Analysis of Reference Ranges of Total Serum Protein in Namibia: Clinical Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Sampling and Data Collection Techniques
2.3. Data Analysis
3. Results
3.1. Analysis of the Subjects
3.2. Reference Range for Total Serum Protein
3.3. Gender-Based Reference Range and Data Distribution
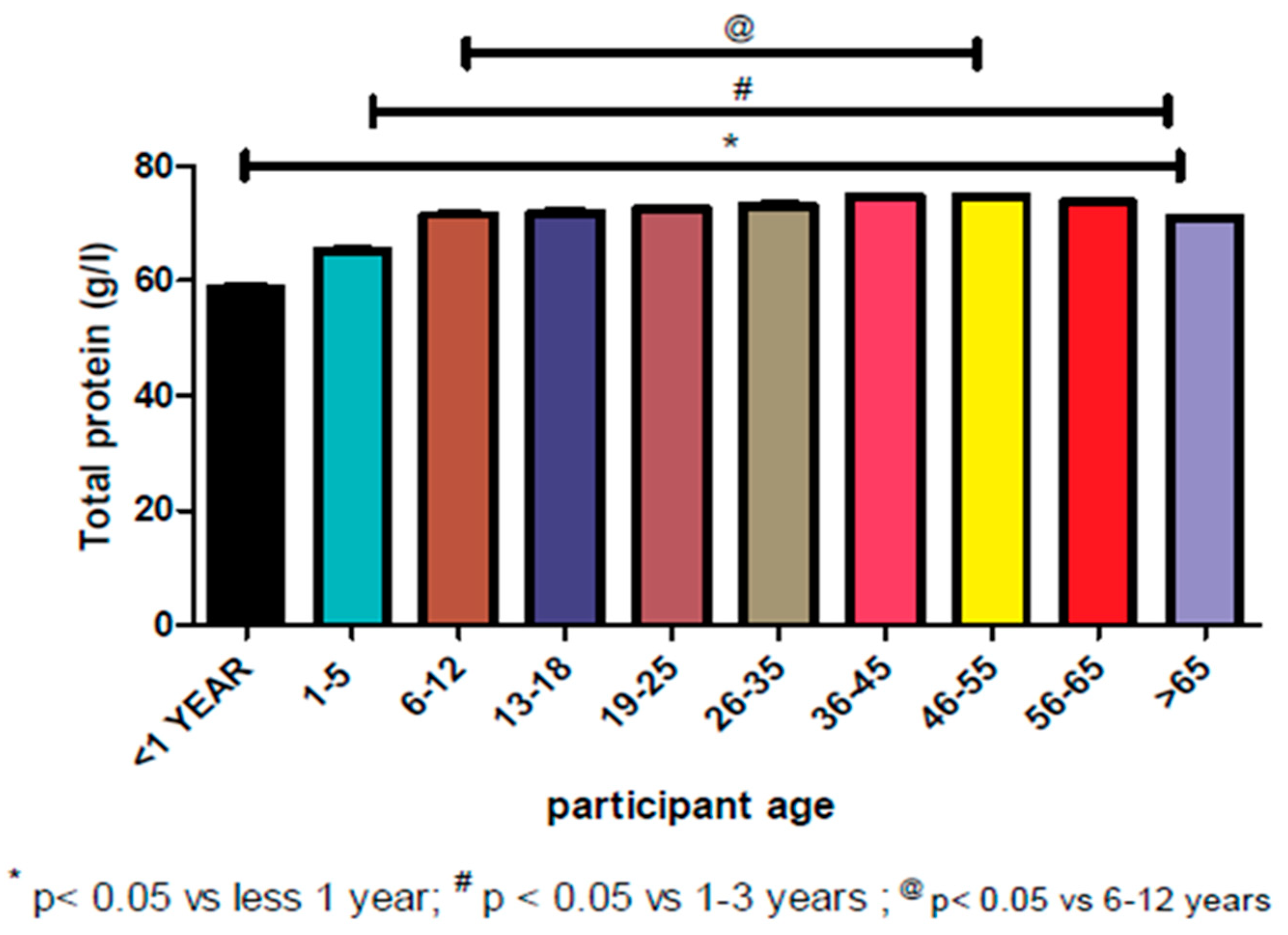
3.4. Reference Range and Data Distribution According to Age Group
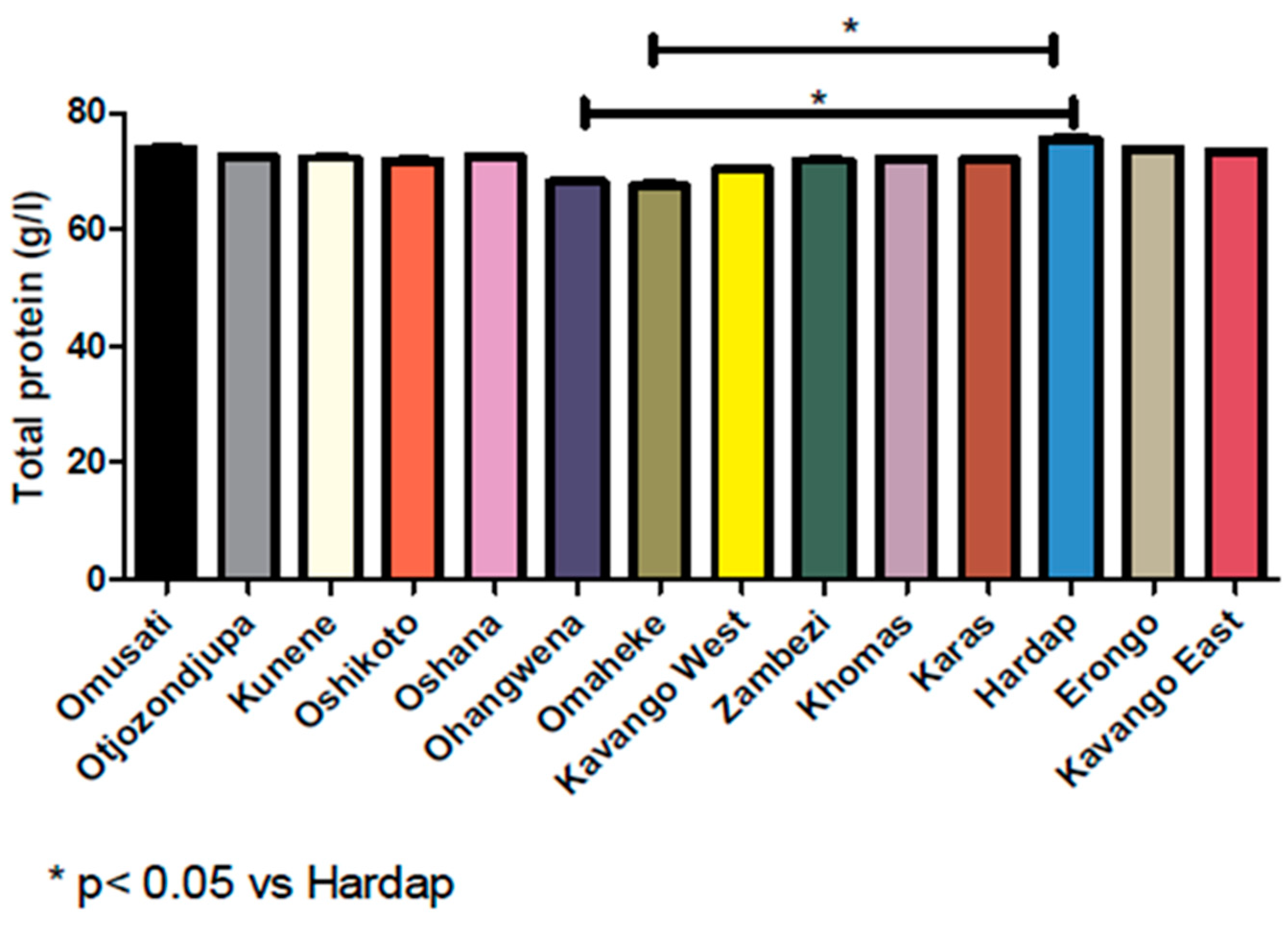
3.5. Reference Range Based on Geographical Location and Symmetric Data Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sabatino, A.; Regolisti, G.; Karupaiah, T.; Sahathevan, S.; Singh, B.K.S.; Khor, B.H.; Salhab, N.; Karavetian, M.; Cupisti, A.; Fiaccadori, E. Protein-energy wasting and nutritional supplementation in patients with end-stage renal disease on hemodialysis. Clin. Nutr. 2017, 36, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.R.; Qian, L.; Shen, X.Z.; Li, J.J.; Wen, J.T. Distribution of serum total protein in elderly Chinese. PLoS ONE 2014, 9, e101242. [Google Scholar] [CrossRef] [PubMed]
- Katayev, A.; Balciza, C.; Seccombe, D.W. Establishing reference intervals for clinical laboratory test results: Is there a better way? Am. J. Clin. Pathol. 2010, 133, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, T.; Mori, K.; Mukoyama, M.; Kasahara, M.; Yokoi, H.; Saito, Y.; Yoshioka, T.; Ogawa, Y.; Imamaki, H.; Kusakabe, T.; et al. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009, 75, 285–294. [Google Scholar] [CrossRef]
- Eunjung, L.; Jill, M.; John, J.C. Racial/ethnic-specific reference intervals for common laboratory tests: A comparison among Asians, Blacks, Hispanics, and Whites. Hawaii J. Med. Pub. Health 2015, 74, 302–310. [Google Scholar]
- Brinkley, T.; Semba, R.; Kritchevsky, S.; Houston, D. Dietary protein intake and serum age/rage levels in the health abc study. Innov. Aging 2018, 2, 707. [Google Scholar] [CrossRef]
- Furruqh, S.; Anitha, D.; Venkatesh, T. Estimation of reference values in liver function test in health plan individuals of an urban South Indian population. Indian J. Clin. Biochem. 2004, 19, 72–79. [Google Scholar] [CrossRef][Green Version]
- Bertholf, R.F. Proteins and albumin. Lab. Med. 2014, 45, 25–41. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Javadov, S.; Grimm, M.; Margreiter, R.; Ausserlechner, M.J.; Hagenbuchner, J. Crosstalk between mitochondria and cytoskeleton in cardiac cells. Cells 2020, 9, 222. [Google Scholar] [CrossRef]
- Horowitz, G.L. Reference intervals: Practical aspects. J. Int. Fed. Clin. Chem. Lab. Med. 2008, 19, 95–105. [Google Scholar]
- Hoffman, R.G. Statistics in the practice of medicine. JAMA 1963, 185, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.T.; Buhr, K.A. Widespread incorrect implementation of the Hoffmann method, the correct approach, and modern alternatives. Am. J. Clin. Pathol. 2019, 151, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Wani, H.; Rahman, S.; Azmi, S.; Sultana, M.; Kour, N.; Khajuria, A. Haemato-biochemical alterations in induced chlorpyrifos toxicity in broiler chickens. Appl. Biol. Res. 2015, 17, 48–54. [Google Scholar] [CrossRef]
- Alemnji, G.A.; Mbuagbaw, J.; Folefac, E.; Teto, G.; Nkengafac, S.; Atems, N.; Kwingwah, B.B.W.; Asonganyi, T. Reference physiological ranges for serum biochemical parameters among healthy Cameroonians to support HIV vaccine and related clinical trials. Afr. J. Health Sci. 2010, 17, 71–82. [Google Scholar]
- Ibrahim, K.; Zuberi, S.J. Serum total protein, albumin, globulin population of various ages and sex in Karachi and their ratio in apparently healthy. J. Pak. Med. Assoc. 1989, 39, 12–16. [Google Scholar]
- Gahutu, J.B.; Wane, J. Reference values for serum protein and electrolyte study from Rwanda. East Afr. Med. J. 2006, 83, 64–67. [Google Scholar] [CrossRef][Green Version]
- Tipton, K.D. Gender differences in protein metabolism. Care 2001, 4, 493–498. [Google Scholar] [CrossRef]
- Weaving, G.; Batstone, G.F.; Jones, R.G. Age and sex variation in serum albumin concentration: An observational study. Assoc. Clin. Biochem. Lab. Med. 2016, 53, 106–111. [Google Scholar] [CrossRef]
- Ignjatovic, V.; Lai, C.; Summerhayes, R.; Mathesius, U.; Tawfilis, S.; Perugini, M.A. Age-related differences in plasma proteins: How plasma proteins change from neonates to adults. PLoS ONE 2011, 6, e17213. [Google Scholar] [CrossRef]
- De Waal-Miller, C. Determination of Reference Ranges for Selected Clinical Laboratory Tests for a Medical Laboratory in Namibia Using Pre-Tested Data. Master’s Thesis, Cape Peninsula University of Technology, Cape Town, South Africa, 2015. [Google Scholar]
- Dorizzi, R.M.; Giannone, G.; Cambiaso, P.; Cappa, M.; Muraca, M. Indirect methods for TSH reference interval: At last fit for purpose? Am. J. Clin. Pathol. 2015, 135, 167–169. [Google Scholar] [CrossRef]
- Zeh, C.E.; Odhiambo, C.O.; Mills, L.A. Laboratory reference intervals in Africa. In Blood Cell-An Overview of Studies in Hematology; IntechOpen: London, UK, 2012; pp. 303–320. [Google Scholar]
- Fraser, C.G. Inherent biological variation and reference values. Clin. Chem. Lab. Med. 2004, 42, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K. Estimating reference ranges. Am. J. Clin. Pathol. 2010, 134, 351. [Google Scholar]
- D’Silva, A.M.; Hyett, J.A.; Coorssen, J.R. A routine top-down approach to analysis of the human serum proteome. Proteomes 2017, 5, 13. [Google Scholar] [CrossRef] [PubMed]
| Measurable Parameters | Values |
|---|---|
| Percentiles | (g/L) |
| 2.5 | 51.00 |
| 5 | 54.00 |
| 95 | 88.00 |
| 97.5 | 91.00 |
| Central tendency and dispersion | |
| N* | 78,477 |
| Mean | 72.58 |
| Median | 73.00 |
| Std. Deviation | 9.925 |
| Asymmetry and existence of outliers | |
| Skewness | −0.229 |
| Std. Error of Skewness | 0.009 |
| Kurtosis | −0.159 |
| Std. Error of Kurtosis | 0.017 |
| Measurable Parameters | Female | Male |
|---|---|---|
| Percentiles | (g/L) | (g/L) |
| 2.5 | 51.00 | 51.00 |
| 5 | 54.00 | 55.00 |
| 95 | 88.00 | 89.00 |
| 97.5 | 91.00 | 92.00 |
| Central tendency and dispersion | ||
| N* | 44,845 | 33,632 |
| Mean | 72.27 | 72.99 |
| Median | 73.00 | 74.00 |
| Std. Deviation | 9.796 | 10.080 |
| Asymmetry and existence of outliers | ||
| Skewness | −0.215 | −0.253 |
| Std. Error of Skewness | 0.012 | 0.013 |
| Kurtosis | −0.129 | −0.191 |
| Std. Error of Kurtosis | 0.023 | 0.027 |
| Age Group | N* | Mean | Median | Standard Deviation | Skewness | Kurtosis | Percentiles (g/L) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 2.5 | 5 | 95 | 97.5 | |||||||
| <1 | 2549 | 61.59 | 58.00 | 9.503 | 0.688 | 0.141 | 48.00 | 48.00 | 79.00 | 83.25 |
| 1–5 | 4257 | 67.62 | 67.00 | 9.155 | −0.062 | −0.100 | 49.00 | 51.00 | 82.00 | 85.55 |
| 6–12 | 2709 | 71.93 | 73.00 | 8.747 | −0.299 | 0.379 | 52.00 | 55.00 | 85.00 | 88.00 |
| 13–18 | 3152 | 72.35 | 73.00 | 9.769 | −0.238 | −0.185 | 51.83 | 54.00 | 88.00 | 91.00 |
| 19–25 | 8027 | 72.82 | 74.00 | 9.927 | −0.251 | −0.182 | 52.00 | 55.00 | 88.00 | 91.00 |
| 26–35 | 18,003 | 73.23 | 74.00 | 10.033 | −0.251 | −0.190 | 52.00 | 55.00 | 89.00 | 92.00 |
| 36–45 | 13,243 | 74.40 | 75.00 | 9.599 | −0.227 | −0.031 | 54.00 | 57.00 | 90.00 | 93.00 |
| 46–55 | 9867 | 74.62 | 75.00 | 9.322 | −0.336 | 0.219 | 53.00 | 57.00 | 89.00 | 92.00 |
| 56–65 | 7420 | 73.64 | 74.00 | 9.052 | −0.281 | 0.176 | 54.00 | 57.00 | 88.00 | 91.00 |
| >65 | 9250 | 71.06 | 72.00 | 9.361 | −0.151 | −0.152 | 51.00 | 54.00 | 86.00 | 89.00 |
| Region | N* | Mean | Median | Standard Deviation | Skewness | Kurtosis | Percentiles | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 2.5 | 5 | 95 | 97.5 | |||||||
| Otjozondjupa | 2925 | 71.96 | 73.00 | 9.858 | −0.123 | −0.214 | 51.00 | 55.00 | 88.00 | 91.00 |
| Khomas | 26,014 | 72.80 | 74.00 | 10.227 | −0.342 | −0.169 | 51.00 | 53.00 | 88.00 | 91.00 |
| Oshikoto | 6021 | 71.95 | 72.00 | 9.952 | −0.137 | −0.249 | 51.00 | 54.00 | 88.00 | 91.00 |
| Oshana | 11,887 | 72.07 | 73.00 | 9.710 | −0.279 | −0.099 | 51.00 | 54.00 | 87.00 | 90.00 |
| Erongo | 2952 | 73.76 | 74.00 | 9.428 | −0.235 | 0.021 | 53.83 | 57.00 | 89.00 | 92.00 |
| Ohangwena | 4114 | 69.78 | 69.00 | 9.728 | −0.013 | −0.204 | 50.00 | 53.00 | 85.00 | 89.00 |
| Kavango West | 979 | 71.56 | 71.00 | 9.979 | −0.054 | −0.266 | 51.50 | 54.00 | 89.00 | 91.00 |
| Karas | 1862 | 72.25 | 73.00 | 9.756 | −0.089 | −0.162 | 52.00 | 56.00 | 88.00 | 91.00 |
| Zambezi | 2523 | 72.86 | 72.00 | 9.868 | −0.082 | −0.241 | 53.00 | 56.00 | 89.00 | 92.00 |
| Hardap | 1532 | 74.45 | 75.00 | 10.060 | −0.314 | −0.223 | 53.00 | 56.00 | 89.35 | 92.67 |
| Kunene | 1681 | 72.34 | 72.00 | 9.684 | −0.011 | 0.028 | 52.00 | 55.00 | 89.00 | 92.00 |
| Kavango East | 12,353 | 73.41 | 74.00 | 9.641 | −0.146 | −0.132 | 53.00 | 57.00 | 89.00 | 92.00 |
| Omusati | 2986 | 73.79 | 74.00 | 9.064 | −0.215 | 0.051 | 54.00 | 58.00 | 88.00 | 91.00 |
| Omaheke | 648 | 70.51 | 68.50 | 9.981 | −0.024 | −0.251 | 51.00 | 53.00 | 87.55 | 91.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henok, J.N.; Okeleye, B.I.; Omodanisi, E.I.; Ntwampe, S.K.O.; Aboua, Y.G. Analysis of Reference Ranges of Total Serum Protein in Namibia: Clinical Implications. Proteomes 2020, 8, 7. https://doi.org/10.3390/proteomes8020007
Henok JN, Okeleye BI, Omodanisi EI, Ntwampe SKO, Aboua YG. Analysis of Reference Ranges of Total Serum Protein in Namibia: Clinical Implications. Proteomes. 2020; 8(2):7. https://doi.org/10.3390/proteomes8020007
Chicago/Turabian StyleHenok, Josephine N., Benjamin I. Okeleye, Elizabeth I. Omodanisi, Seteno K. O. Ntwampe, and Yapo G. Aboua. 2020. "Analysis of Reference Ranges of Total Serum Protein in Namibia: Clinical Implications" Proteomes 8, no. 2: 7. https://doi.org/10.3390/proteomes8020007
APA StyleHenok, J. N., Okeleye, B. I., Omodanisi, E. I., Ntwampe, S. K. O., & Aboua, Y. G. (2020). Analysis of Reference Ranges of Total Serum Protein in Namibia: Clinical Implications. Proteomes, 8(2), 7. https://doi.org/10.3390/proteomes8020007






