Quantitative Differences in Rumen Epithelium Proteins in Lambs Fed Wheat, Perennial Wheat, or Perennial Wheat plus Lucerne
Abstract
:1. Introduction
2. Methods and Materials
2.1. Feeding Regime
2.2. Dietary Intake, Mineral Composition, and Growth
2.3. Rumen Epithelium Isolation, Protein Extraction, and Labelling for Quantification
2.4. Statistical and Bioinformatics Analysis
3. Results
3.1. Dietary Intake of Energy, Minerals, and Growth
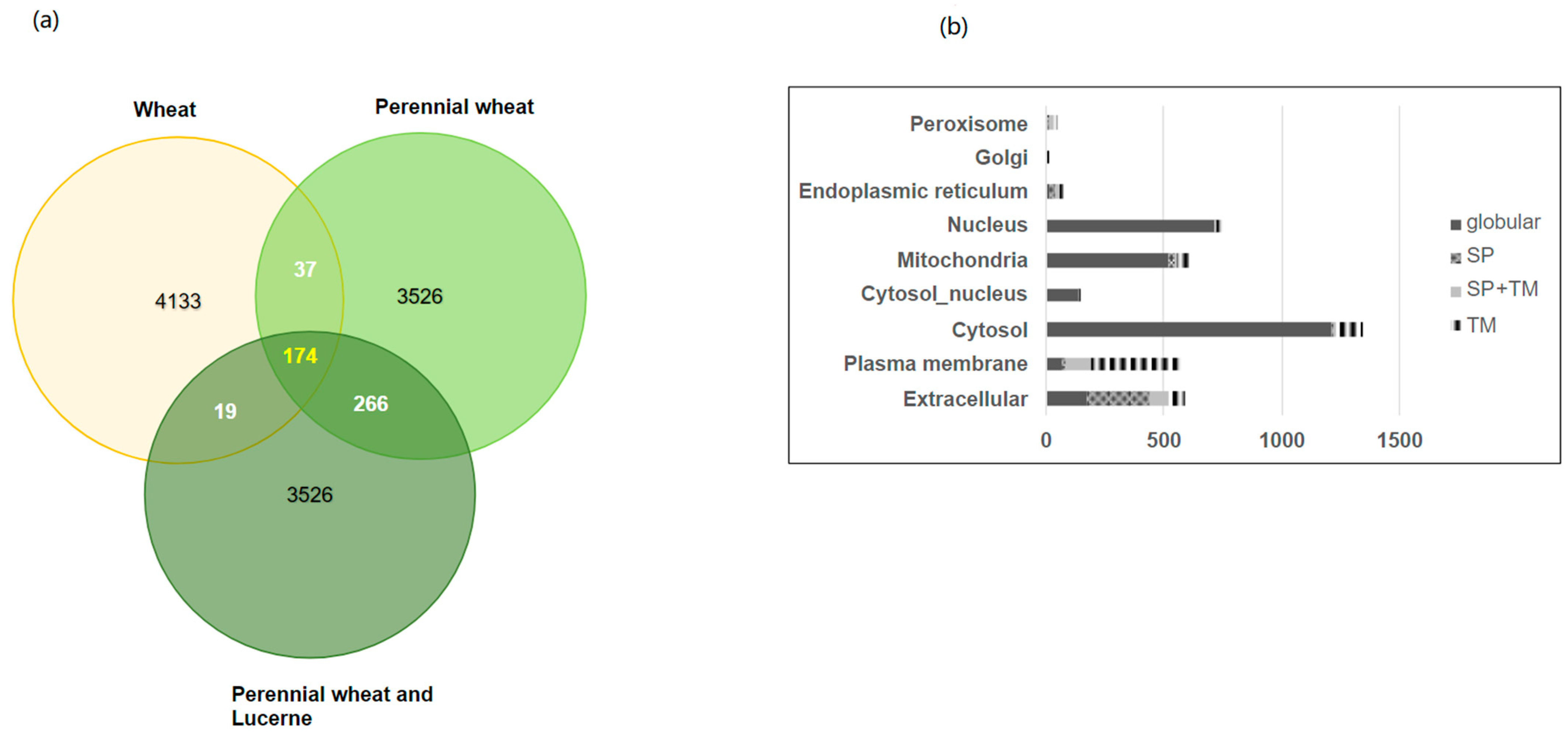
3.2. Protein Identifications and Predicted Sub Cellular Location
3.3. Biological Function of Quantitative Differences in Protein Abundance with Diet
3.4. Significant Differences in Abundance of Nutrient Transporters between Diets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, L.W.; Moore, A.D.; Kirkegaard, J.A. Evolution in crop-livestock integration systems that improve farm productivity and environmental performance in Australia. Eur. J. Agron. 2014, 57, 10–20. [Google Scholar] [CrossRef]
- Newell, M.T.; Hayes, R.C. An initial investigation of forage production and feed quality of perennial wheat derivatives. Crop. Pasture Sci. 2017, 68, 1141–1148. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, G.; Zhang, Y.; Lu, X.; Wan, K.; Liang, J.; Feng, Y.; Dao, J.; Wu, S.; Zhang, L.; et al. Sustained productivity and agronomic potential of perennial rice. Nat. Sustain. 2023, 6, 28–38. [Google Scholar] [CrossRef]
- Crews, T.E.; Blesh, J.; Culman, S.W.; Hayes, R.C.; Jensen, E.S.; Mack, M.C.; Peoples, M.B.; Schipanski, M.E. Going where no grains have gone before: From early to mid-succession. Agric. Ecosyst. Environ. 2016, 223, 223–238. [Google Scholar] [CrossRef]
- Ryan, M.R.; Crews, T.E.; Culman, S.W.; DeHaan, L.R.; Hayes, R.C.; Jungers, J.M.; Bakker, M.G. Managing for Multifunctionality in Perennial Grain Crops. BioScience 2018, 68, 294–304. [Google Scholar] [CrossRef]
- Hayes, R.C.; Newell, M.T.; Crews, T.E.; Peoples, M.B. Perennial cereal crops: An initial evaluation of wheat derivatives grown in mixtures with a regenerating legume. Renew. Agric. Food Syst. 2017, 32, 276–290. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Hayes, R.C.; Newell, M.T.; Refshauge, G.; McGrath, S.R.; Fowler, S.M.; Shanley, A.R.; Hopkins, D.L. The quality and mineral composition of the longissimus lumborum and semimembranosus muscles from lambs fed perennial or annual wheat forage with or without lucerne. Meat Sci. 2021, 180, 108564. [Google Scholar] [CrossRef]
- Refshauge, G.; Newell, M.T.; Hopkins, D.L.; Holman, B.W.B.; Morris, S.; Hayes, R.C. The plasma and urine mineral status of lambs offered diets of perennial wheat or annual wheat, with or without lucerne. Small Rumin. Res. 2022, 209, 106639. [Google Scholar] [CrossRef]
- Muller, F.; Huber, K.; Pfannkuche, H.; Aschenbach, J.R.; Breves, G.; Gabel, G. Transport of ketone bodies and lactate in the sheep ruminal epithelium by monocarboxylate transporter 1. Am. J. Physiol. Gastrointest Liver Physiol. 2002, 283, G1139–G1146. [Google Scholar] [CrossRef]
- Stevens, C.E.; Settler, B.K. Factors affecting the transport of volatile fatty acids across rumen epithelium. Am. J. Physiol. 1966, 210, 365–372. [Google Scholar] [CrossRef]
- Chen, H.; Wong, E.A.; Webb, K.E. Tissue distribution of a peptide transporter mRNA in sheep, dairy cows, pigs, and chickens. J. Anim. Sci. 1999, 77, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Sehested, J.; Diernaes, L.; Moller, P.D.; Skadhauge, E. Ruminal transport and metabolism of short-chain fatty acids (SCFA) in vitro: Effect of SCFA chain length and pH. Comp. Biochem. Physiol. 1999, 123, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef] [PubMed]
- Masters, D.G.; Hancock, S.; Refshauge, G.; Robertson, S.; Bhanugopan, M.; Friend, M.; Thompson, A.N. Mineral status of reproducing ewes grazing vegetative cereal crops. Anim. Prod. Sci. 2018, 58, 2049–2060. [Google Scholar] [CrossRef]
- Dove, H.; Kelman, W.M. Liveweight gains of young sheep grazing dual-purpose wheat with sodium and magnesium supplied as direct supplement, or with magnesium supplied as fertiliser. Anim. Prod. Sci. 2015, 55, 1217–1229. [Google Scholar] [CrossRef]
- Dove, H.; Masters, D.G.; Thompson, A.N. New perspectives on the mineral nutrition of livestock grazing cereal and canola crops. Anim. Prod. Sci. 2016, 56, 1350–1360. [Google Scholar] [CrossRef]
- Bond, J.J.; Donaldson, A.J.; Woodgate, S.; Kamath, K.S.; Mckay, M.J.; Wheeler, D.; Tucker, D.; Oddy, V.H. Quantification of cytosol and membrane proteins in rumen epithelium of sheep with low or high CH4 emission phenotype. PLoS ONE 2022, 17, e0273184. [Google Scholar] [CrossRef]
- NHMRC. Australian Code of Practice for the Care and Use of Animals for Scientific Purposes; National Health and Medical Research Council. Australian Government: Canberra, Australia, 2013.
- Newell, M.T.; Holman, B.W.B.; Refshauge, G.; Shanley, A.R.; Hopkins, D.L.; Hayes, R.C. The effect of a perennial wheat and lucerne biculture diet on feed intake, growth rate and carcass characteristics of Australian lambs. Small Rumin. Res. 2020, 192, 106235. [Google Scholar] [CrossRef]
- AFIA—Laboratory Methods Manual V8 April; Australian Fodder Industry Association Inc.: Ascot Vale, Australia, 2014; ISBN 0 642 58599 7.
- Rayment, G.E.; Lyons, D.J. Soil Chemical Methods; CSIRO Publishing: Clayton, Australasia, 2011. [Google Scholar]
- Bond, J.J.; Donaldson, A.J.; Coumans, J.V.F.; Austin, K.; Ebert, D.; Wheeler, D.; Oddy, V.H. Protein profiles of enzymatically isolated rumen epithelium in sheep fed a fibrous diet. J. Anim. Sci. Biotech. 2019, 10, 5. [Google Scholar] [CrossRef]
- Kumar, R.; Kamath, K.S.; Carroll, L.; Hoffmann, P.; Gecz, J.; Jolly, L.A. Endogenous protein interactomes resolved through immunoprecipitation-coupled quantitative proteomics in cell lines. STAR Protoc. 2022, 3, 101693. [Google Scholar] [CrossRef]
- Kamath, K.S.; Krisp, C.; Chick, J.; Pascovici, D.; Gygi, S.P.; Molloy, M.P. Pseudomonas aeruginosa Proteome under hypoxic stress conditions mimicking the cystic fibrosis lung. J. Proteome Res. 2017, 16, 3917–3928. [Google Scholar] [CrossRef] [PubMed]
- Minitab Statistical Software (2010) v 20. Available online: www.minitab.com (accessed on 23 May 2023).
- Hallgren, J.; Tsirigos, K.D.; Pedersen, M.D.; Armenteros, J.J.A.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. bioRxiv 2022, 4, 487609. [Google Scholar]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- NRC Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007.
- Bhanugopan, M.; Fulkerson, W.; Hyde, M.; Fraser, D. Effect of dietary potassium supplementation on the calcium absorption capacity in the rumen and abomasum and fractional excretion of urinary minerals in sheep. Anim. Prod. Sci. 2014, 55, 508–514. [Google Scholar] [CrossRef]
- Hediger, M.A.; Clémençon, B.; Burrier, R.E.; Bruford, E.A. The ABCs of membrane transporters in health and disease (SLC series): Introduction. Mol. Asp. Med. 2013, 34, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, F. The mitochondrial transport family SLC25: Identification, properties and physiology. Mol. Asp. Med. 2012, 34, 465–484. [Google Scholar] [CrossRef]
- Kuzinski, J.; Zitnan, R.; Warnke-gurgel, C.; Schweigel, M. The vaculolar-type H+ -ATPase in ovine rumen epithelium is regulated by metabolic signals. J. Biomed. Biotechnol. 2010, 525034, 1–12. [Google Scholar] [CrossRef]
- Goff, J.P. Invited review: Mineral absorption mechanisms, mineral interactions that affect acid-base and antioxidant status, and diet considerations to improve mineral status. J. Dairy Sci. 2018, 101, 2763–2813. [Google Scholar] [CrossRef]
- Jentsch, T.J.; Lutter, D.; Planells-cases, R.; Ullrich, F.; Voss, F.K. VRAC: Molecular identification as LRRC8 heteromers with differential functions. Pflug. Arch.–Eur. J. Physiol. 2016, 468, 385–393. [Google Scholar] [CrossRef]
- Allen, M.S. Relationship between fermentation acid production in the rumen and the requirement for physically effective fibre. J. Dairy Sci. 1997, 80, 1447–1462. [Google Scholar] [CrossRef]
- Bennink, M.R.; Tyler, T.R.; Ward, G.M.; Johnson, D.E. Ionic milieu of bovine and ovine rumen as affected by diet. J. Dairy Sci. 1978, 61, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Gemmel, R.T.; Stacey, B.D. Effects of ruminal hyperosmolarity on the ultrastructure of ruminal epithelium and their relevance to sodium transport. J. Exp. Physiol. 1973, 58, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Aschenbach, J.R.; Bhatia, S.K.; Pfannkuche, H.; Gäbel, G. Glucose is absorbed in a sodium-dependent manner from forestomach contents of sheep. J. Nutr. 2000, 130, 2797–2801. [Google Scholar] [CrossRef] [PubMed]
- Stumpff, F. A look at the smelly side of physiology: Transport of short chain fatty acids. Pflug. Arch.-Eur. J. Physiol. 2018, 470, 571–598. [Google Scholar] [CrossRef]
- Graham, C.; Gatherer, I.; Haslam, I.; Glanville, M.; Simmons, N.L. Expression and localisation of monocarboxylate transporters and sodium/proton exchangers in bovine rumen epithelium. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R997–R1007. [Google Scholar] [CrossRef]
- Sabirov, R.Z.; Merzlyak, P.G.; Okada, T.; Islam, R.; Uramoto, H.; Mori, T.; Makino, Y.; Matsuura, H.; Xie, Y.; Okada, Y. The organic anion transporter SLCO2A1 constitutes the core component of the Maxi-Cl channel. EMBO J. 2017, 36, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Georgi, M.-I.; Rosendahl, J.; Ernst, F.; Günzel, D.; Aschenbach, J.R.; Martens, H.; Stumpff, F. Epithelia of the ovine and bovine forestomach express basolateral maxi-anion channels permeable to the anions of short chain fatty acids. Pflug. Arch.–Eur. J. Physiol. 2014, 466, 1689–1712. [Google Scholar] [CrossRef]
- Rogers, P.A.M.; van’t Klooster, A. The Fate of Na, K, Ca, Mg and P in the Digesta. Ph.D. Thesis, Mededeling Landbouwhogeschool, Wageningen, The Netherlands, 1969. [Google Scholar]
- Hayes, R.C.; Dear, B.S.; Orchard, B.A.; Peoples, M.B.; Eberbach, P.L. Response of subterranean clover, balansa clover, and gland clover to lime when grown in mixtures on an acid soil. Aust. J. Agric. Res. 2008, 59, 824–835. [Google Scholar] [CrossRef]



| W n = 10 | PW n = 8 | PWL n = 12 | SEM 1 | p-Value | Minimum Dietary Requirement 2 | |
|---|---|---|---|---|---|---|
| DMI (kg) | 1.28 a | 1.21 b | 1.32 a | 0.015 | 0.001 | na |
| MEI (MJ ME/kg DM) | 15.70 a | 14.58 b | 15.1 b | 0.184 | 0.001 | na |
| CP (%) intake | 33.53 a | 29.48 b | 25.04 c | 0.355 | 0.001 | na |
| NDF % | 53.56 a | 54.39 a | 45.29 b | 0.436 | 0.001 | na |
| Na (%) | 0.009 a | 0.005 b | 0.012 c | 0.0003 | 0.001 | 0.07–0.12% |
| K (%) | 4.24 a | 4.70 b | 3.47 c | 0.032 | 0.001 | 0.5% |
| Ca (%) | 0.31 a | 0.34 a | 0.82 b | 0.006 | 0.001 | 0.3–0.5% |
| Mg (%) | 0.12 a | 0.12 a | 0.14 b | 0.001 | 0.001 | 0.09–0.2% |
| K:Na | 524 a | 937 b | 313 c | 0.081 | 0.001 | 6–7 ‡ |
| DCAD (meq/100 g DM) | 67.5 a | 77.9 b | 53.3 c | 0.63 | 0.001 | <12–35 † |
| Liveweight (kg) | 47.8 | 47.4 | 47.6 | 0.62 | ns | na |
| ADG (g/d) | 127.4 a | 84.3 b | 82.2 b | 36.80 | 0.02 | na |
| Pathway | UniProt Accession | Protein Name | Gene Name | FC | p-Value |
|---|---|---|---|---|---|
| Gluconeogenesis | |||||
| O18751 | Glycogen phosphorylase, muscle form (EC 2.4.1.1) (Myophosphorylase) | PYGM | 1.38 | 0.006 | |
| W5PFT7 | Fructose-bisphosphatase 2 | FBP2 | 1.37 | 0.002 | |
| Glycolysis | |||||
| W5P5C0 | Enolase 2 | ENO2 | 1.51 | 0.01 | |
| W5NZZ4 | Protein serine/threonine kinase (EC 2.7.11.) | PDK4 | 1.55 | 0.05 | |
| Methylglyoxal shunt | W5Q540 | Hydroxyacylglutathione hydrolase | HAGH | 1.20 | 0.005 |
| TCA cycle | W5QAA9 | Aconitate hydratase, mitochondrial (Aconitase) (EC 4.2.1.) | ACO2 | 1.22 | 0.02 |
| Isocitrate dehydrogenase [NAD] subunit B | IDH3B | ||||
| Fatty acid degradation | |||||
| W5PUC2 | Acyl-CoA dehydrogenase short chain | ACADS | 1.42 | 0.005 | |
| W5PHF2 | Acyl-CoA dehydrogenase short/branched chain | ACADSB | 1.27 | 0.01 | |
| W5PW04 | Acyl-CoA dehydrogenase family member 8 | ACAD8 | 1.23 | 0.02 | |
| Fatty acid degradation | |||||
| W5PYE3 | Lipase | LIPN | 1.50 | 0.004 PWL> | |
| W5PT19 | Phospholipid-transporting ATPase | ATP8B3 | 1.61 | 0.003 | |
| W5P1M4 | Acetyl-CoA acyltransferase 2 | ACAA2 | 1.44 | 0.014 | |
| W5Q9G8 | Enoyl-CoA delta isomerase 1 | ECI1 | 1.23 | 0.008 | |
| W5NWE0 | Mitochondrial trans-2-enoyl-CoA reductase | MECR | 1.22 | 0.02 | |
| Fatty acid biosynthesis | |||||
| W5QH76 | Elongation of very-long-chain fatty acids protein 1 (EC 2.3.1.199) (very-long-chain 3-ketoacyl-CoA synthase 1) | ELOVL1 | 1.36 | ns | |
| W5QG36 | Very-long-chain (3R)-3-hydroxyacyl-CoA dehydratase (EC 4.2.1.134) | HACD2 | 1.58 | 0.02 PWL> | |
| W5PG98 | Hydroxysteroid 17-beta dehydrogenase 8 | HSD17B8 | 1.31 | 0.03 PW> | |
| W5QHK3 | Methylcrotonoyl-CoA carboxylase 1 | MCCC1 | 1.22 | ns | |
| W5NTN7 | Patatin-like phospholipase domain containing 6 | PNPLA6 | 1.20 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bond, J.J.; Refshauge, G.; Newell, M.T.; Holman, B.W.B.; Wheeler, D.; Woodgate, S.; Kamath, K.S.; Hayes, R.C. Quantitative Differences in Rumen Epithelium Proteins in Lambs Fed Wheat, Perennial Wheat, or Perennial Wheat plus Lucerne. Proteomes 2023, 11, 27. https://doi.org/10.3390/proteomes11030027
Bond JJ, Refshauge G, Newell MT, Holman BWB, Wheeler D, Woodgate S, Kamath KS, Hayes RC. Quantitative Differences in Rumen Epithelium Proteins in Lambs Fed Wheat, Perennial Wheat, or Perennial Wheat plus Lucerne. Proteomes. 2023; 11(3):27. https://doi.org/10.3390/proteomes11030027
Chicago/Turabian StyleBond, Jude Jessie, Gordon Refshauge, Matthew T. Newell, Benjamin W. B. Holman, David Wheeler, Serey Woodgate, Karthik S. Kamath, and Richard C. Hayes. 2023. "Quantitative Differences in Rumen Epithelium Proteins in Lambs Fed Wheat, Perennial Wheat, or Perennial Wheat plus Lucerne" Proteomes 11, no. 3: 27. https://doi.org/10.3390/proteomes11030027
APA StyleBond, J. J., Refshauge, G., Newell, M. T., Holman, B. W. B., Wheeler, D., Woodgate, S., Kamath, K. S., & Hayes, R. C. (2023). Quantitative Differences in Rumen Epithelium Proteins in Lambs Fed Wheat, Perennial Wheat, or Perennial Wheat plus Lucerne. Proteomes, 11(3), 27. https://doi.org/10.3390/proteomes11030027







