Comparison of Different Label-Free Techniques for the Semi-Absolute Quantification of Protein Abundance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Cultures
2.2. Total Protein Extraction and In-Gel Digestion
2.3. Preparation of the UPS2 Samples
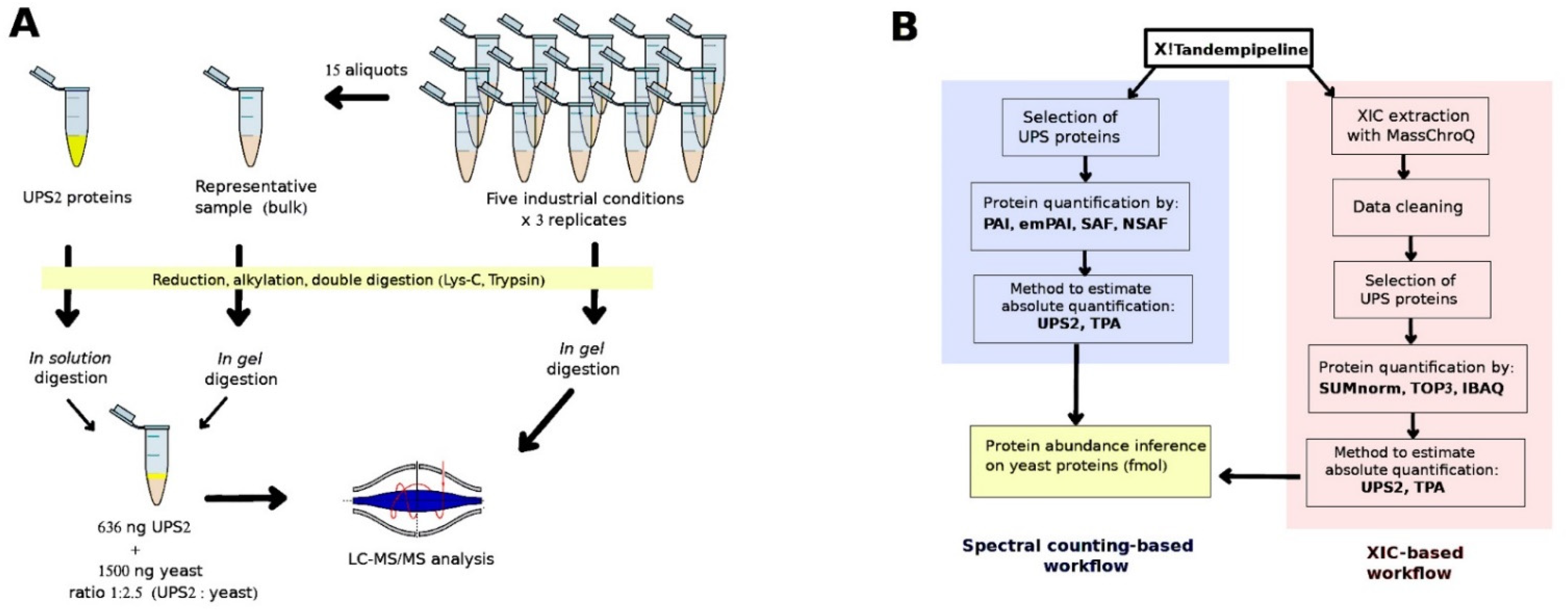
2.4. Sample Preparation for Method Validation
2.5. Mass Spectrometry Analysis
2.6. Protein Identification
2.7. Protein Quantification
2.8. Data Analysis
3. Results and Discussion
3.1. Implementation of the UPS2-Based Strategy in Yeast
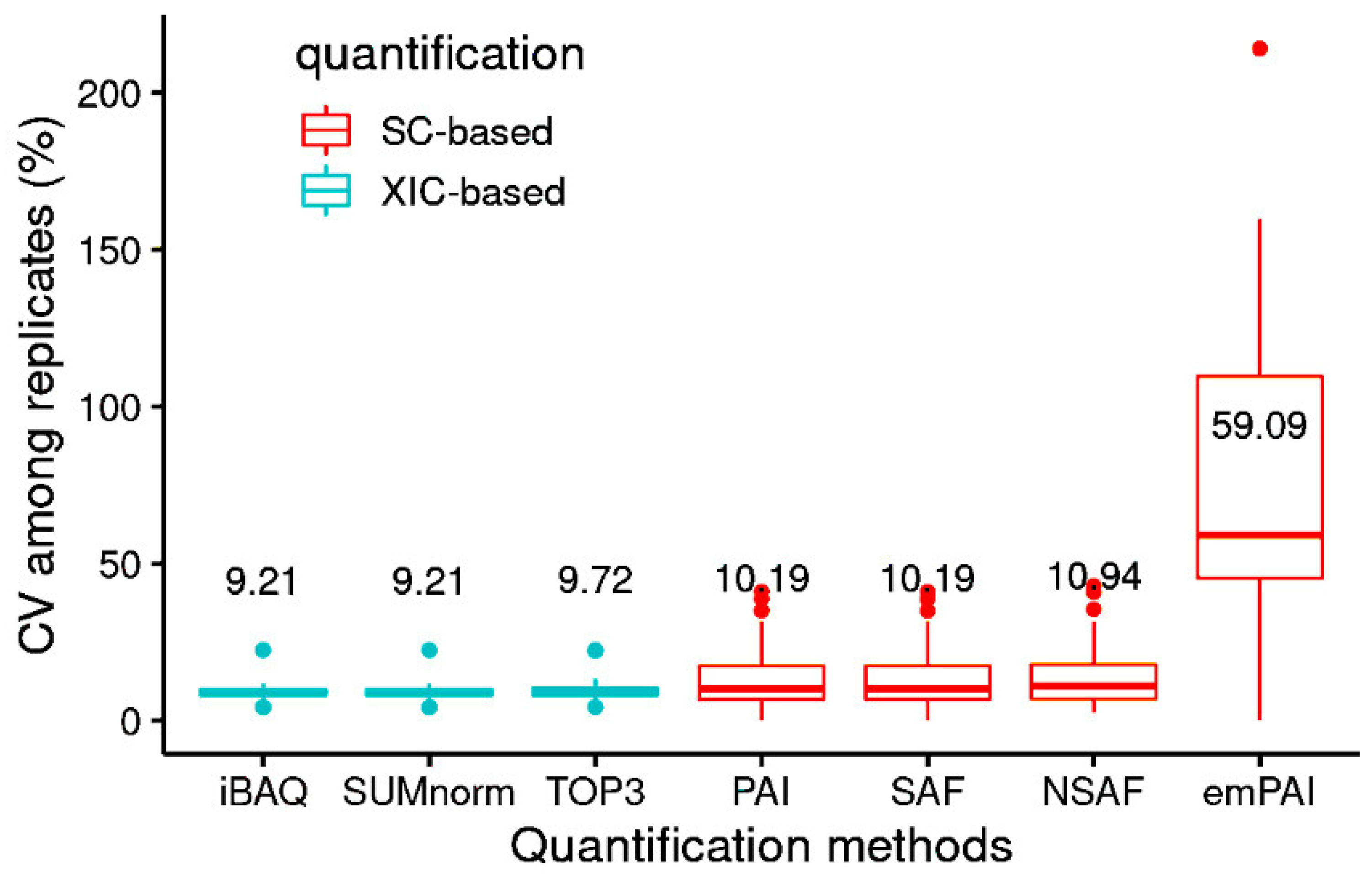
3.2. Performance of the Quantification Methods with the UPS2 Proteins
3.3. Performance of the Semi-Absolute Quantification Techniques with the UPS2 Proteins
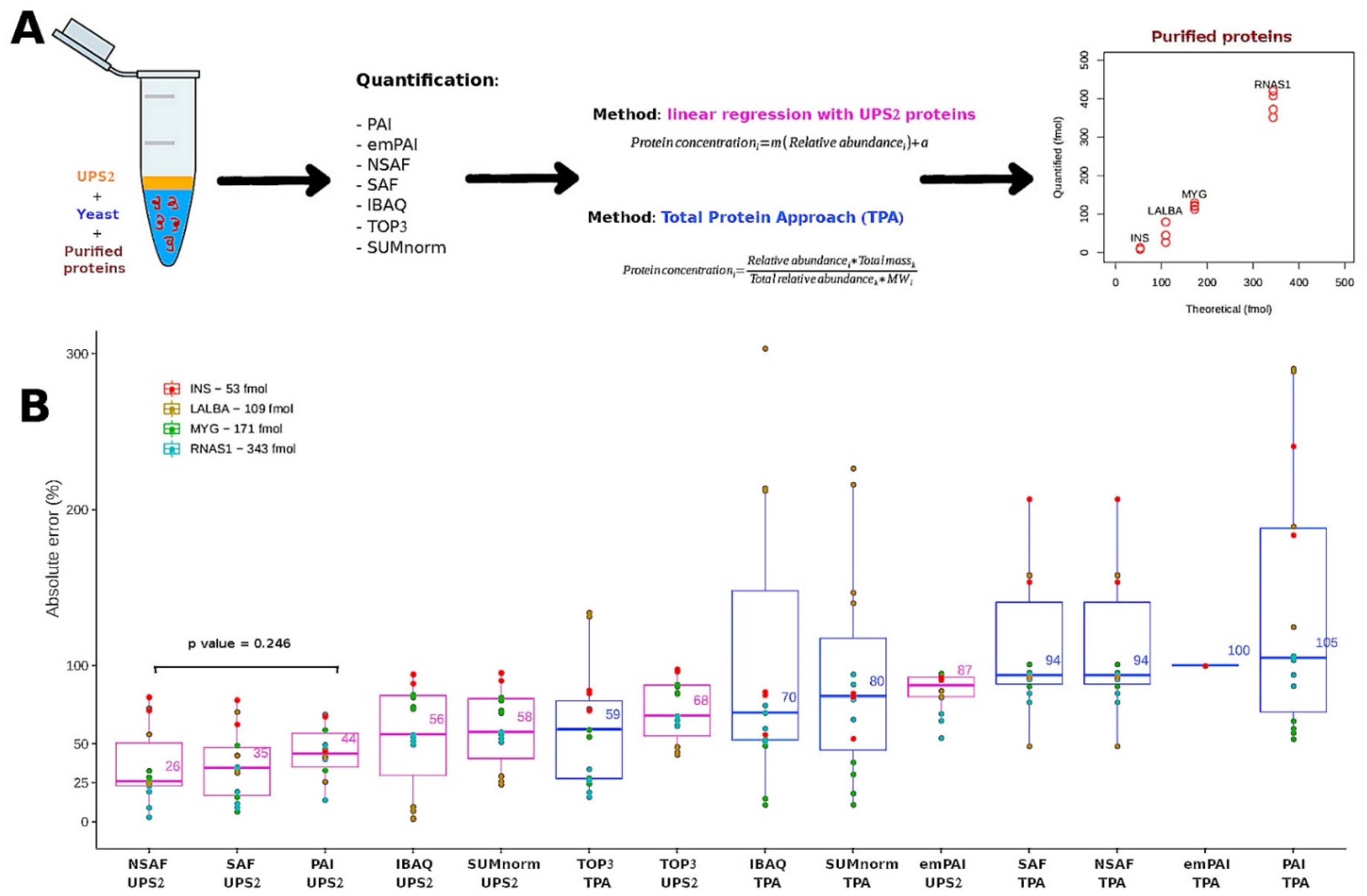
3.4. Performance of the Semi-Absolute Quantification Techniques with External Proteins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ankney, J.A.; Muneer, A.; Chen, X. Relative and Absolute Quantitation in Mass Spectrometry–Based Proteomics. Annu. Rev. Anal. Chem. 2018, 11, 49–77. [Google Scholar] [CrossRef]
- Blein-Nicolas, M.; Zivy, M. Thousand and one ways to quantify and compare protein abundances in label-free bottom-up proteomics. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2016, 1864, 883–895. [Google Scholar] [CrossRef]
- Rappsilber, J.; Ryder, U.; Lamond, A.I.; Mann, M. Large-Scale Proteomic Analysis of the Human Spliceosome. Genome Res. 2002, 12, 1231–1245. [Google Scholar] [CrossRef] [Green Version]
- Ishihama, Y.; Oda, Y.; Tabata, T.; Sato, T.; Nagasu, T.; Rappsilber, J.; Mann, M. Exponentially Modified Protein Abundance Index (emPAI) for Estimation of Absolute Protein Amount in Proteomics by the Number of Sequenced Peptides per Protein. Mol. Cell. Proteom. 2005, 4, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Bubis, J.; Levitsky, L.; Ivanov, M.; Tarasova, I.; Gorshkov, M.V. Comparative evaluation of label-free quantification methods for shotgun proteomics. Rapid Commun. Mass Spectrom. 2017, 31, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Dowle, A.A.; Wilson, J.; Thomas, J.R. Comparing the Diagnostic Classification Accuracy of iTRAQ, Peak-Area, Spectral-Counting, and emPAI Methods for Relative Quantification in Expression Proteomics. J. Proteome Res. 2016, 15, 3550–3562. [Google Scholar] [CrossRef] [PubMed]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Gerber, S.A.; Rush, J.; Stemman, O.; Kirschner, M.W.; Gygi, S.P. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc. Natl. Acad. Sci. USA 2003, 100, 6940–6945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkpatrick, D.; Gerber, S.; Gygi, S.P. The absolute quantification strategy: A general procedure for the quantification of proteins and post-translational modifications. Methods 2005, 35, 265–273. [Google Scholar] [CrossRef]
- Brun, V.; Dupuis, A.; Adrait, A.; Marcellin, M.; Thomas, D.; Court, M.; Vandenesch, F.; Garin, J. Isotope-labeled Protein Standards: Toward Absolute Quantitative Proteomics. Mol. Cell. Proteom. 2007, 6, 2139–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bär, C.; Mathis, D.; Neuhaus, P.; Dürr, D.; Bisig, W.; Egger, L.; Portmann, R. Protein profile of dairy products: Simultaneous quantification of twenty bovine milk proteins. Int. Dairy J. 2019, 97, 167–175. [Google Scholar] [CrossRef]
- Sánchez, B.J.; Nielsen, J. Genome scale models of yeast: Towards standardized evaluation and consistent omic integration. Integr. Biol. 2015, 7, 846–858. [Google Scholar] [CrossRef] [Green Version]
- Monk, J.M.; Nogales, J.; Palsson, B.O. Optimizing genome-scale network reconstructions. Nat. Biotechnol. 2014, 32, 447–452. [Google Scholar] [CrossRef]
- Ross, P.L.; Huang, Y.N.; Marchese, J.N.; Williamson, B.; Parker, K.; Hattan, S.; Khainovski, N.; Pillai, S.; Dey, S.; Daniels, S.; et al. Multiplexed Protein Quantitation in Saccharomyces cerevisiae Using Amine-reactive Isobaric Tagging Reagents. Mol. Cell. Proteom. 2004, 3, 1154–1169. [Google Scholar] [CrossRef] [Green Version]
- Choe, L.; D’Ascenzo, M.; Relkin, N.R.; Pappin, D.; Ross, P.; Williamson, B.; Guertin, S.; Pribil, P.; Lee, K.H. 8-Plex quantitation of changes in cerebrospinal fluid protein expression in subjects undergoing intravenous immunoglobulin treatment for Alzheimer’s disease. Proteomics 2007, 7, 3651–3660. [Google Scholar] [CrossRef] [Green Version]
- Werner, T.; Becher, I.; Sweetman, G.; Doce, C.; Savitski, M.M.; Bantscheff, M. High-Resolution Enabled TMT 8-plexing. Anal. Chem. 2012, 84, 7188–7194. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Ostasiewicz, P.; Duś, K.; Zielińska, D.F.; Gnad, F.; Mann, M. Extensive quantitative remodeling of the proteome between normal colon tissue and adenocarcinoma. Mol. Syst. Biol. 2012, 8, 611. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Hein, M.; Cox, J.; Mann, M. A “Proteomic Ruler” for Protein Copy Number and Concentration Estimation without Spike-in Standards. Mol. Cell. Proteom. 2014, 13, 3497–3506. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, B.J.; Lahtvee, P.-J.; Campbell, K.; Kasvandik, S.; Yu, R.; Domenzain, I.; Zelezniak, A.; Nielsen, J. Benchmarking Accuracy and Precision of Intensity-Based Absolute Quantification of Protein Abundances in Saccharomyces cerevisiae. Proteomics 2020, 21, 2000093. [Google Scholar] [CrossRef] [PubMed]
- Vildhede, A.; Wiśniewski, J.R.; Norén, A.; Karlgren, M.; Artursson, P. Comparative Proteomic Analysis of Human Liver Tissue and Isolated Hepatocytes with a Focus on Proteins Determining Drug Exposure. J. Proteome Res. 2015, 14, 3305–3314. [Google Scholar] [CrossRef]
- Belouah, I.; Nazaret, C.; Pétriacq, P.; Prigent, S.; Bénard, C.; Mengin, V.; Blein-Nicolas, M.; Denton, A.K.; Balliau, T.; Augé, S.; et al. Modeling Protein Destiny in Developing Fruit. Plant Physiol. 2019, 180, 1709–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belouah, I.; Bénard, C.; Denton, A.; Blein-Nicolas, M.; Balliau, T.; Teyssier, E.; Gallusci, P.; Bouchez, O.; Usadel, B.; Zivy, M.; et al. Transcriptomic and proteomic data in developing tomato fruit. Data Brief 2020, 28, 105015. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, P.; Saei, A.A.; Wang, S.; Zubarev, R.A. Dynamic Proteomics Reveals High Plasticity of Cellular Proteome: Growth-Related and Drug-Induced Changes in Cancer Cells are comparable. Proteomics 2018, 18, e1800118. [Google Scholar] [CrossRef]
- Esoufi, B.; Ekrug, K.; Eharst, A.; Emacek, B. Characterization of the E. coli proteome and its modifications during growth and ethanol stress. Front. Microbiol. 2015, 6, 103. [Google Scholar]
- Carpy, A.; Krug, K.; Graf, S.; Koch, A.; Popic, S.; Hauf, S.; Macek, B. Absolute Proteome and Phosphoproteome Dynamics during the Cell Cycle of Schizosaccharomyces pombe (Fission Yeast). Mol. Cell. Proteom. 2014, 13, 1925–1936. [Google Scholar] [CrossRef] [Green Version]
- Ahrné, E.; Molzahn, L.; Glatter, T.; Schmidt, A. Critical assessment of proteome-wide label-free absolute abundance estimation strategies. Proteomics 2013, 13, 2567–2578. [Google Scholar] [CrossRef]
- Trudgian, D.C.; Ridlova, G.; Fischer, R.; Mackeen, M.M.; Ternette, N.; Acuto, O.; Kessler, B.M.; Thomas, B. Comparative evaluation of label-free SINQ normalized spectral index quantitation in the central proteomics facilities pipeline. Proteomics 2011, 11, 2790–2797. [Google Scholar] [CrossRef]
- Krey, J.F.; Wilmarth, P.A.; Shin, J.-B.; Klimek, J.; Sherman, N.; Jeffery, E.D.; Choi, D.; David, L.L.; Barr-Gillespie, P. Accurate Label-Free Protein Quantitation with High- and Low-Resolution Mass Spectrometers. J. Proteome Res. 2014, 13, 1034–1044. [Google Scholar] [CrossRef]
- Wu, Q.; Shan, Y.; Qu, Y.; Jiang, H.; Yuan, H.; Liu, J.; Zhang, S.; Liang, Z.; Zhang, L.; Zhang, Y. Improved accuracy for label-free absolute quantification of proteome by combining the absolute protein expression profiling algorithm and summed tandem mass spectrometric total ion current. Analyst 2014, 139, 138–146. [Google Scholar] [CrossRef]
- Smits, A.H.; Lindeboom, R.G.; Perino, M.; Van Heeringen, S.J.; Veenstra, G.J.C.; Vermeulen, M. Global absolute quantification reveals tight regulation of protein expression in single Xenopus eggs. Nucleic Acids Res. 2014, 42, 9880–9891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilani, J.; Davanture, M.; Simon, A.; Zivy, M.; Fillinger, S. Comparative quantitative proteomics of osmotic signal transduction mutants in Botrytis cinerea explain mutant phenotypes and highlight interaction with cAMP and Ca2+ signalling pathways. J. Proteom. 2020, 212, 103580. [Google Scholar] [CrossRef]
- Doughty, T.W.; Domenzain, I.; Millan-Oropeza, A.; Montini, N.; de Groot, P.A.; Pereira, R.; Nielsen, J.; Henry, C.; Daran, J.-M.G.; Siewers, V.; et al. Stress-induced expression is enriched for evolutionarily young genes in diverse budding yeasts. Nat. Commun. 2020, 11, 2144. [Google Scholar] [CrossRef]
- Langella, O.; Valot, B.; Balliau, T.; Blein-Nicolas, M.; Bonhomme, L.; Zivy, M. X!TandemPipeline: A Tool to Manage Sequence Redundancy for Protein Inference and Phosphosite Identification. J. Proteome Res. 2017, 16, 494–503. [Google Scholar] [CrossRef]
- Ferry-Dumazet, H.; Houel, G.; Montalent, P.; Moreau, L.; Langella, O.; Negroni, L.; Vincent, D.; Lalanne, C.; De Daruvar, A.; Plomion, C.; et al. PROTICdb: A web-based application to store, track, query, and compare plant proteome data. Proteomics 2005, 5, 2069–2081. [Google Scholar] [CrossRef]
- Langella, O.; Valot, B.; Jacob, D.; Balliau, T.; Flores, R.; Hoogland, C.; Joets, J.; Zivy, M. Management and dissemination of MS proteomic data with PROTICdb: Example of a quantitative comparison between methods of protein extraction. Proteomics 2013, 13, 1457–1466. [Google Scholar] [CrossRef]
- Langella, O.; Zivy, M.; Joets, J. The PROTICdb Database for 2-DE Proteomics. Methods Mol. Bio. 2007, 355, 279–303. [Google Scholar]
- Vizcaino, J.A.; Deutsch, E.W.; Wang, R.; Csordas, A.; Reisinger, F.; Ríos, D.; Dianes, J.A.; Sun, Z.; Farrah, T.; Bandeira, N.; et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 2014, 32, 223–226. [Google Scholar] [CrossRef]
- Zybailov, B.; Mosley, A.L.; Sardiu, M.E.; Coleman, M.K.; Florens, L.; Washburn, M.P. Statistical Analysis of Membrane Proteome Expression Changes in Saccharomyces cerevisiae. J. Proteome Res. 2006, 5, 2339–2347. [Google Scholar] [CrossRef]
- Valot, B.; Langella, O.; Nano, E.; Zivy, M. MassChroQ: A versatile tool for mass spectrometry quantification. Proteomics 2011, 11, 3572–3577. [Google Scholar] [CrossRef]
- Millan-Oropeza, A.; Henry, C.; Blein-Nicolas, M.; Aubert-Frambourg, A.; Moussa, F.; Bleton, J.; Virolle, M.-J. Quantitative Proteomics Analysis Confirmed Oxidative Metabolism Predominates in Streptomyces coelicolor versus Glycolytic Metabolism in Streptomyces lividans. J. Proteome Res. 2017, 16, 2597–2613. [Google Scholar] [CrossRef] [PubMed]
- Ning, K.; Fermin, D.; Nesvizhskii, A.I. Comparative Analysis of Different Label-Free Mass Spectrometry Based Protein Abundance Estimates and Their Correlation with RNA-Seq Gene Expression Data. J. Proteome Res. 2012, 11, 2261–2271. [Google Scholar] [CrossRef]
- Silva, J.C.; Gorenstein, M.V.; Li, G.-Z.; Vissers, J.P.C.; Geromanos, S.J. Absolute Quantification of Proteins by LCMSE: A Virtue of Parallel MS Acquisition. Mol. Cell. Proteom. 2006, 5, 144–156. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Tsou, C.-C.; Avtonomov, D.; Larsen, B.; Tucholska, M.; Choi, H.; Gingras, A.-C.; Nesvizhskii, A. DIA-Umpire: Comprehensive computational framework for data-independent acquisition proteomics. Nat. Methods 2015, 12, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Cook, R.D. Detection of Influential Observation in Linear Regression. Technometrics 2000, 42, 65. [Google Scholar] [CrossRef]
- Muntel, J.; Fromion, V.; Goelzer, A.; Maaß, S.; Mäder, U.; Büttner, K.; Hecker, M.; Becher, D. Comprehensive Absolute Quantification of the Cytosolic Proteome of Bacillus subtilis by Data Independent, Parallel Fragmentation in Liquid Chromatography/Mass Spectrometry (LC/MSE). Mol. Cell. Proteom. 2014, 13, 1008–1019. [Google Scholar] [CrossRef] [Green Version]
- Trauchessec, M.; Enjalbert, Q.; Bardet, C.; Homo-Prault, X.; Jacquet, C.; Herment, L.; Fortin, T. A Universal Approach for Individual Identification and Quantities Assessment of Host Cell Proteins with LC-MS. 2019. Available online: https://www.anaquant.com/wp-content/uploads/2019/05/POSTER-ANAQUANT_HCP-analysis_cellandgenetherapy.pdf (accessed on 28 November 2021).

| Quantification Methods | Linearity (r2) | CV among Proteins (%) | CV among Replicates (%) | |
|---|---|---|---|---|
| SC-based | PAI | 0.89 | 48.8 | 10.2 |
| emPAI | 0.61 | 161.4 | 59.1 | |
| SAF | 0.90 | 48.0 | 10.2 | |
| NSAF | 0.90 | 48.0 | 10.9 | |
| XIC-based | SUMnorm | 0.96 | 52.9 | 10.0 |
| TOP3 | 0.91 | 62.6 | 10.5 | |
| iBAQ | 0.96 | 51.3 | 10.0 | |
| Quantification Methods | Purified Proteins | Spiked UPS2 Proteins | ||
|---|---|---|---|---|
| UPS2 | TPA | UPS2 | TPA | |
| iBAQ | 0.15 | 0.65 | 0.69 | 0.96 |
| SUMnorm | 0.16 | 0.89 | 0.74 | 0.95 |
| TOP3 | 0.21 | 0.53 | 1.09 | 0.96 |
| NSAF | 0.21 | 0.16 | 1.21 | 0.92 |
| SAF | 0.22 | 0.16 | 1.19 | 0.92 |
| PAI | 0.17 | 0.15 | 1.17 | 0.92 |
| emPAI | 0.12 | 3.67 | 84.83 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millán-Oropeza, A.; Blein-Nicolas, M.; Monnet, V.; Zivy, M.; Henry, C. Comparison of Different Label-Free Techniques for the Semi-Absolute Quantification of Protein Abundance. Proteomes 2022, 10, 2. https://doi.org/10.3390/proteomes10010002
Millán-Oropeza A, Blein-Nicolas M, Monnet V, Zivy M, Henry C. Comparison of Different Label-Free Techniques for the Semi-Absolute Quantification of Protein Abundance. Proteomes. 2022; 10(1):2. https://doi.org/10.3390/proteomes10010002
Chicago/Turabian StyleMillán-Oropeza, Aarón, Mélisande Blein-Nicolas, Véronique Monnet, Michel Zivy, and Céline Henry. 2022. "Comparison of Different Label-Free Techniques for the Semi-Absolute Quantification of Protein Abundance" Proteomes 10, no. 1: 2. https://doi.org/10.3390/proteomes10010002
APA StyleMillán-Oropeza, A., Blein-Nicolas, M., Monnet, V., Zivy, M., & Henry, C. (2022). Comparison of Different Label-Free Techniques for the Semi-Absolute Quantification of Protein Abundance. Proteomes, 10(1), 2. https://doi.org/10.3390/proteomes10010002






