Vesiculation of Red Blood Cells in the Blood Bank: A Multi-Omics Approach towards Identification of Causes and Consequences
Abstract
1. Introduction
2. Materials and Methods
3. Results
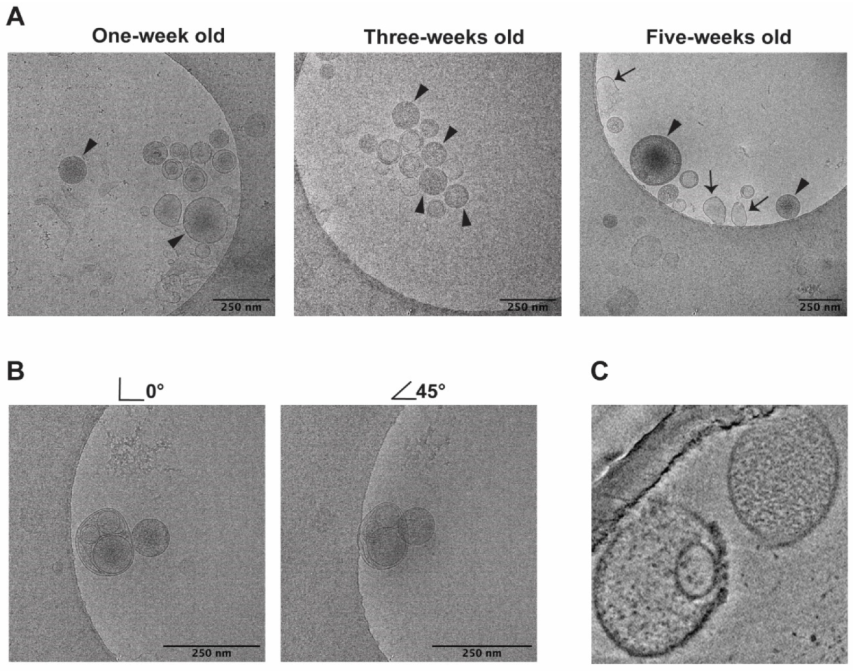
3.1. Morphology by cryo-EM
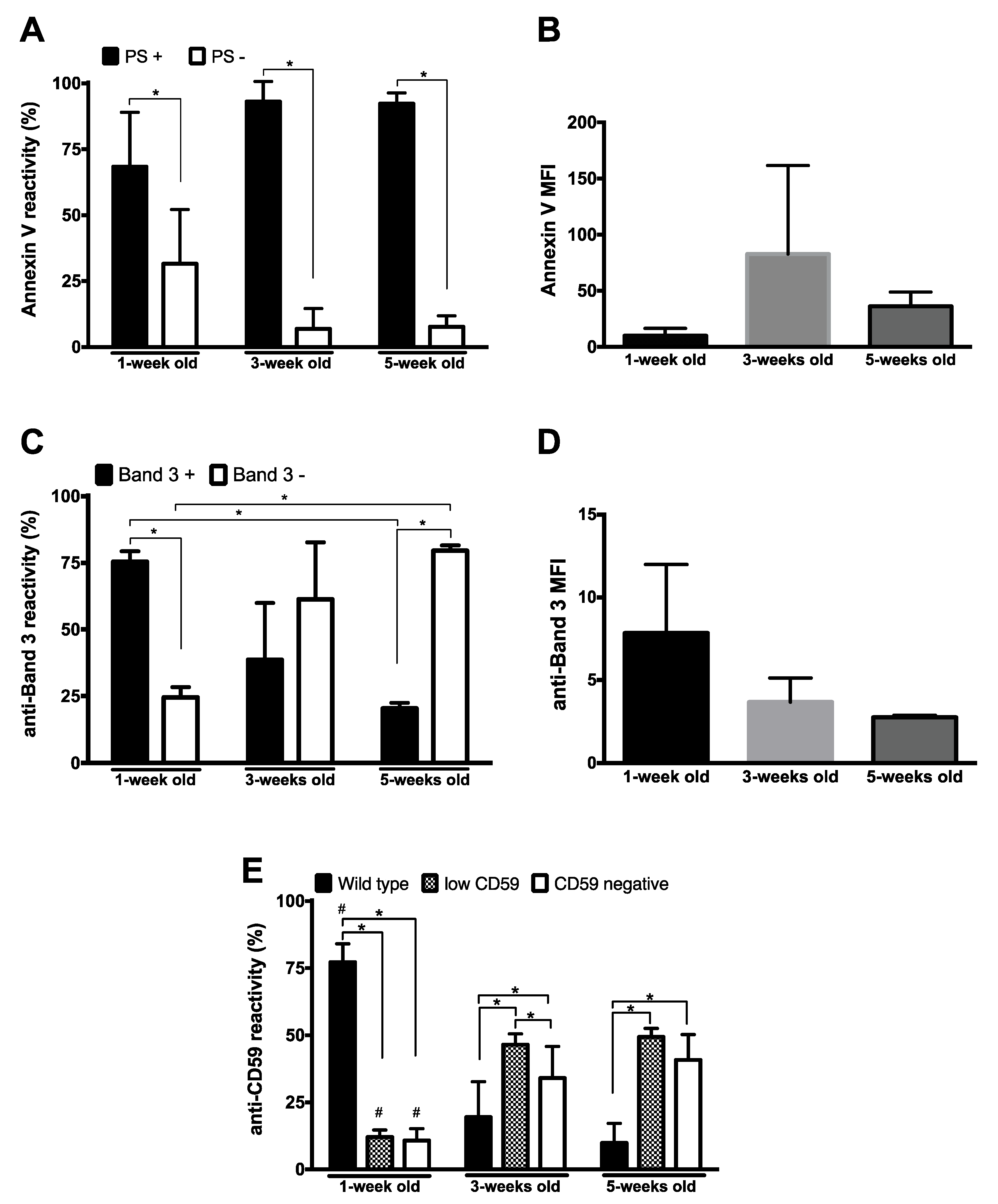
3.2. Membrane Organization by Flow Cytometry
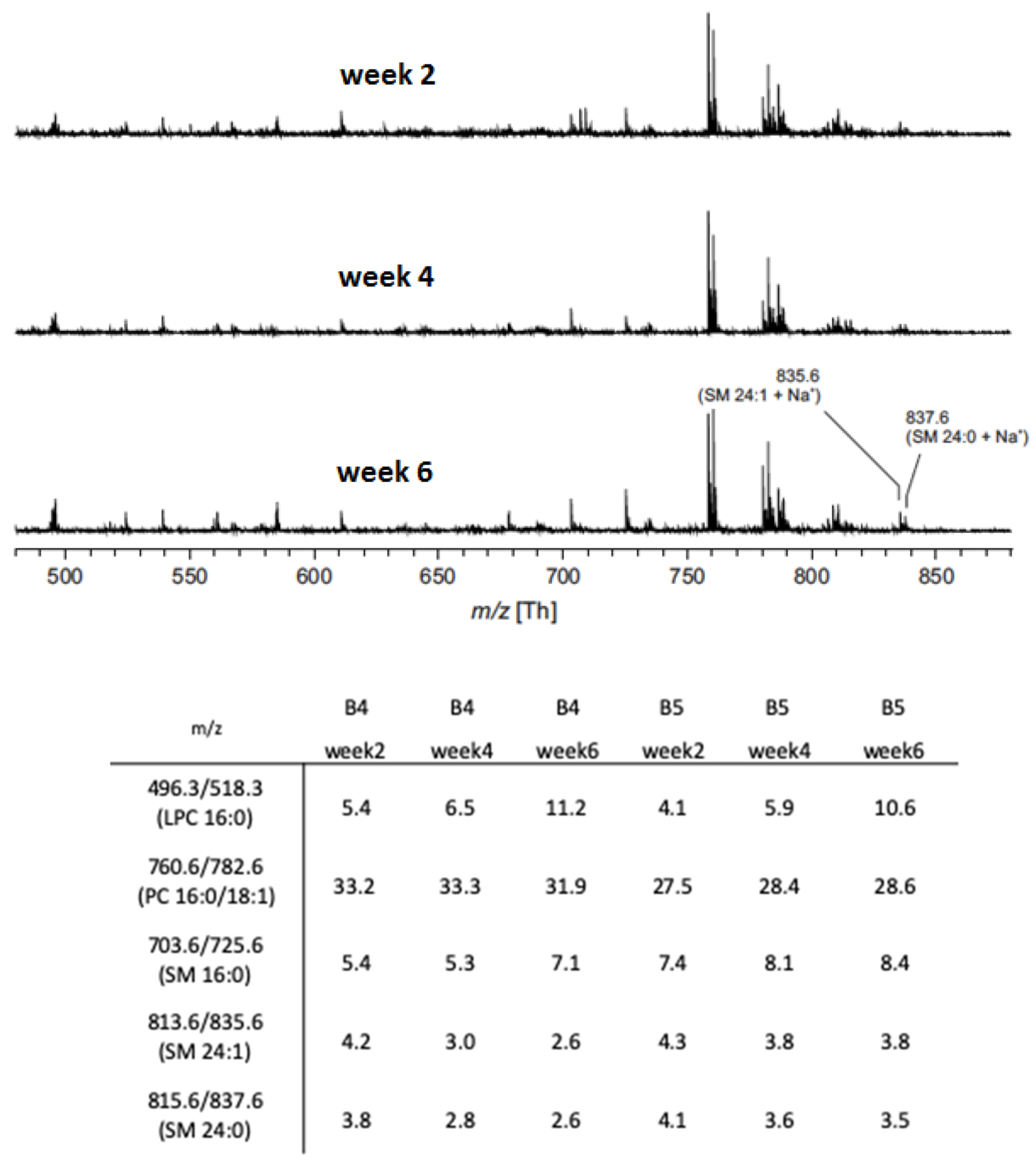
3.3. Phospholipid Composition by Mass Spectrometry
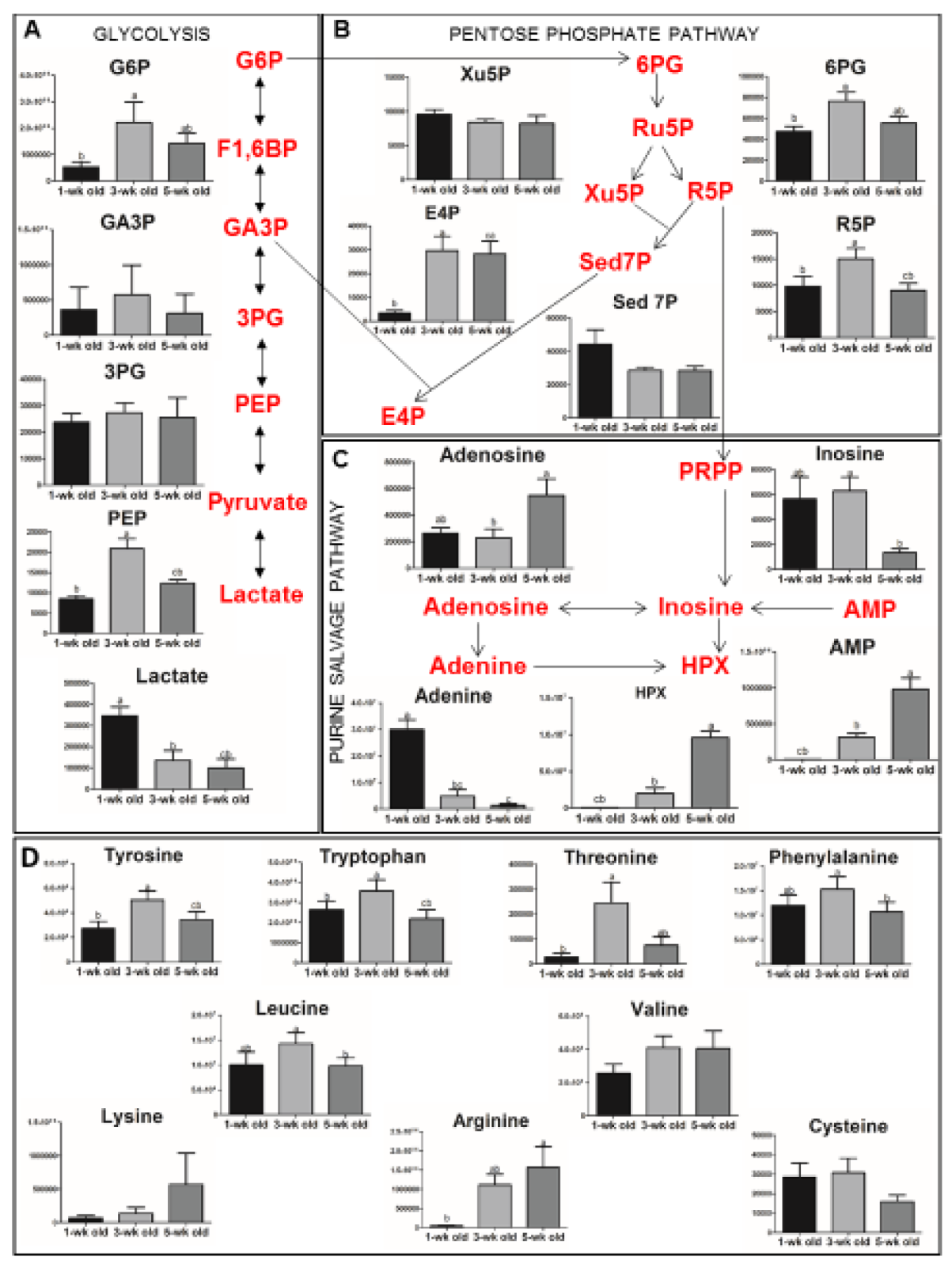
3.4. Storage-Related Changes in the Vesicular Metabolome
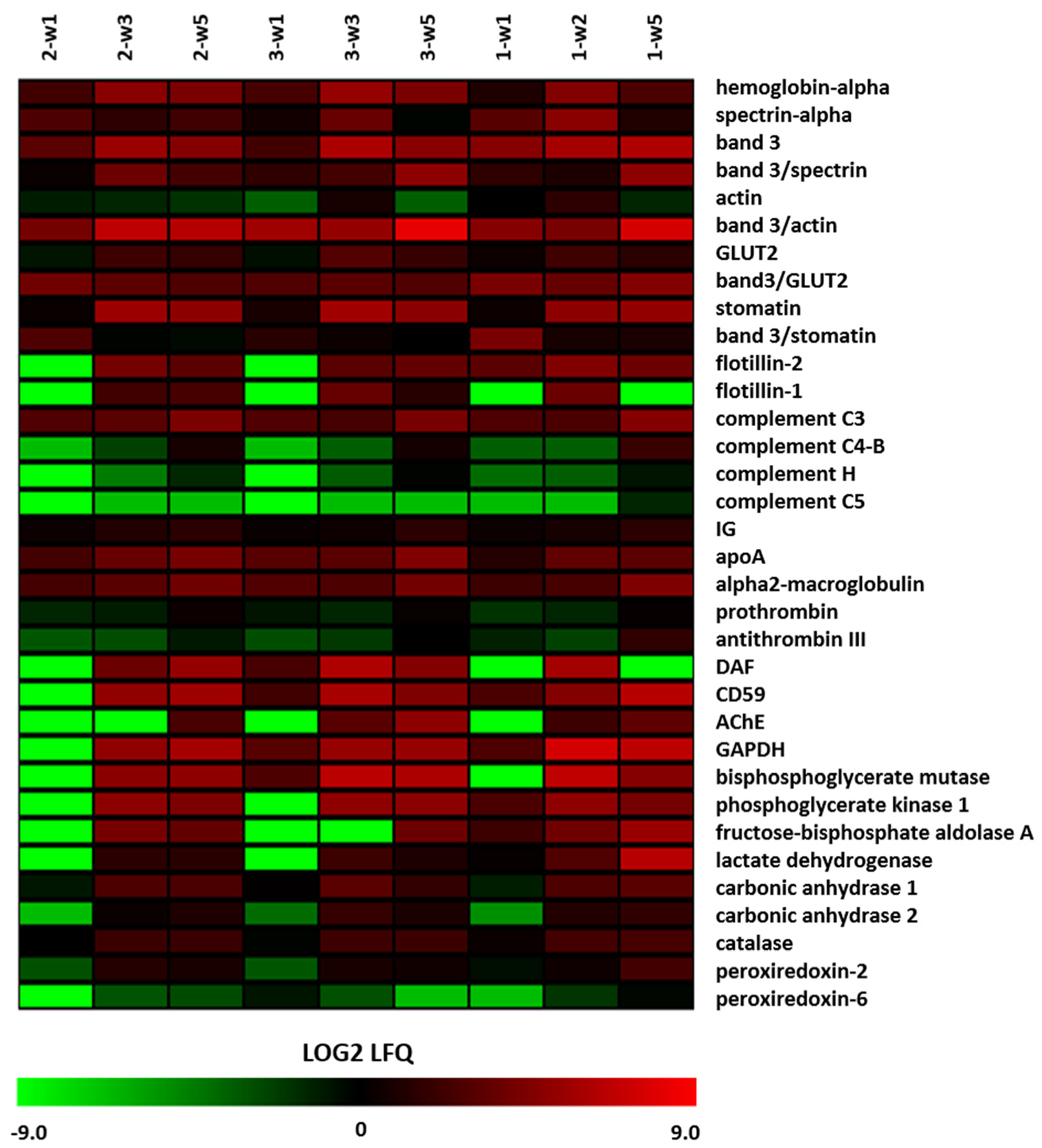
3.5. Storage-Dependent Changes in the Microvesicle Proteome
4. Discussion
4.1. Morphology (cryo-EM)
4.2. Membrane Organization 1: Flow Cytometry and Phospholipid Analysis
4.3. Membrane Organization 2: Flow Cytometry and Proteomics
4.4. Metabolome Changes as Clues to Vesiculation Mechanisms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blasi, B.; D’Alessandro, A.; Ramundo, N.; Zolla, L. Red blood cell storage and cell morphology. Transfus. Med. 2012, 22, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Cluitmans, J.C.; Hardeman, M.R.; Dinkla, S.; Brock, R.; Bosman, G.J.C.G.M. Red blood cell deformability during storage: Towards functional proteomics and metabolomics in the Blood Bank. High Speed Blood Transfus. Equip. 2012, 10, s12–s18. [Google Scholar]
- Frank, S.M.; Abazyan, B.; Ono, M.; Hogue, C.W.; Cohen, D.B.; Berkowitz, D.E.; Ness, P.M.; Barodka, V.M.; Berkowitz, D.E. Decreased Erythrocyte Deformability After Transfusion and the Effects of Erythrocyte Storage Duration. Anesth. Analg. 2013, 116, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Hovav, T.; Yedgar, S.; Manny, N.; Barshtein, G. Alteration of red cell aggregability and shape during blood storage. Transfusion 1999, 39, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Anniss, A.M.; Sparrow, R. Storage duration and white blood cell content of red blood cell (RBC) products increases adhesion of stored RBCs to endothelium under flow conditions. Transfusion 2006, 46, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Neuman, R.; Hayek, S.S.; Rahman, A.; Poole, J.C.; Menon, V.; Sher, S.; Newman, J.L.; Karatela, S.; Polhemus, D.; Lefer, D.J.; et al. Effects of storage-aged red blood cell transfusions on endothelial function in hospitalized patients. Transfusion 2014, 55, 782–790. [Google Scholar] [CrossRef]
- Rapido, F.; Brittenham, G.M.; Bandyopadhyay, S.; La Carpia, F.; L’Acqua, C.; McMahon, N.J.; Rebbaa, A.; Wojczyk, B.S.; Netterwald, J.; Wang, H.; et al. Prolonged red cell storage before transfusion increases extravascular hemolysis. J. Clin. Investig. 2016, 127, 375–382. [Google Scholar] [CrossRef]
- Kay, M.M.; Flowers, N.; Goodman, J.; Bosman, G. Alteration in membrane protein band 3 associated with accelerated erythrocyte aging. Proc. Natl. Acad. Sci. USA 1989, 86, 5834–5838. [Google Scholar] [CrossRef]
- Lutz, H.U. Naturally occurring anti-band 3 antibodies in clearance of senescent and oxidatively stressed human red blood cells. Transfus. Med. Hemotherapy 2012, 39, 321–327. [Google Scholar] [CrossRef]
- Burger, P.; Kostova, E.; Bloem, E.; Meijer, A.B.; Berg, T.K.V.D.; Verhoeven, A.J.; De Korte, D.; Van Bruggen, R.; Hilarius-Stokman, P. Potassium leakage primes stored erythrocytes for phosphatidylserine exposure and shedding of pro-coagulant vesicles. Br. J. Haematol. 2012, 160, 377–386. [Google Scholar] [CrossRef]
- Dinkla, S.; Peppelman, M.; Van Der Raadt, J.; Atsma, F.; Novotný, V.M.; Van Kraaij, M.G.; Joosten, I.; Bosman, G.J.C.G.M. Phosphatidylserine exposure on stored red blood cells as a parameter for donor-dependent variation in product quality. High Speed Blood Transfus. Equip. 2013, 12, 204–209. [Google Scholar]
- Anniss, A.M.; Sparrow, R.L. Expression of CD47 (integrin-associated protein) decreases on red blood cells during storage. Transfus. Apher. Sci. 2002, 27, 233–238. [Google Scholar] [CrossRef]
- Stewart, A.; Urbaniak, S.; Turner, M.; Bessos, H. The application of a new quantitative assay for the monitoring of integrin-associated protein CD47 on red blood cells during storage and comparison with the expression of CD47 and phosphatidylserine with flow cytometry. Transfusion 2005, 45, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Kriebardis, A.; Rinalducci, S.; Antonelou, M.; Hansen, K.C.; Papassideri, I.S.; Zolla, L. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion 2014, 55, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Delobel, J.; Barelli, S.; Canellini, G.; Prudent, M.; Lion, N.; Tissot, J.-D. Red blood cell microvesicles: A storage lesion or a possible salvage mechanism. Isbt Sci. Ser. 2016, 11, 171–177. [Google Scholar] [CrossRef]
- Bosman, G.J.C.G.M.; Lasonder, E.; Luten, M.; Roerdinkholder-Stoelwinder, B.; Novotný, V.M.J.; Bos, H.; De Grip, W.J. The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion 2008, 48, 827–835. [Google Scholar] [PubMed]
- Willekens, F.L.A.; Werre, J.M.; Groenen-Döpp, Y.A.M.; Roerdinkholder-Stoelwinder, B.; De Pauw, B.; Bosman, G.J.C.G.M. Erythrocyte vesiculation: A self-protective mechanism? Br. J. Haematol. 2008, 141, 549–556. [Google Scholar] [CrossRef]
- Jy, W.; Ricci, M.; Shariatmadar, S.; Gomez-Marin, O.; Horstman, L.H.; Ahn, Y.S. Microparticles in stored red blood cells as potential mediators of transfusion complications. Transfusion 2011, 51, 886–893. [Google Scholar] [CrossRef]
- Dinkla, S.; Novotny, V.M.J.; Joosten, I.; Bosman, G.J.C.G.M. Storage-Induced Changes in Erythrocyte Membrane Proteins Promote Recognition by Autoantibodies. PLoS ONE 2012, 7, e42250. [Google Scholar] [CrossRef]
- Said, A.S.; Rogers, S.; Doctor, A. Physiologic Impact of Circulating RBC Microparticles upon Blood-Vascular Interactions. Front. Physiol. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Rubin, O.; Crettaz, D.; Canellini, G.; Tissot, J.-D.; Lion, N. Microparticles in stored red blood cells: An approach using flow cytometry and proteomic tools. Vox Sang. 2008, 95, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Bosman, G.J.C.G.M.; Lasonder, E.; Groenen-Döpp, Y.; Willekens, F.; Werre, J.; Novotny, V. Comparative proteomics of erythrocyte aging in vivo and in vitro. J. Proteom. 2010, 73, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Bosman, G. The involvement of erythrocyte metabolism in organismal homeostasis in health and disease. Proteom.-Clin. Appl. 2016, 10, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Nemkov, T.; Reisz, J.A.; Xia, Y.; Zimring, J.C.; D’Alessandro, A. Red blood cells as an organ? How deep omics characterization of the most abundant cell in the human body highlights other systemic metabolic functions beyond oxygen transport. Expert Rev. Proteom. 2018, 15, 855–864. [Google Scholar] [CrossRef]
- Yoshida, T.; Prudent, M.; D’Alessandro, A. Red blood cell storage lesion: Causes and potential clinical consequences. Blood Transfus. 2019, 17, 27–52. [Google Scholar]
- Dinkla, S.; Brock, R.; Joosten, I.; Bosman, G.J. Gateway to understanding microparticles: Standardized isolation and identification of plasma membrane-derived vesicles. Nanomedicine 2013, 8, 1657–1668. [Google Scholar] [CrossRef]
- Derksen, J.; Janssen, G.-J.; Wolters-Arts, M.; Lichtscheidl, I.; Adlassnig, W.; Ovečka, M.; Doris, F.; Steer, M. Wall architecture with high porosity is established at the tip and maintained in growing pollen tubes of Nicotiana tabacum. Plant J. 2011, 68, 495–506. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Rappsilber, J.; Ishihama, Y.; Mann, M. Stop and Go Extraction Tips for Matrix-Assisted Laser Desorption/Ionization, Nanoelectrospray, and LC/MS Sample Pretreatment in Proteomics. Anal. Chem. 2003, 75, 663–670. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.; Mann, M. Andromeda: A Peptide Search Engine Integrated into the MaxQuant Environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef] [PubMed]
- Bresler, K.; Pyttel, S.; Paasch, U.; Schiller, J. Parameters affecting the accuracy of the MALDI-TOF MS determination of the phosphatidylcholine/lysophosphatidylcholine (PC/LPC) ratio as potential marker of spermatozoa quality. Chem. Phys. Lipids 2011, 164, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Dinkla, S.; Van Eijk, L.T.; Fuchs, B.; Schiller, J.; Joosten, I.; Brock, R.; Pickkers, P.; Bosman, G. Inflammation-associated changes in lipid composition and the organization of the erythrocyte membrane. BBA Clin. 2016, 5, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Tzounakas, V.L.; Gevi, F.; Georgatzakou, H.T.; Zolla, L.; Papassideri, I.S.; Kriebardis, A.; Rinalducci, S.; Antonelou, M. Redox Status, Procoagulant Activity, and Metabolome of Fresh Frozen Plasma in Glucose 6-Phosphate Dehydrogenase Deficiency. Front. Med. 2018, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, W.H.; Sung, L.P.; Chien, S. Quantitative relationship between Heinz body formation and red blood cell deformability. Blood 1986, 68, 1376–1383. [Google Scholar] [CrossRef]
- Leal, J.; Preijers, F.; Brock, R.; Adjobo-Hermans, M.; Bosman, G. Red Blood Cell Homeostasis and Altered Vesicle Formation in Patients with Paroxysmal Nocturnal Hemoglobinuria. Front. Physiol. 2019, 10, 578. [Google Scholar] [CrossRef]
- Ferru, E.; Giger, K.; Pantaleo, A.; Puchulu-Campanella, E.; Grey, J.; Ritchie, K.; Vono, R.; Turrini, F.; Low, P.S. Regulation of membrane-cytoskeletal interactions by tyrosine phosphorylation of erythrocyte band 3. Blood 2011, 117, 5998–6006. [Google Scholar] [CrossRef]
- Azouzi, S.; Romana, M.; Arashiki, N.; Takakuwa, Y.; El Nemer, W.; Peyrard, T.; Colin, Y.; Amireault, P.; Le Van Kim, C. Band 3 phosphorylation induces irreversible alterations of stored red blood cells. Am. J. Hematol. 2018, 93, E110–E112. [Google Scholar] [CrossRef]
- Leal, J.; Adjobo-Hermans, M.J.; Brock, R.; Bosman, G. Acetylcholinesterase provides new insights into red blood cell ageing in vivo and in vitro. High Speed Blood Transfus. Equip. 2017, 15, 232–238. [Google Scholar]
- Salzer, U.; Zhu, R.; Luten, M.; Isobe, H.; Pastushenko, V.; Perkmann, T.; Hinterdorfer, P.; Bosman, G.J. Vesicles generated during storage of red cells are rich in the lipid raft marker stomatin. Transfusion 2008, 48, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, A.J.; Hilarius, P.M.; Dekkers, D.W.C.; Lagerberg, J.W.M.; De Korte, D. Prolonged storage of red blood cells affects aminophospholipid translocase activity. Vox Sang. 2006, 91, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Campanella, M.E.; Chu, H.; Low, P.S. Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. Proc. Natl. Acad. Sci. USA 2005, 102, 2402–2407. [Google Scholar] [CrossRef]
- Lewis, I.A.; Campanella, M.E.; Markley, J.L.; Low, P.S. Role of band 3 in regulating metabolic flux of red blood cells. Proc. Natl. Acad. Sci. USA 2009, 106, 18515–18520. [Google Scholar] [CrossRef] [PubMed]
- Rinalducci, S.; Marrocco, C.; Zolla, L. Thiol-based regulation of glyceraldehyde-3-phosphate dehydrogenase in blood bank-stored red blood cells: A strategy to counteract oxidative stress. Transfusion 2014, 55, 499–506. [Google Scholar] [CrossRef]
- Gevi, F.; D’Alessandro, A.; Rinalducci, S.; Zolla, L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD–SAGM. J. Proteom. 2012, 76, 168–180. [Google Scholar] [CrossRef]
- Kriebardis, A.; Antonelou, M.; Stamoulis, K.E.; Margaritis, L.H.; Papassideri, I.S.; Economou-Petersen, E. RBC-derived vesicles during storage: Ultrastructure, protein composition, oxidation, and signaling components. Transfusion 2008, 48, 1943–1953. [Google Scholar] [CrossRef]
- Sorkin, R.; Huisjes, R.; Bošković, F.; Vorselen, D.; Pignatelli, S.; Ofir-Birin, Y.; Leal, J.; Schiller, J.; Mullick, D.; Roos, W.H.; et al. Nanomechanics of Extracellular Vesicles Reveals Vesiculation Pathways. Small 2018, 14, e1801650. [Google Scholar] [CrossRef]
- Laurén, E.; Sahle, F.T.; Valkonen, S.; Westberg, M.; Valkeajärvi, A.; Eronen, J.; Siljander, P.; Pettilä, V.; Käkelä, R.; Laitinen, S.; et al. Phospholipid composition of packed red blood cells and that of extracellular vesicles show a high resemblance and stability during storage. Biochim. Et Biophys. Acta (Bba)-Mol. Cell Boil. Lipids 2018, 1863, 1–8. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Gray, A.D.; Szczepiorkowski, Z.M.; Hansen, K.; Herschel, L.H.; Dumont, L.J.; Sczcepiorkowski, Z.M. Red blood cell metabolic responses to refrigerated storage, rejuvenation, and frozen storage. Transfusion 2017, 57, 1019–1030. [Google Scholar] [CrossRef]
- Antonelou, M.H.; Kriebardis, A.G.; Stamoulis, K.E.; Trougakos, I.P.; Papassideri, I.S. Apolipoprotein cJ/clusterin in human erythrocytes is involved in the molecular process of defect material disposal during vesiculation. PLoS ONE 2011, 6, e26033. [Google Scholar]
- Willekens, F.L.A.; Werre, J.M.; Kruijt, J.K.; Roerdinkholder-Stoelwinder, B.; Groenen-Döpp, Y.A.; Bos, A.G.V.D.; Bosman, G.J.C.G.M.; Van Berkel, T.J.C. Liver Kupffer cells rapidly remove red blood cell–derived vesicles from the circulation by scavenger receptors. Blood 2005, 105, 2141–2145. [Google Scholar] [CrossRef] [PubMed]
- Peter, C.; Waibel, M.; Radu, C.G.; Yang, L.V.; Witte, O.N.; Schulze-Osthoff, K.; Wesselborg, S.; Lauber, K. Migration to Apoptotic "Find-me" Signals Is Mediated via the Phagocyte Receptor G2A. J. Boil. Chem. 2007, 283, 5296–5305. [Google Scholar] [CrossRef]
- Fens, M.H.; Van Wijk, R.; Andringa, G.; Van Rooijen, K.L.; Dijstelbloem, H.M.; Rasmussen, J.T.; De Vooght, K.M.; Schiffelers, R.M.; Gaillard, C.A.; Van Solinge, W. A role for activated endothelial cells in red blood cell clearance: Implications for vasopathology. Haematologica 2011, 97, 500–508. [Google Scholar] [CrossRef]
- Prudent, M.; Delobel, J.; Hübner, A.; Benay, C.; Lion, N.; Tissot, J.-D. Proteomics of Stored Red Blood Cell Membrane and Storage-Induced Microvesicles Reveals the Association of Flotillin-2 With Band 3 Complexes. Front. Physiol. 2018, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Lapatsina, L.; Brand, J.; Poole, K.; Daumke, O.; Lewin, G.R. Stomatin-domain proteins. Eur. J. Cell Boil. 2012, 91, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Rungaldier, S.; Oberwagner, W.; Salzer, U.; Csaszar, E.; Prohaska, R. Stomatin interacts with GLUT1/SLC2A1, band 3/SLC4A1, and aquaporin-1 in human erythrocyte membrane domains. Biochim. Et Biophys. Acta (Bba)-Bioenerg. 2012, 1828, 956–966. [Google Scholar] [CrossRef]
- Gov, N.; Müllner, E.W.; Salzer, U. Cytoskeletal connectivity may guide erythrocyte membrane ex- and invagination–A discussion point how biophysical principles might be exploited by a parasite invading erythrocytes. Blood Cells Mol. Dis. 2017, 65, 78–80. [Google Scholar] [CrossRef]
- Kodippili, G.C.; Spector, J.; Hale, J.; Giger, K.; Hughes, M.R.; McNagny, K.; Birkenmeier, C.; Peters, L.; Ritchie, K.; Low, P.S. Analysis of the Mobilities of Band 3 Populations Associated with Ankyrin Protein and Junctional Complexes in Intact Murine Erythrocytes*. J. Boil. Chem. 2011, 287, 4129–4138. [Google Scholar] [CrossRef]
- Hess, J.R. Measures of stored red blood cell quality. Vox Sang. 2014, 107, 1–9. [Google Scholar] [CrossRef]
- Puchulu-Campanella, E.; Chu, H.; Anstee, D.J.; Galan, J.A.; Tao, W.A.; Low, P.S. Identification of the components of a glycolytic metabolon on the human red lood cell membrane. J Biol. Chem. 2013, 288, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Puchulu-Campanella, E.; Turrini, F.M.; Li, Y.-H.; Low, P.S. Global transformation of erythrocyte properties via engagement of an SH2-like sequence in band 3. Proc. Natl. Acad. Sci. USA 2016, 113, 13732–13737. [Google Scholar] [CrossRef] [PubMed]
- Pallotta, V.; Rinalducci, S.; Zolla, L. Red blood cell storage affects the stability of cytosolic native protein complexes. Transfusion 2015, 55, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Cluitmans, J.C.A.; Gevi, F.; Siciliano, A.; Matte, A.; Leal, J.; De Franceschi, L.; Zolla, L.; Brock, R.; Adjobo-Hermans, M.J.; Bosman, G. Red Blood Cell Homeostasis: Pharmacological Interventions to Explore Biochemical, Morphological and Mechanical Properties. Front. Mol. Biosci. 2016, 3, 4516. [Google Scholar] [CrossRef] [PubMed]
- Darghouth, D.; Koehl, B.; Heilier, J.F.; Madalinski, G.; Bovee, P.; Bosman, G.; Delaunay, J.; Junot, C.; Romeo, P.-H. Alterations of red blood cell metabolome in overhydrated hereditary stomatocytosis. Haematologica 2011, 96, 1861–1865. [Google Scholar] [CrossRef] [PubMed]
- Darghouth, D.; Koehl, B.; Madalinski, G.; Heilier, J.-F.; Bovee, P.; Xu, Y.; Olivier, M.-F.; Bartolucci, P.; Benkerrou, M.; Pissard, S.; et al. Pathophysiology of sickle cell disease is mirrored by the red blood cell metabolome. Blood 2011, 117, e57–e66. [Google Scholar] [CrossRef]
- Pertinhez, T.A.; Casali, E.; Lindner, L.; Spisni, A.; Baricchi, R.; Berni, P. Biochemical assessment of red blood cells during storage by 1H nuclear magnetic resonance spectroscopy. Identification of a biomarker of their level of protection against oxidative stress. High Speed Blood Transfus. Equip. 2014, 12, 548–556. [Google Scholar]
- Casali, E.; Berni, P.; Spisni, A.; Baricchi, R.; Pertinhez, T.A. Hypoxanthine: A new paradigm to interpret the origin of transfusion toxicity. High Speed Blood Transfus. Equip. 2015, 14, 555–556. [Google Scholar]
- Paglia, G.; D’Alessandro, A.; Rolfsson, Ó.; Sigurjónsson, Ó.E.; Bordbar, A.; Palsson, S.; Nemkov, T.; Hansen, K.C.; Gudmundsson, S.; Palsson, B.O. Biomarkers defining the metabolic age of red blood cells during cold storage. Blood 2016, 128, e43–e50. [Google Scholar] [CrossRef]
- Nemkov, T.; Sun, K.; Reisz, J.A.; Song, A.; Yoshida, T.; Dunham, A.; Wither, M.J.; Francis, R.O.; Roach, R.C.; Dzieciatkowska, M.; et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine duringe refrigerated storage. Haematologica 2018, 103, 361–372. [Google Scholar]
- Pollet, H.; Conrard, L.; Cloos, A.-S.; Tyteca, D. Plasma Membrane Lipid Domains as Platforms for Vesicle Biogenesis and Shedding? Biomolecules 2018, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Smith, N.H.; Zimring, J.C.; Fu, X.; Palmer, A.; Fontes, J.; Banerjee, U.; Yazer, M.H. Addition of ascorbic acid solution to stored murine red blood cells increases posttransfusion recovery and decreases microparticles and alloimmunization. Transfusion 2013, 53, 2248–2257. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas Leal, J.K.; Lasonder, E.; Sharma, V.; Schiller, J.; Fanelli, G.; Rinalducci, S.; Brock, R.; Bosman, G. Vesiculation of Red Blood Cells in the Blood Bank: A Multi-Omics Approach towards Identification of Causes and Consequences. Proteomes 2020, 8, 6. https://doi.org/10.3390/proteomes8020006
Freitas Leal JK, Lasonder E, Sharma V, Schiller J, Fanelli G, Rinalducci S, Brock R, Bosman G. Vesiculation of Red Blood Cells in the Blood Bank: A Multi-Omics Approach towards Identification of Causes and Consequences. Proteomes. 2020; 8(2):6. https://doi.org/10.3390/proteomes8020006
Chicago/Turabian StyleFreitas Leal, Joames K., Edwin Lasonder, Vikram Sharma, Jürgen Schiller, Giuseppina Fanelli, Sara Rinalducci, Roland Brock, and Giel Bosman. 2020. "Vesiculation of Red Blood Cells in the Blood Bank: A Multi-Omics Approach towards Identification of Causes and Consequences" Proteomes 8, no. 2: 6. https://doi.org/10.3390/proteomes8020006
APA StyleFreitas Leal, J. K., Lasonder, E., Sharma, V., Schiller, J., Fanelli, G., Rinalducci, S., Brock, R., & Bosman, G. (2020). Vesiculation of Red Blood Cells in the Blood Bank: A Multi-Omics Approach towards Identification of Causes and Consequences. Proteomes, 8(2), 6. https://doi.org/10.3390/proteomes8020006










