same as (2) in the absence of the viral infection (). This model predicts the competitive exclusion; that is, under Assumption 1, at most, the first species (which has the best affinity with the substrate) avoids extinction; however, the second species goes to extinction (see, for example, [19,38,40,41]). Let us define the steady-states of system (6) on the non-negative quadrant by , , and with:
where (according to Lemma 1). Therefore, we have:
Proposition 2.
The equilibrium point is globally asymptotically stable [38].
Note that by introducing a virus that affects only species 1, which has the best affinity with the nutriment, we aim to give a possibility of the coexistence of both competing species.
4. Reduction to Three-Dimensional System
Note that all solutions of the 4D-dynamics (2) converge toward . Now, because we are interested by the asymptotic behavior of the dynamics (2), we will restrict the study to . Thanks to Thieme’s results [42], the asymptotic behavior of the reduced dynamics will be informative for the dynamics (2); see [19,43] for other applications. The reduced dynamics of (2) on is given by:
where the functions , and are given by:
Thus, for (7) the state-vector belongs to the following subset of :
Formally, let , , , and be the four equilibrium points of dynamics (7) on . reflects the extinction of all species and predators, and reflects the extinction of the infected first species and the second species while the non-infected first species is present. reflects the extinction of the first species (either infected or not) while the second species is present. Finally, reflects the coexistence of both species including the first species in its two forms, infected or not.
, , , and are given by:
- .
- , where is the unique solution of the equation .
- , where is the unique solution of the equation .
- , where is the solution of the three-dimensional system given by:
From the third equation of system (9), and by Assumption 1, there exists a unique value , such that . Thus, and the system (9) is reduced to:
From the second equation of (10), we have . Let . Therefore, from the first equation of (10), we have:
The derivative of is given by:
Furthermore, we have:
Therefore, the equation admits a unique solution and, thus, the existence and uniqueness of the equilibrium point corresponding to the coexistence of all species:
The following equilibrium points are either not generic or not possible; that is why they are neglected.
- , where is the solution of the two-dimensional system given by:This case will be ignored since it is non-generic because we obtain (classical model of bacterial competition in a chemostat).
- , where is the solution of the two-dimensional system given by:This case will be ignored since we obtain , which is impossible because .
- , where is the solution of the equation . Again, this equilibrium is not possible since .
- , where is the solution of the two-dimensional system given by:Let:andand are non-empty and can intersect at a finite number of positive equilibrium points of the form , such that and . Functions and are decreasing. Therefore, the isoclines are the graphs of two functions and and, then, and . is solution of , where . The derivatives of and are given by and . According to Assumption 2, we have , and by Lemma 2, we have ; therefore, one deduces that:Note that , since by Assumption 3. Therefore, there is no equilibrium points of the form if .
Therefore, we will consider only the equilibrium points , , , and to be the four equilibrium points of dynamics (7) on and we resume them in Proposition 3.
Proposition 3.
Under Assumptions 1–3, the dynamics (7) admit four equilibrium points , , , and .
4.1. Local Stability
The Jacobian matrix at a point solution of system (7) is given by:
- The Jacobian matrix calculated at the steady-state is given by:admits three eigenvalues: , , and . Then, the steady-state is a saddle point.
- The Jacobian matrix calculated at the steady-state is given by:where and are expressed at . admits three eigenvalues: , , and . Then, the steady-state is a saddle point.
- The Jacobian matrix calculated at the steady-state is given by:where and are expressed at . admits three eigenvalues: , , and . Thus, the steady-state is a saddle point.
- The Jacobian matrix calculated at the steady-state is given by:where , , and are expressed at .admits three eigenvalues: , , and , roots of the characteristic polynomial given by:with:We can verify, by using Maple, that , and . Then, the steady-state is locally asymptotically stable once it exists.
According to Assumptions 1–3, we resume the local stability of equilibrium points in the following proposition.
Proposition 4.
, , and are saddle points; however, is stable node.
4.2. No Periodic Orbits on the Faces
We start by excluding the possibility of periodic trajectory in one of the faces of the invariant set .
- Note that the axes and are invariant. Let us apply the transformation and for . Then, one gets the following new system:Note that using Lemma 2, we have:
- Note that the axes and are invariant. Let ua apply the transformation and for . Then, one gets the following new system:Note that:
- Note that the axes and are invariant. Let ua apply the transformation and for . Then, one gets the following new system:Note that:
4.3. Persistence
In this subsection, we aim to prove the coexistence of both species 1 (either infected or not) and species 2 by proving the uniform persistence of dynamics (7). The saddle points , , and are the only boundary steady states for the dynamics (7). Then, we apply the proof used in [37,43,44] using the Butler-McGehee Lemma [38] frequently to prove the persistence of system (7).
Theorem 1.
Dynamics (7) is persistent.
Proof.
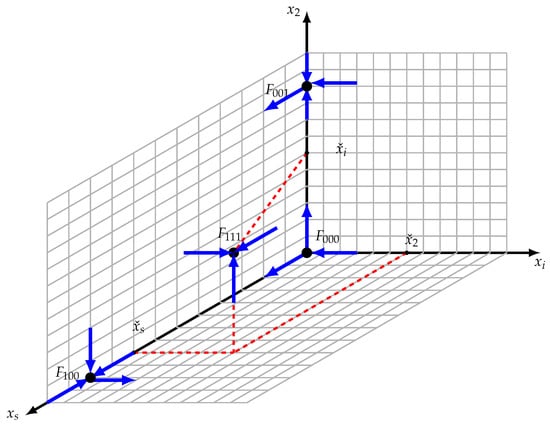
All the faces , , and are invariant. Furthermore, stable and unstable manifolds of the boundary equilibrium points are represented in Figure 4.

Figure 4.
Equilibria configuration. , , and are saddle points; however, is an asymptotically stable interior equilibrium.
Consider a solution with an initial condition , where , , and are given data. Let us denote to be the omega limit set of , where is the positive semi-orbit passing through . We aim to prove that the omega limit set has no points on each of the three faces.
- Assume that . Then, inside . The stable manifold is one-dimensional and is restricted to the -axis. Therefore, the entire orbit passing through , which is inside , becomes unbounded, which contradicts the existence of .
- Assume that . is a saddle point with a stable manifold, , of dimension two, restricted to the -plane. Therefore, is not the entire omega limit set . Using the Butler-McGehee Lemma [38], there exists a point inside . Since lies entirely in the -plane, and since the entire orbit through is in , this orbit is unbounded, which contradicts the fact that is inside .
- Assume that . Since is a saddle point where its stable manifold is of dimension two and is restricted to the -plane, then is not the entire omega limit set . Therefore, using the Butler-McGehee Lemma [38], there exists a point inside . Since lies entirely in the -plane, and since the entire orbit through is in , this orbit is unbounded, which contradicts the fact that is inside .
Now, let with at least one of the components , , and is zero, and suppose that . Thus, the entire orbit passing through should be inside . However, since the orbit should lie entirely inside either , , or faces, it should converge to one of the boundary equilibrium points, since there is no periodic trajectory. Therefore, this boundary equilibrium point is inside , which contradicts the fact that all boundary equilibrium points are saddle points. Therefore, each of the components of the trajectory is greater than zero:
and then system (7) is persistent (see Section 4.3 in [44] for another example). □
4.4. Uniform Persistence of System (7)
Persistence and uniform persistence [45] are equivalent in many examples of mathematical models. Recall a theory in [45] stating that if is a dynamical system, such that and are both invariant, then is uniformly persistent if it satisfies the following statements.
- is weakly persistent;
- is dissipative;
- is isolated, where be the restriction of to ;
- is acyclic.
Consider the dynamics on the invariant attractor bounded set . We can apply the theorem given in [45] if and is invariant on , but repelling into the interior of on if Conditions 3 and 4 are satisfied when restricting to . It is clear that condition 1 is satisfied. Condition 2 is also satisfied according to Theorem 1. Condition 3 is satisfied because all boundary equilibrium points are hyperbolic and then their union forms a covering of the omega limit sets of . Condition 4 is also satisfied because the boundary equilibrium points are not linked cyclically. Thus, we conclude on the uniform persistence of system (7).
Theorem 2.
Dynamics (7) is uniformly persistent, i.e., , such that:
5. Uniform Persistence of System (2)
Return to the main mathematical model (2) describing the competition of two bacteria in a chemostat in the presence of a virus that affects only the first bacteria. System (2) admits , , , and as equilibrium points. , , and are saddle points; however, is locally asymptotically stable. We need to prove the uniform persistence of the main system (2). Let with and ; then, . Furthermore, assume that such that the the solution converges to . This is not possible since is a global attractor according to proposition 1. Now, suppose that contains a point on one of the faces where one of the variables , or is zero; therefore, the entire trajectory passing through this point should be inside . Thus, the omega limit set should be entirely inside .
Theorem 3.
Dynamics (2) is uniformly persistent, i.e., , such that:
6. Numerical Simulations
We cofirm the theoretical findings by some numerical results using Monod functions (or Holling’s functions type II) to express all growth rates and the incidence rate:
where , and k are Monod constants. , , , and are positive constants. We used Holling type-II functions as typical examples [46,47] since they are nonlinear and satisfied all our assumptions on growth rates and incidence rates. All constants are chosen such that the functions , , , and satisfy Assumptions 1–3.
Consider the parameters values given in the following Table 2.

Table 2.
The parameter values are used to illustrate the theoretical results, but they have no biological significance.
We give two examples that satisfy Assumptions 1–3, which ensures the persistence of both species 1 (either infected or not) and species 2, as seen in Figure 5 and Figure 6.
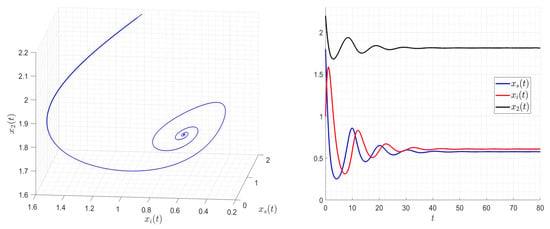
Figure 5.
and and then Assumptions 1–3 are satisfied. The solution of the system (2) converges to the equilibrium where the two species coexist (either infected or not).
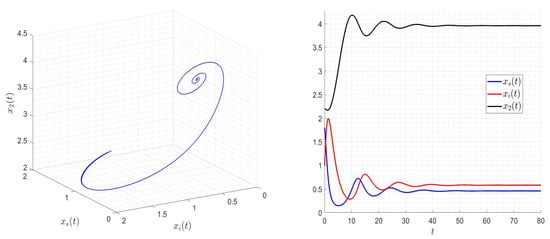
Figure 6.
, and then Assumptions 1–3 are satisfied. The solution of the system (2) converges to the equilibrium where the two species coexist (either infected or not).
7. Conclusions
Since we aim to prove that the competitive exclusion principle is not usually valid when two competitors grow on a single essential resource, we add, in this paper, an additional mechanism of competition by adding a virus in the chemostat that affects only the first species, and then the coexistence becomes possible. We propose a mathematical model that describes the competition of two species in a chemostat in the presence of a virus. We suppose that only one population is affected by the virus. We suppose also that the substrate is continuously added to the bioreactor. We obtain a model taking the form of an ’SI’ epidemic model. The stability of the steady states was carried out. The system can have multiple steady states with which we can determine the necessary and sufficient conditions for both existence and local stability. We exclude the possibility of periodic orbits and we prove the uniform persistence of both species. Finally, we give some numerical simulations that validate the obtained results.
The main result of this work is that the presence of the virus allows the coexistence of the two bacterial species when the species cannot coexist unless the virus is present. A biological explanation of this result is that the virus affects the species which should win the competition and then it gives the opportunity to the second species to persist.
Author Contributions
Conceptualization, M.E.H. and A.H.A.; methodology, M.E.H. and A.H.A.; writing—original draft, M.E.H. and A.H.A.; writing—review and editing, M.E.H. and A.H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was funded by Institutional Fund Projects under grant no. IFPIP: 672-130-1443.
Data Availability Statement
Not applicable.
Acknowledgments
This research work was funded by Institutional Fund Projects under grant no. IFPIP: 672-130-1443. The authors gratefully acknowledge the technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia. The authors are also grateful to the unknown referees for the many constructive suggestions, which helped to improve the presentation of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bingtuan, L. Global Asymptotic Behavior of the Chemostat: General Response Functions and Different Removal Rates. SIAM J. Appl. Math. 1998, 59, 411–422. [Google Scholar] [CrossRef]
- Hsu, S.B.; Hubbell, S.; Waltman, P. A mathematical theory for single-nutrient competition in continuous cultures of micro-organisms. SIAM J. Appl. Math. 1977, 32, 366–383. [Google Scholar] [CrossRef]
- Butler, G.J.; Wolkowicz, G.S.K. A mathematical model of the chemostat with a general class of functions describing nutrient uptake. SIAM J. Appl. Math. 1985, 45, 138–151. [Google Scholar] [CrossRef]
- Wolkowicz, G.S.K.; Lu, Z. Global dynamics of a mathematical model of competition in the chemostat: General response functions and differential death rates. SIAM J. Appl. Math. 1992, 52, 222–233. [Google Scholar] [CrossRef]
- Smith, H.L.; Waltman, P. Competition for a single limiting resource in continuous culture: The variable-yield model. SIAM J. Appl. Math. 1994, 54, 1113–1131. [Google Scholar] [CrossRef]
- Korytowski, D.; Smith, H. Permanence and Stability of a Kill the Winner Model in Marine Ecology. Bull. Math. Biol. 2017, 79, 995–1004. [Google Scholar] [CrossRef]
- Browne, C.; Smith, H. Dynamics of virus and immune response in multi-epitope network. J. Math. Biol. 2018, 77, 1833–1870. [Google Scholar] [CrossRef]
- Vandermeer, J.; Pascual, M. Competitive coexistence through intermediate polyphagy. Ecol. Complex. 2006, 3, 37–43. [Google Scholar] [CrossRef]
- El Hajji, M.; Mazenc, F.; Harmand, J. A mathematical study of a syntrophic relationship of a model of anaerobic digestion process. Math. Biosci. Eng. 2010, 7, 641–656. [Google Scholar] [CrossRef]
- Sari, T.; El Hajji, M.; Harmand, J. The mathematical analysis of a syntrophic relationship between two microbial species in a chemostat. Math. Biosci. Eng. 2012, 9, 627–645. [Google Scholar] [CrossRef]
- Albargi, A.H.; El Hajji, M. Mathematical analysis of a two-tiered microbial food-web model for the anaerobic digestion process. Math. Biosci. Eng. 2023, 20, 6591–6611. [Google Scholar] [CrossRef]
- Hsu, S.B. A competition model for a seasonally fluctuating nutrient. J. Math. Biol. 1980, 9, 115–132. [Google Scholar] [CrossRef]
- Butler, G.J.; Hsu, S.B.; Waltman, P. A mathematical model of the chemostat with periodic washout rate. SIAM J. Appl. Math. 1985, 45, 435–449. [Google Scholar] [CrossRef]
- Wolkowicz, G.S. Successful invasion of a food web in a chemostat. Math. Biosci. 1989, 93, 249–268. [Google Scholar] [CrossRef]
- Bratbak, G.; Heldal, M. Viruses rule the waves—The smallest and most abundant members of marine ecosystems. Microbiol. Today 2000, 27, 171–173. [Google Scholar]
- Das, K.P.; Roy, P.; Karmakar, P.; Sarkar, S. Role of Viral Infection in Controlling Planktonic Blooms-Conclusion Drawn from a Mathematical Model of Phytoplankton-Zooplankton System. Differ. Equ. Dyn. Syst. 2020, 28, 381–400. [Google Scholar] [CrossRef]
- Larsen, A.; Castberg, T.; Sandaa, R.A.; Brussaard, C.P.D.; Egge, J.; Heldal, M.; Paulino, A.; Thyrhaug, R.; van Hannen, E.J.; Bratbak, G. Population dynamics and diversity of phytoplankton, bacteria and viruses in a seawater enclosure. Mar. Ecol. Prog. Ser. 2001, 221, 47–57. [Google Scholar] [CrossRef]
- Proctor, L.M.; Fuhrman, J. Viral mortality of marine bacteria and cyanobacteria. Nature 1990, 343, 60–62. [Google Scholar] [CrossRef]
- El Hajji, M. How can inter-specific interferences explain coexistence or confirm the competitive exclusion principle in a chemostat. Int. J. Biomath. 2018, 11, 1850111. [Google Scholar] [CrossRef]
- Hethcote, H.W. The mathematics of infectious diseases. SIAM Rev. 2000, 42, 599–653. [Google Scholar] [CrossRef]
- Moneim, I.A. An SEIR model with infectious latent and a periodic vaccination strategy. Math. Modell. Anal. 2021, 26, 236–252. [Google Scholar] [CrossRef]
- Martsenyuk, V.; Bernas, M.; Klos-Witkowska, A. Two-Strain COVID-19 Model Using Delayed Dynamic System and Big Data. IEEE Access 2021, 9, 113866–113878. [Google Scholar] [CrossRef]
- El Hajji, M.; Zaghdani, A.; Sayari, S. Mathematical analysis and optimal control for Chikungunya virus with two routes of infection with nonlinear incidence rate. Int. J. Biomath. 2022, 15, 2150088. [Google Scholar] [CrossRef]
- Alshehri, A.; El Hajji, M. Mathematical study for Zika virus transmission with general incidence rate. AIMS Math. 2022, 7, 7117–7142. [Google Scholar] [CrossRef]
- Kermack, F.; McKendrick, D. A contribution to the mathematical theory of epidemics. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 1927, 115, 700–721. [Google Scholar]
- Nkamba, L.; Ntaganda, J.; Abboubakar, H.; Kamgang, J.; Castelli, L. Global Stability of a SVEIR Epidemic Model: Application to Poliomyelitis Transmission Dynamics. Open J. Model. Simul. 2017, 5, 98–112. [Google Scholar] [CrossRef]
- Tang, Y.; Xiao, D.; Zhang, W.; Zhu, D. Dynamics of epidemic models with asymptomatic infection and seasonal succession. Math. Biosci. Eng. 2017, 14, 1407–1424. [Google Scholar] [CrossRef] [PubMed]
- Adda, P.; Nkague Nkamba, L.; Sallet, G.; Castelli, L. A SVEIR model with Imperfect Vaccine. In Proceedings of the CMPD 3 Conference on Computational and Mathematical Population Dynamics, Bordeaux, France, 31 May–4 June 2010. [Google Scholar]
- Momoh, A.A.; Ibrahim, M.O.; Uwanta, I.J.; Manga, S.B. Mathematical model for control of measles epidemiology. Int. J. Pure Appl. Math. 2013, 87, 707–718. [Google Scholar] [CrossRef]
- Edward, S.; Raymond, E.K.; Gabriel, T.K.; Nestory, F.; Godfrey, G.M.; Arbogast, P.M. A mathematical model for control and elimination of the transmission dynamics of measles. Appl. Comput. Math. 2015, 4, 396–408. [Google Scholar] [CrossRef]
- Aldila, D.; Asrianti, D. A deterministic model of measles with imperfect vaccination and quarantine intervention. J. Phys. Conf. Ser. 2019, 1218, 012044. [Google Scholar] [CrossRef]
- El Hajji, M.; Albargi, A.H. A mathematical investigation of an “SVEIR” epidemic model for the measles transmission. Math. Biosci. Eng. 2022, 19, 2853–2875. [Google Scholar] [CrossRef] [PubMed]
- El Hajji, M.; Alshaikh, D.M.; Almuallem, N.A. Periodic behaviour of an epidemic in a seasonal environment with vaccination. Mathematics 2023, 11, 2350. [Google Scholar] [CrossRef]
- Northcott, K.; Imran, M.; Wolkowicz, G. Competition in the presence of a virus in an aquatic system: An SIS model in the chemostat. J. Math. Biol. 2012, 64, 1043–1086. [Google Scholar] [CrossRef] [PubMed]
- Mestivier, D.; Pakdaman, K.; Boelle, P.Y.; Nicolas, J.C.; Lebaron, P. Viral regulation of bacterial biodiversity. In Proceedings of the Ecology of Marine Viruses, Banyuls, French, 19–22 March 2003; Volume 21. CIEMS Workshop Monographs. [Google Scholar]
- Weitz, J.S.; Hartman, H.; Levin, S.A. Coevolutionary arms races between bacteria and bacteriophage. Proc. Natl. Acad. Sci. USA 2005, 102, 9535–9540. [Google Scholar] [CrossRef] [PubMed]
- Alsolami, A.A.; El Hajji, M. Mathematical Analysis of a Bacterial Competition in a Continuous Reactor in the Presence of a Virus. Mathematics 2023, 11, 883. [Google Scholar] [CrossRef]
- Smith, H.L.; Waltman, P. The Theory of the Chemostat. Dynamics of Microbial Competition; Cambridge Studies in Mathematical Biology; Cambridge University Press: Cambridge, UK, 1995; Volume 13. [Google Scholar]
- Diekmann, O.; Heesterbeek, J. On the definition and the computation of the basic reproduction ratio in models for infectious diseases in heterogeneous populations. J. Math. Biol. 1990, 28, 365–382. [Google Scholar] [CrossRef]
- Hardin, G. The competition exclusion principle. Science 1960, 131, 1292–1298. [Google Scholar] [CrossRef]
- Aris, R.; Humphrey, A.E. Dynamics of a chemostat in which two organisms compete for a common substrate. Biotechnol. Bioeng. 1977, 19, 1375–1386. [Google Scholar] [CrossRef]
- Thieme, H.R. Convergence results and a Poincaré-Bendixson trichotomy for asymptotically autonomous differential equations. J. Math. Biol. 1992, 30, 755–763. [Google Scholar] [CrossRef]
- El Hajji, M.; Chorfi, N.; Jleli, M. Mathematical modelling and analysis for a three-tiered microbial food web in a chemostat. Electron. J. Differ. Equ. 2017, 2017, 1–13. [Google Scholar]
- Sobieszek, S.; Wade, M.J.; Wolkowicz, G.S.K. Rich dynamics of a three-tiered anaerobic food-web in a chemostat with multiple substrate inflow. Math. Biosci. Eng. 2020, 17, 7045–7073. [Google Scholar] [CrossRef] [PubMed]
- Butler, G.J.; Freedman, H.I.; Waltman, P. Uniformly persistent systems. Proc. Am. Math. Soc. 1986, 96, 425–429. [Google Scholar] [CrossRef]
- Monod, J. Croissance des populations bactériennes en fonction de la concentration de l’aliment hydrocarboné. Comptes Rendus L’Acad. Sci. 1941, 212, 771–773. [Google Scholar]
- Lobry, J.R.; Flandrois, J.P.; Carret, G.; Pave, A. Monod’s bacterial growth revisited. Bull. Math. Biol. 1992, 54, 117–122. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).