Abstract
Curricula related to instrumental analysis aim for competency-based education to promote the development of teaching strategies that encourage students to successfully tackle the problem-solving and tasks inherent to their profession. However, this endeavor is constrained by the complexity of equipment and the lack of time in laboratory classes. The objective of this work is to present an alternative high-performance liquid chromatography (HPLC) practice that adapts the desired competencies to a shorter and more effective timeframe. It seeks more active participation from the student and contextualizes chromatographic analysis within a real-world problem that encompasses the entire analytical process, from sample to final result. In this scenario, the student receives a solid sample of spicy paprika from a supermarket and must report the level of spiciness in terms of the total amount of capsaicinoids. To achieve this, they must first apply different experimental conditions for extracting the analytes of interest (varying temperatures and solvents) and selecting the most optimal condition. This practice is designed for short sessions, specifically conducted in two 2.5 h laboratory sessions, and has been implemented in the subject “Advanced Techniques in Instrumental Analysis (ATIA)” in the fourth year of the Degree in Biotechnology at the University of Cadiz. The results obtained demonstrated a significant increase in student motivation and an improvement in the acquisition of skills; 100% of the students achieved a grade higher than seven in the final evaluation of their learning process.
1. Introduction
Currently, the globalization process has brought about many advancements of all kinds, primarily technological. Therefore, human beings are now required to have the ability to confront these changes by developing a series of skills, abilities, and critical thinking in order to achieve a proper education [1].
Nowadays, there is a strong consensus suggesting that, to promote improvement in thinking skills, it is necessary to create a conducive learning environment for students to take an active role in the process. According to Travieso et al. [2], there are three principles related to learning. The first one is to value learning by the students as a system of construction rather than passivity. The second principle is based on the impact of metacognition on knowledge utilization, with metacognition understood as an awareness of one’s own thought processes and understanding the patterns behind them. Lastly, the third principle emphasizes the social nature of learning. This implies that students can explore complex problem situations by posing open-ended questions that guide discussion, exchange of ideas, and meaning construction.
These active methodologies aim to foster knowledge construction through inquiry, reflection, creativity, and problem solving [3]. Their effectiveness is contingent upon role changes throughout the learning process, with the teacher taking on the role of facilitator or guide, while still recognizing that the teacher also has to teach, as not everything can be learned autonomously. The methodological proposal of Problem-Based Learning (PBL) is an appealing alternative to implement this active methodology. It is the means by which the activities aimed at promoting active and direct participation of students throughout the teaching–learning process are made possible. PBL stimulates interpersonal relationships and communication skills, develops research skills, and promotes reflection and analysis [4,5].
One of the current premises in curricula, concerning content related to Instrumental Analysis, is to promote the development of teaching strategies that encourage students to acquire basic skills in the field of chemistry, such as sample preparation, handling of materials, instrumentation, and data analysis. These competencies aim to foster their creativity and autonomy [6], and therefore, new methodologies are considered that enable competency-based education, allowing for comprehensive development of the individual [7]. One of the main objectives of laboratory sessions is for students not only to acquire isolated knowledge of different techniques but also to have the ability to correctly apply the acquired knowledge in solving real analytical problems within their field of study and with autonomy [8]. The role of the laboratory in the teaching–learning process, in addition to consolidating conceptual concepts, provides students with the procedural content and basic skills they will need in their future professional endeavors. However, the complexity of the equipment, the limited time dedicated to laboratory practice in the curricula, and the cost of materials make it difficult to acquire these skills. These limitations result in conventional laboratory practices, where procedures similar to a “cookbook” are followed, ensuring that they are completed within the stipulated time without deviations in results and increased costs [9]. Students are provided with a protocol they must strictly adhere to, leaving no room for improvisation, thus creating a learning situation that does not foster student autonomy and limits the meaningful learning of content. For this reason, it is necessary to establish training strategies in these subjects where students take on a more active role in their learning, make decisions, and construct their own knowledge [10]. These proposals must be everyday real problems of their profession but ensure, at the same time, that they can be solved in the laboratory session and at no greater cost than conventional ones [11]. However, despite the theoretical simplicity of these problems, they should serve as the fundamental basis for achieving the learning objectives established as requirements for passing the course. Additionally, through the use of this methodology, students will develop other competencies, such as collaborative work, learning to work in teams, dividing tasks, learning communication strategies, and promoting peer teaching (explaining to others) [12,13]. Thus, this work is generally established as Problem-Based Learning (PBL) adapted to the context of instrumental analysis within analytical chemistry. This learning methodology has been widely used at various educational levels [14,15,16] with the aim of enabling students to develop self-directed learning habits, problem-solving skills, and deep disciplinary knowledge [17,18,19].
Based on all of the above, a practical exercise has been designed to develop students’ autonomy and cooperation skills while being motivating and adapting to the infrastructure and limitations of an educational institution. In this way, it can be conducted in a few hours, successfully contextualizing to the students’ environment, the chromatographic analysis within the analytical process, starting from a real problem [20]. In this practice, the student takes on the role of a quality manager in a laboratory, who must provide a client with the total amount of spiciness of a batch of supermarket paprika samples to add this information to its labeling. To do this, they need to determine the total concentration (mg/g) of capsaicinoids in the paprika. Many authors advocate for the utility of case studies, as using real-life examples from their profession in the teaching–learning process increases interest and the degree of knowledge assimilation and fosters students’ attitudes and aptitudes [21].
Thus, the objectives pursued by this practice are:
- -
- Understanding the concept of extraction and the optimization of the most influential parameters to obtain the greatest number of compounds of interest from a sample. Knowing extraction methods for natural compounds in samples of biotechnological interest and being able to choose the most appropriate method according to the nature of the sample;
- -
- Familiarizing the student with high-performance liquid chromatography (HPLC), including its application, handling the instrumentation, preparing calibration curves, learning to separate and detect compounds, and the pre-treatment of sample extraction;
- -
- Working in teams, developing communication skills, and interaction among students;
- -
- Learning to interpret, compare, and explain the results obtained;
- -
- Understanding the importance of the different stages of the analytical process to solve an initial analytical problem by reporting the analytical results.
In this way, the laboratory session proposed as a model employs a real-life situation where students identify fundamental problems they are unfamiliar with, obtain new learning objectives that allow them to deepen their understanding of the content, retain information, and develop additional competencies beyond those of the subject itself [22].
Thus, the objective of this practice is for students not to view instrumental techniques in isolation and analyze samples following a standard protocol but rather to face decision-making and to assess the importance of sample pretreatment, the amount of sample to be taken, the selection of experimental conditions in extraction, and critical interpretation of the results, while simultaneously developing their skill in laboratory operations.
2. Methodology
2.1. Sample
The starting sample is a commercial container of spicy paprika obtained from a local store. Spicy paprika was chosen because it is easy to obtain and handle and is non-contaminating and non-hazardous. Additionally, it is economical, and the fact that it is a sample known to the students further arouses their curiosity, as they see it as a more familiar product. The student decides how much of the sample, between 1 and 2 g, to take from the container for the practical exercise.
2.2. Materials and Reagents
To carry out this experiment, a specialized laboratory for analytical chemistry is required, equipped with balances, spatulas, thermometers, wash bottles, stirring plates, an ice bath, scissors, filter paper, a high-performance liquid chromatography instrument, and an adequate supply of glassware. Specifically, in addition to the common equipment and materials, for each pair of students, the following material is necessary:
- -
- Two 50 mL volumetric flasks;
- -
- Three 100 mL beakers;
- -
- A 600 mL beaker;
- -
- A 50 mL graduated cylinder;
- -
- A 5 mL syringe with a needle;
- -
- Two nylon syringe filters;
- -
- Two Pasteur pipettes and 2 rubber bulbs;
- -
- A conical funnel;
- -
- A Teflon-coated magnet;
- -
- Two HPLC vials with their respective caps.
For the preparation of the extracts, Milli-Q water is used, in this case obtained through a Millipore water purification system (Bedford, MA, USA). And, HPLC-grade methanol was acquired from Panreac Quimica S.A.U. (Castellar del Vallés, Spain). For chromatographic separations, acetic acid is employed, in this case obtained from Merck (Darmstadt, Germany).
2.3. HPLC Instrumentation
The sample analysis is conducted at room temperature using a high-performance liquid chromatography (HPLC) system from JASCO (Tokyo, Japan), composed of an autosampler (Intelligent Sampler AS-2055 Plus), a pump (Intelligent HPLC Pump PU-1580), a mixing unit (Quaternary Gradient Unit LG-1580-04), a solvent degasser (Degasys Populaire), a UV-Vis detector (Intelligent-UV-Vis Detector UV-1575), a LiChrospher® 100 RP-18 column (Merck KGaA 64271, Darmstadt, Germany) (250 mm × 4 mm, 5 μm particle size), and a control interface (LC-NetII/ADC).
The method for separating capsaicinoids has been previously developed by the subject instructors. It employs two elution solvents: water (solvent A) and methanol (solvent B), both acidified with 0.1% acetic acid. The separation gradient used is shown in Table 1:

Table 1.
Separation gradient employed for the chromatographic separation.
The solvent flow rate was 1 mL/min. The detection of capsaicinoids was carried out through UV-Vis spectrophotometry, measuring these compounds at their maximum absorption wavelength (280 nm).
The HPLC chromatograms were processed using DropView software (Version 1.1), and manual integration was performed following well-accepted criteria applied to chromatographic methods [23].
2.4. Practice Organization and Timetable
Table 2 displays the tasks carried out during the two practical sessions, along with the allocated time for each to achieve the set objectives. It also outlines the skills that students should overcome and acquire.

Table 2.
Temporal organization of the contents developed in the practice along with assimilated competencies.
As can be seen in Table 2, the laboratory practice is divided into two sessions of 2.5 h each. In addition, each session is divided into 15 min intervals to understand the temporal organization of the tasks carried out within them.
- -
- Session 1 (150 min): Sample collection, preparation, and treatment. The isolation and separation of the compounds of interest from the sample matrix are prerequisites for any analysis process and involve several stages, such as extraction, preconcentration, and purification [24]. This laboratory session begins with an introduction to the importance of sample collection and the extraction of the target analytes from paprika prior to HPLC analysis. Paprika is a solid substance, so solid–liquid extraction is necessary to make the target analytes (in this case, capsaicinoids) accessible for analysis. Currently, there are many sophisticated solid–liquid extraction techniques. However, by using magnetic stirring, a simple technique with equipment available in any university laboratory, very realistic results can be obtained in a short period of time [25]. Students follow a general protocol for the experimental procedure, including the parameters to be studied. They will assess different extraction conditions to ultimately determine the optimal one. With the guidance of the professor, students have the freedom to make some decisions, such as the quantity of the starting sample or the volume of the extract to prepare. Once the extracts are prepared, students move on to the HPLC equipment, where the instructor introduces them to the most important parts of the equipment (type of solvents, column, pump, autosampler, degasser, and detector) and the connection between them, and emphasizes the importance of the analysis procedure conditions. A sequence is set up with all the students’ samples under various conditions for analysis;
- -
- Session 2 (150 min): Analysis and interpretation of results. Students visualize the results of their samples, for which they must integrate the obtained chromatograms. Once the target analytes are integrated, students record the area data for each one. Next, using the data provided by the instructor, they calculate the calibration curve using an external standard and proceed to calculate the amount of spiciness obtained in their samples. Students also have the data obtained by all the students, allowing them to compare the optimal extraction conditions. Once these conditions are selected, they proceed to calculate the amount of spiciness provided by each compound, identifying the predominant one and the total amount of capsaicinoids of the starting sample (mg/g).
2.5. Experimental Procedure
The practice script is given to the students, and they are paired up to carry out the experiments. First, different conditions are optimized to determine which one is the most suitable for extracting a greater amount of the compounds of interest (capsaicinoids). To achieve this, two extraction solvents (water and methanol) and different temperatures (0 °C, room temperature 20 °C, and 50 °C) are evaluated. All temperatures are controlled. To maintain the temperature at 0 °C, an ice bath is used, while a heating plate is used to reach 50 °C.
2.5.1. Step 1: Extraction of Capsaicinoids Using Magnetic Stirring
The sample preparation procedure is shown in Figure 1 and consists of the following steps: 1–2 g paprika are weighed in a beaker using a precision balance (Figure 1A). Each student records the weight, as the capsaicinoid concentration obtained will depend on the amount of starting sample. Next, approximately 30 mL of solvent (water or methanol, depending on the experiment) are added using a graduated cylinder. To initialize the magnetic stirring extraction, a magnet is introduced, and the mixture is placed on a heating plate for 10 min. It is very important to take into account the extraction conditions that will be carried out to place an ice-water bath or a heating plate to control the appropriate temperature (Figure 1B). Subsequently, to remove solid residues, gravity filtration is performed in a 50 mL volumetric flask using a funnel and a conical or folded filter (student’s choice) (Figure 1C). It is convenient to emphasize the importance of thoroughly rinsing the beaker to prevent any sample loss that could lead to errors in the measurement. Once filtered, the flask is filled to the mark with the same solvent used for the extraction. To prevent any impurities that may clog the chromatographic column, it is necessary to filter the extract using a 0.45 µm diameter nylon filter (Figure 1D). For this, 2–3 mL of the extract are taken with a syringe and filtered into a 2 mL chromatographic vial, which is labeled and placed in the HPLC equipment’s autosampler for subsequent analysis.
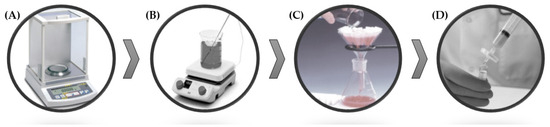
Figure 1.
Diagram of the experimental procedure followed by the students in the laboratory. (A) Weighing of the sample; (B) extraction of the compounds of interest; (C) gravity filtration of the extract; and (D) filtration using a syringe filter.
2.5.2. Step 2: Separation and Quantification of Capsaicinoids
First, the separated compounds are identified based on their retention time, using prior knowledge from the instructors. The spicy flavor of peppers is due to a group of molecules exclusive to these fruits, the capsaicinoids. They are acidic amides formed from branched-chain fatty acids C9–C11 and vanillylamine [26]. Although more than 20 capsaicinoids have been found in peppers, the most predominant are capsaicin (C) and dihydricapsaicin (DHC), representing around 90% of the total content. The rest is mainly represented by nor-dihydrocapsaicin (n-DHC), homo-capsaicin (h-C), and homo-dihydrocapsaicin (h-DHC). In Figure 2, the chemical structures of the five most abundant capsaicinoids found in nature can be observed, which differ in the length of the carbon chain and the presence of unsaturations [27].
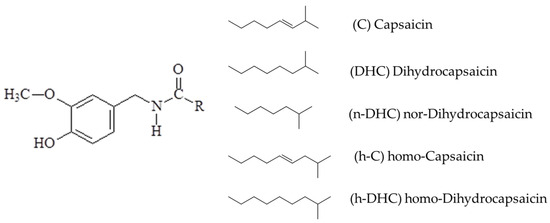
Figure 2.
Structures of the main capsaicinoids identified in peppers.
The five major capsaicinoids identified in the analyzed paprika sample elute in the following order: n-DHC, C, DHC, h-C, and h-DHC. These are relative non-polar compounds because, despite having a phenol group in their structure, they have an aromatic ring and a hydrocarbon chain. It is observed that, as the length of the aliphatic chain increases, as in the case of h-C or h-DHC, the retention time also increases. This is because the stationary phase (C18) is a reverse phase, so the higher the number of carbon atoms in the side chain, the lower the polarity of the structure. Consequently, it is more retained by the C18 column and takes more time for elution. Subsequently, the quantity of these capsaicinoids is determined from the chromatogram obtained at 280 nm, which is the wavelength of maximum absorption for this compound family (Figure 3).
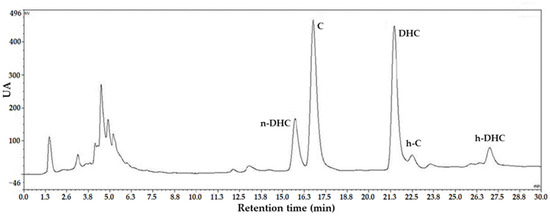
Figure 3.
Chromatogram of the most abundant capsaicinoids present in the pepper (n-DHC (nor-dihydrocapsaicin), C (capsaicin), DHC (dihydrocapsaicin), h-C (homo-capsaicin), and h-DHC (homo-dihydrocapsaicin)) obtained with the equipment used to conduct this laboratory practice.
For the quantification of the analytes, it is necessary to first develop a calibration curve. Due to the difficulty and cost of obtaining standard substances for the capsaicinoids under study, the instructors have previously prepared the calibration curve using an external standard of nonivamide, a type of capsaicinoids not as abundant in peppers as the ones studied but with similar characteristics. The data obtained for the calibration curve are provided to the students and are shown in Table 3.

Table 3.
Data provided by the teaching group for the calculation of the calibration curve of the nonivamide standard.
After that, the students are required to calculate the calibration curve in the range of 1–100 mg/L of spiciness through least squares linear regression. As this is an external standard calibration, students can interpolate the value of the areas obtained in each case to calculate the concentration of each of the capsaicinoids under different extraction conditions in the starting sample, as well as the total capsaicinoids concentration (mg/g), which is the goal of this practical exercise [28].
2.5.3. Step 3: Conclusions, Final Explanation, and Sharing of Results
The limited time in laboratory sessions prevents students from carrying out all experiments in duplicate, and each student may not be able to perform all extraction conditions. However, considering the importance of replicates in analysis, instructors put in common the data obtained by all students. In this way, students have the information to calculate the average value of the total capsaicinoid concentration alongside its standard deviation under each condition, allowing them to assess the most optimal extraction condition. With access to all the data, students must critically interpret the results, making decisions, such as discarding any outlier, justified statistically.
Students must report the final value of the total capsaicinoid concentration (mg/g), which is the overall objective of the practical exercise. To achieve this, they prepare a lab report explaining the methodology they have followed, as well as a discussion of the results. Additionally, in order to assess the assimilation of concepts related to the development of the chromatographic method and the fundamental principles of chromatography, students must answer some questions related to the laboratory session, which will be presented below with the correct answer for each of them.
2.6. Teaching Strategies
Some considerations that need to be emphasized to the students during the development of the practical exercise are as follows:
- -
- Weigh between 1–2 g of the sample. Emphasize that it is not necessary to weigh a fixed amount; what is important is to accurately record the weight taken for later inclusion in the calculations. The capsaicinoid concentration will depend on the initially weighed quantity;
- -
- Measure the extraction volume with a graduated cylinder: This volume can be approximate; what is crucial is that, subsequently, the obtained extract is brought to an exactly known volume for reference. This is why it is made up to the mark in a 50 mL volumetric flask.
Moreover, some recommendations to be taken into account:
- -
- Given the time allocated for the practice, students should start by using water as the first solvent, as this process involves a longer filtration time, and finish with a hot methanol extraction, as the cooling of the heating plate is a rather slow process;
- -
- Monitor temperature very carefully to prevent boiling and solvent losses due to splashing or evaporation, which could lead to errors in the analysis.
2.7. Security Considerations
This practice does not involve exceptional safety measures. Students should use the appropriate personal protection equipment: splash-resistant protective glasses, latex or nitrile gloves, and a laboratory coat.
Spicy paprika is a common food; however, it is true that capsaicin, the major component of hot peppers, is highly irritating if it comes into contact with the skin and eyes. So, if that happens, it should be washed with plenty of water.
Methanol will be used in the fume hood due to the hazards defined in its labeling. It is a liquid that can generate harmful and highly flammable organic vapors (H225). Additionally, it is toxic if ingested, comes into contact with the skin, or is inhaled (H301 + H311 + H331). Finally, it can cause damage to the central nervous system and visual organs (H370).
The waste generated in this practical exercise is limited to a small amount of leftover extracts with methanol, which will be disposed of in the non-halogenated organic solvent safety container. Filters with remnants of paprika can be discarded in the regular trash bin.
3. Results and Discussion
HPLC is commonly used as a successful tool for the separation, determination, and quantification of biological compounds in a wide variety of complex matrices [29]. Additionally, it has become the dominant analytical technique for achieving rapid separations in all industries, such as pharmaceutical [30], agrifood [31], environmental [32], and forensic chemistry [33]. For this reason, chromatography is an important chapter within any degree related to instrumental analysis or analytical chemistry, requiring students to acquire skills for their future careers. In this section, the results of the laboratory practice, as described and carried out in the Advanced Techniques in Instrumental Analysis course in the fourth year of the Biotechnology Degree at the University of Cadiz, are presented. Specifically, the results obtained by students during the years 2014 to 2022 are included, involving a total of approximately 400 students. The two practical sessions, each lasting 2.5 h, have been implemented to complement the knowledge acquired during the theoretical part of the course. Each session has had a total of students ranging between 20–30 that have been divided into pairs. Students had to prepare the sample, create a calibration curve, analyze the samples, and interpret the final results. Below are the average results obtained at different stages.
3.1. Obtaining the Optimal Extraction Condition
As mentioned earlier, the main goal is that students take a more active role. Therefore, a real-world problem is presented: reporting the data on the total spicy content to label the product. In this experiment, students explored the following extraction conditions: water at room temperature, methanol at 0 °C, methanol at room temperature 20 °C, and methanol at 50 °C. Due to the limited time in the session, not all students performed all extractions. Instead, they were divided into two groups, with each group conducting two extraction conditions. Thus, each condition was carried out by a total of 10, 12, or 14 students, depending on the number of students enrolled each year when the experiment took place. All extracts were analyzed by HPLC. Subsequently, the results obtained by the different groups were discussed to conclude which extraction conditions are the most suitable for this type of analyte.
In the resulting chromatograms, several peaks were observed; however, only five of them corresponded to capsaicinoids: n-DHC, C, DHC, h-C, and h-DHC. The remaining compounds are sugar and other analytes that also absorb at 280 nm. As mentioned earlier, the compounds were previously identified by the professors based on their retention times, and students had access to this information. On the other hand, with the information provided in Table 3 on the external standard, the students calculated the calibration curve (y = 27403x − 50133, regression coefficient R2 = 0.9996, LOD = 0.58 mg L–1, and LOQ = 1.30 mg L–1). The negative value of the intersection of the axis on the calibration curve means that a minimum analyte concentration is needed to be detected by the equipment. So, once the compounds of interest were identified, they proceeded to integrate the peaks and apply the calibration curve they calculated the concentration of all capsaicinoids under the different conditions. Based on the provided information, they selected only those compounds from the chromatogram under study in this practical exercise. In this way, they understood the importance of knowing retention times for compound identification because, as mentioned, other analytes can also absorb energy at the same wavelength as capsaicinoids. Table 4 displays the average results obtained by one of the practice groups for each condition. Students were asked to fill in the table according to their group and condition, and then a collective discussion of all classmate’s results was conducted. The presented results are based on peak integration (areas).

Table 4.
Experimental data generated by students for different extraction conditions.
From the obtained areas and by interpolation on the calibration curve, taking into account the initially weighed amount of paprika, the students determined the concentrations of each capsaicinoid as well as the total concentration in the solid sample (mg/g) under different extraction conditions (Table 5). It is crucial for each student to use their initial weight and recognize the importance of accurate weighing, as the total capsaicinoid concentration depends on the quantity of the starting sample. At this stage, students also grasp the significance of proper calibration curve usage and the importance of the final data, which must be of high quality and interpretable for the client. They cannot, for instance, directly report the data obtained from the calibration curve, as the quantity of the starting sample and any stage in the entire analytical process can influence the result. If done correctly, any stage prior to analysis should not interfere with or alter the final data.

Table 5.
Capsaicinoid concentration (mg/g) in the solid paprika sample for each of the extraction conditions.
All students had access to their peers’ data to determine the optimal extraction conditions for any compounds of interest. Prior to performing the calculations, they had to identify if there were any outliers by applying the Q-Dixon test. To this end, they compared the calculated Q with the tabulated Q, and in cases where Qcalculated > Qtabulated, they rejected the data. A p-value less than 0.05 was set as statistically significant. The outlier values excluded from the analysis are highlighted in bold in Table 5.
Based on the results obtained, students had to create a comparative graph of the average (and standard deviation) concentration of each individual capsaicinoid for each characteristic of the extraction method, using all the data from all groups (Figure 4). With this, the students observed and discussed, using critical reasoning, which was the optimal condition or the trend they followed. In doing so, they developed some of the aforementioned competencies, such as learning to interpret results through graphing, sharing results, or using their own knowledge and critical reasoning skills to draw conclusions from the obtained results.
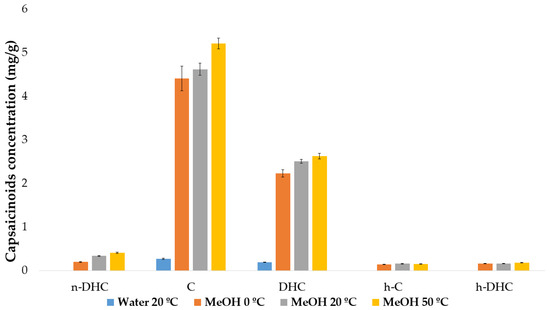
Figure 4.
Average concentration of individual capsaicinoids for different extraction conditions.
After comparing and discussing the results, the students concluded that, regardless of the extraction conditions, capsaicin was the predominant capsaicinoid, followed by dihydrocapsaicin, with the latter being approximately half the concentration of C. Furthermore, as mentioned in the introduction, it was confirmed that both major capsaicinoids together represent around 90% of the total concentration of capsaicinoids in the analyzed sample. Finally, they observed that the other three capsaicinoids, n-DHC, h-C, and h-DHC, were present in very similar concentrations (with n-DHC slightly higher than the other two) and significantly lower than the major ones.
Finally, students were asked to create a graph with the average (and standard deviation) concentration obtained under each extraction condition (Figure 5). In this way, they confirmed that the total amount of capsaicinoids in the paprika sample analyzed by HPLC ranged from 0.45 to 8.60 mg/g of the sample depending on the conditions.
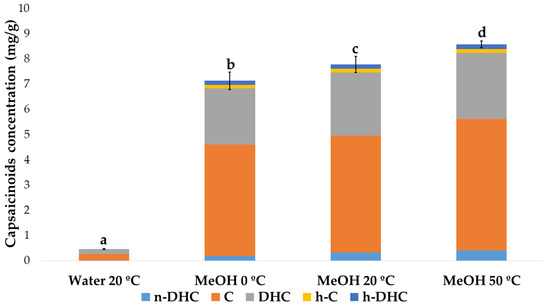
Figure 5.
Average concentration of capsaicinoids (mg/g) for different extraction conditions. Different letters (a, b, c, and d) indicate the presence of significant differences between the conditions.
Through visual inspection, all of them noted that a smaller amount of capsaicinoids was extracted with water compared to methanol. However, to make an objective comparison of the results and determine if there were significant differences between the use of solvent and/or temperature, the students conducted an ANOVA, specifically the Student’s t-test (p-value < 0.05), that supported the results statistically.
Based on the results obtained, the students concluded that methanol extracts are better at any temperature than water for any of the analyzed capsaicinoids, since the concentration obtained is much higher. This is due to the high polarity of water compared to that of the compounds of interest, which have an aromatic ring and a hydrocarbon chain in their structure [34]. On the other hand, they observed that, as the extraction temperature increases in the case of methanol, the concentration of extracted capsaicinoids also increases. This is because higher extraction temperatures accelerate movement, penetration, dissolution, and molecular diffusion to promote the release of bioactive compounds [35]. Moreover, a higher temperature also causes structural denaturing of proteins, e.g., cell-wall degradation, and thus greater access to the contents of the cells. Based on the ANOVA results, the students concluded that there were no significant differences between 0 °C and 20 °C, but there were significant differences at 50 °C. Therefore, the optimal condition reported by the students for the extraction of capsaicinoids was methanol at 50 °C, and thus, the final value reported to the client was 8.58 ± 0.13 mg/g.
3.2. Student Experience and Evaluation
The assessment of the learning situation was initially conducted with a series of questions during the theoretical explanation to understand the students’ prior knowledge and to build upon their existing understanding. Throughout the activity, this learning process was also evaluated. At the end of the first day of practical sessions, the students were gathered to receive an explanation about the HPLC equipment used. In this way, they could physically see each part of the equipment they had been studying in the theory class and become familiar with it. Furthermore, group discussion was encouraged to support reflection on the results they expected to obtain based on the appearance of the samples under each of the conditions and the polarity of the solvents and temperatures used. At this point, the professor can identify if there are any misconceptions, for example, if they understand the relationship between polarity and temperature with the color of the vial and, therefore, with the amount of compounds extracted under each condition, the importance of optimizing different conditions, how the separation and quantification of compounds are carried out, or why it is necessary to know the exact initial weight of the sample, which will be addressed to ensure full comprehension.
When all the content had been covered, in the second session, a final assessment was requested in the form of a laboratory report, which helped reinforce the acquired knowledge and was used to evaluate the learning objectives. In this report, students were required to process all the data and engage in a critical discussion thereof. Additionally, they had to answer a series of questions to assess whether they had correctly understood the foundation of the practice and the concepts under study. The report grading heavily relies on the analysis and interpretation of results, enabling the drawing of conclusions from the analytical data obtained.
The questions they had to answer in the report, along with their correct responses were as follows.
Q1. What effect does the extraction solvent have on the extraction of capsaicinoids? Explain the values observed as a function of the polarity of the analytes studied and the extraction solvents used.
The main objective of using the solvent in extraction is to release the analytes of interest contained in the sample, thus obtaining the analytes in solution for subsequent LC analysis. Depending on the nature of the solvent, a higher or lower concentration of these analytes will be obtained. Capsaicinoids are considered analytes of intermediate polarity because they have both polar and nonpolar components. For a successful extraction, it is necessary for the solvent used to have a polarity similar to that of the analyte to be extracted. The solvents used in this practice are methanol and water. Based on the results obtained, it is observed that methanol extracts a greater amount of capsaicinoids compared to water because it has a polarity more similar to that of the analyte under study. Both are polar solvents; however, water, being more polar, will have less affinity for less polar capsaicinoids, resulting in a poorer extraction.
Q2. What effect does the extraction temperature have on the extraction of capsaicinoids? Justify the answer.
In the experiment, extractions were carried out at different temperatures: 0, 20, and 50 °C. Observing the results obtained, the efficiency of the extraction increases with temperature, meaning that higher temperatures result in higher concentrations of capsaicinoids. This is because, as the temperature increases, both the extraction rate and the solubility of the analyte in the solvent increase, facilitating its extraction. However, it should be noted that excessive temperature increase can lead to degradation of the analytes and/or evaporation of the solvent.
Q3. What type of column have you used? Why? Comment in this regard, the solvent used in the separation, as well as their elution order.
In this chromatographic analysis, a C18 column has been employed, as the work is performed in reverse phase, meaning that the column used in the extraction as the stationary phase is nonpolar, and the mobile phase eluents are polar (water and methanol, both acidified with 0.1% acetic acid).
For effective elution, the order in which solvents are added should be water first because it has a polarity more different from the stationary phase, and later methanol gradually, as its polarity is more similar to the stationary phase. The solvent, depending on its polarity, will carry those analytes with more affinity for it through the column faster, allowing the separation of different analytes based on their retention time on the column. Since water is more polar, analytes with a higher polarity (greater affinity for the solvent) will be carried out through the column first, and after adding methanol, analytes with a lower polarity begin to elute. More apolar compounds will remain retained on the column for a longer time due to hydrophobic interactions because of their greater affinity.
Q4. Indicate the type of elution used in the chromatographic separation and represent it graphically (%methanol vs. time). In view of the graph, make comments on it (ramps, periods in isocratic, column washing, column conditioning, etc.).
For the separation of the target capsaicinoids present in the sample, a gradient chromatographic separation method has been employed (Figure 6).
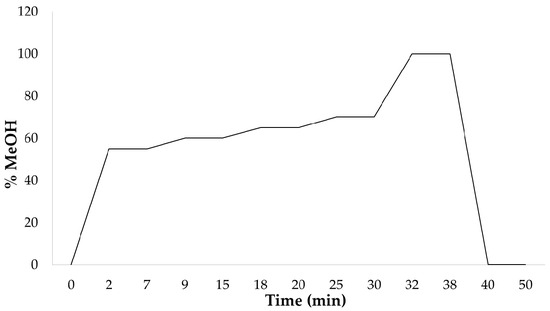
Figure 6.
Graphical representation of the chromatographic gradient used for the separation of the target capsaicinoids present in the paprika sample.
Through the graph, it can be observed that the elution of the sample occurs during the first 32 min. Within this time frame, there are four isocratic periods, moments when the solvent concentration remains constant (slope 0): from minute 2 to 7 (55%), minute 9 to 15 (60%), minute 18 to 20 (65%), and minute 25 to 30 (70%). After this, the column-washing phase begins until minute 38, reaching 100% methanol to ensure the complete elution of any remaining analyte in the column. Subsequently, the methanol concentration is decreased to 0% to return to the initial conditions, resulting in column conditioning until minute 40. Finally, from minute 40 to 50, column equilibration takes place to prepare the equipment for the injection of the next sample.
Q5. Why was a small amount of acetic acid added to the solvents?
Adding a small amount of acetic acid prevents the deprotonation of the analytes of interest (the hydroxyl group tends to deprotonate, potentially resulting in different conformations if the pH is not appropriate due to the release of a proton). In this way, splitting of the analyte peaks in the chromatogram is avoided due to the equilibrium that would occur between the protonated and deprotonated forms.
All students answered all questions proposed and passed the experimental reports submitted; in fact, over 95% of the students received grades higher than 7 over 10 in all cases. This indicated that they all had understood the main objectives of the practice correctly, namely the most important chromatographic concepts and the influence of parameters on the extraction of bioactive compounds of interest. The main mistakes detected were related to data interpretation, as certain students failed to recognize outliers. Consequently, the resulting outcomes were inaccurate, leading to flawed conclusions regarding the optimal conditions. Finally, it is worth noting the fact that the analytical results obtained agreed with the known value of the sample that was also evaluated, that is with the value that appeared on the labeling of the commercial container of spicy paprika (it is very important to take into account that the content of these compounds can be affected by several factors, including genotype, water availability, light, temperature, climatic and growth conditions, cultivation techniques, or maturity stage). Once this was demonstrated, in addition to having carried out the practical situation correctly, they knew how to process the collected data to obtain the analytical results. This feedback indicated that the learning objectives and instructional goals were met.
The results of this laboratory practice reinforce the importance of experimental work carried out in teaching laboratories, as they play a fundamental role in the construction of scientific knowledge. Well-designed laboratory practices provide an excellent opportunity to establish connections between theory and practice, that is, the real-world application of chemical science [36]. A dual approach (classroom learning alongside laboratory exercises with real-world problems) provides students with a deeper understanding of the practical utility of multiple analytical techniques for solving real-world problems during chemical analysis. Laboratory sessions allow students to reinforce theory directly through experience and develop conceptual depth. However, often due to a lack of time or the availability of materials, it is not easy to design a practice where students have some freedom and can put into practice the abstract concepts studied theoretically with their own experimental results, making them more concrete and visual [37].
Given a lower instructor–student ratio and a less formal environment, the laboratory is a conducive place for learning and provides opportunities for students to practice behaviors like scientists, such as asking questions, analyzing and interpreting data, or constructing explanations [38]. Furthermore, the informal atmosphere and smaller number of students allow them to feel freer for social interaction, both among themselves and with the professor, and less intimidated when asking questions, resulting in healthier learning and a more meaningful understanding of scientific concepts [39]. Therefore, it is important to leverage this environment so that students take a more active role in their decisions, truly fostering their skills, and not merely following established protocols.
This type of unsupervised laboratory practical exercise (meaning student-led but with the availability of a professor for any questions or needs), in addition to solidifying the theoretical concepts covered in class and acquiring technical skills, is a valuable tool for stimulating the acquisition of students’ critical thinking skills, problem identification, and problem-solving abilities that mimic real-life challenges. These exercises can foster increased motivation, interest, and self-confidence, leading to improved performance. They also contribute to the development of scientific attitudes and the innovative spirit of students in their future careers [40].
However, the main limitation of implementing this practice in any university laboratory is time, as it is a rather complex technique that encompasses many aspects, thus requiring a focus on the most important fundamentals. Additionally, there is the acquisition and maintenance of chromatography equipment, which can be costly, and the complexity of the technique for students with little experience.
The laboratory sessions presented in this article serve as an example of how a classic and robust tool, which has been used for a long time in the (bio)chemistry laboratories of many universities, can be ‘reformed’ by considering the principles of interdisciplinary and research-based learning approaches [41]. One of the competencies related to Sustainable Development Goals (SDGs) in this course involves critically contextualizing knowledge, and establishing interrelations with social, economic, and environmental issues. Thanks to this simple laboratory practice, the proposed premises are fulfilled, including the minimal preliminary preparation required, the analysis of a sample commonly consumed today, cost-effectiveness, low generation of waste (only small aqueous samples are used), calibration with different types of standards, short analysis times, and low solvent consumption [42]. Additionally, this practice promotes the use of instrumental analysis that aligns with current sustainable development goals, thus contributing to the establishment of what Agenda 2030 outlines as Education for Sustainable Development [43], promoted by the Conference of Rectors of Spanish Universities. More specifically, this practical session has fostered the development of transversal competencies, such as critically contextualizing the knowledge to be acquired, thereby establishing social interrelationships and also the sustainable use of resources in preventing negative impacts on the natural and social environment. All these features make the current HPLC protocol for quantifying capsaicinoids in paprika samples an environmentally responsible technique for analytical chemistry and instrumental analysis laboratories and suitable for both individual and collaborative work.
4. Conclusions
Based on the results obtained, it can be concluded that the proposed practice, aimed at reporting the total spicy-content data to label a batch of spicy peppers, satisfactorily meets the objectives and competencies pursued in an instrumental analysis practice. It is a simple, short-duration, low-cost practice that allows students to apply and complement the theoretical knowledge acquired.
The implemented methodology represents a successful approach, as it allows students to gain a better understanding of liquid chromatography applied in analytical measurements starting from a real-world problem. The fact that they had to work through the different stages of the analytical process, from problem identification and sample collection to the final result, has helped them contextualize and gain a holistic view of the importance of instrumental analysis. Furthermore, it has fostered the acquisition of competencies, such as the importance of critical data analysis, strengthening the assimilation of concepts, and applying theoretical knowledge to the analysis of a commercial product. On the other hand, the low generation of waste has allowed contextualizing the economic, social, and environmental issues pursued with the SDGs (Sustainable Development Goals).
These significant pedagogical aspects were confirmed through the evaluation of the laboratory reports, where students achieved the competencies established for this procedure, with grades higher than seven in all cases. Finally, by working in groups and sharing common objectives, the students learned firsthand the benefits of teamwork, which is often a necessity in scientific research.
Author Contributions
Conceptualization, G.F.B. and M.F.-G.; Methodology, M.V.-E., A.V.G.-d.-P., G.F.B. and M.F.-G.; Software, M.F.-G.; Validation, M.V.-E. and A.V.G.-d.-P.; Formal analysis, M.V.-E., A.V.G.-d.-P., J.L.P.C. and A.R.-R.; Investigation, G.F.B. and M.F.-G.; Resources, G.F.B. and M.F.-G.; Data curation, M.V.-E., P.S.-G., A.V.G.-d.-P., J.L.P.C. and A.R.-R.; Writing—original draft, M.V.-E. and P.S.-G.; Writing—review & editing, G.F.B. and M.F.-G.; Visualization, G.F.B. and M.F.-G.; Supervision, G.F.B. and M.F.-G.; Project administration, G.F.B. and M.F.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bermudez-Mendieta, J. El aprendizaje basado en problemas para mejorar el pensamiento crítico: Revision sistemática. INNOVA Res. J. 2021, 6, 77–89. [Google Scholar] [CrossRef]
- Travieso-Valdés, D.; Ortiz-Cárdenas, T. Aprendizaje basado en problemas y enseñanza por proyectos: Alternativas diferentes para enseñar. Rev. Cuba Educ. Super. 2018, 1, 124–133. [Google Scholar]
- Solórzano-López, J.B.; Lituma-Alejandro, L.A.; Espinoza-Freire, E.E. Estrategias de enseñanza e estudiantes de educación física. Rev. Metrop. Ciencias Apl. 2020, 3, 158–165. [Google Scholar]
- Espinoza-Freire, E.E. El aprendizaje basado en problemas, un reto a la enseñanza superior. Rev. Conrado. 2021, 17, 295–303. [Google Scholar]
- Szalay, L.; Tóth, Z. An Inquiry-Based Approach of Traditional ‘Step-by-Step’ Experiments. Chem. Educ. Res. Pract. 2016, 17, 923–961. [Google Scholar] [CrossRef]
- Lin, X.; Jin, X.; Xu, C.; Lai, H.; Lin, M.; Ren, N.; Cai, L. Iodometric Titration on Microfluidic Paper-Based Analytical Devices for Determination of Ascorbic Acid: A Laboratory Experiment for Chemical Education Undergraduates. J. Chem. Educ. 2023, 100, 1997–2002. [Google Scholar] [CrossRef]
- Guitert-Catasus, M.; Martín-Rodrigo, M.J.; Pérez-González, M.; Puig-Puig, L.; Sancha Fernández, C. Cuadernos de Pedagogía Ignaciana Universitaria. Aprendizaje Basado en Problemas. 2022. Universidad Pontificia Comillas. ISBN (Version Impresa): 978-84-8468-936-2. Available online: https://unijes.net/innovacion-educativa-y-pedagogia-ignaciana/ (accessed on 22 April 2024).
- Mutambuki, J.M.; Fynewever, H.; Douglass, K.; Cobern, W.W.; Obare, S.O. Integrating Authentic Research Experiences into the Quantitative Analysis Chemistry Laboratory Course: STEM Majors’ Self-Reported Perceptions and Experiences. J. Chem. Educ. 2019, 96, 1591–1599. [Google Scholar] [CrossRef]
- Moloney, J.G.; Campbell, C.D.; Worrall, A.F.; Stewart, M.I. Hands-on Inquiry-Based Qualitative Identification of Metals in Coins Utilizing Atmospheric Pressure Chemical Ionization Mass Spectrometry. J. Chem. Educ. 2022, 99, 2697–2703. [Google Scholar] [CrossRef]
- Hmelo-Silver, C.E. Proble-based learning: What and how do students learn. Educ. Psychol. Rev. 2004, 16, 235–265. [Google Scholar] [CrossRef]
- Akkuzu, N.; Arzu Uyulgan, M. Step by step learning using the I diagram in the systematic qualitative analyses of cations within a guided inquiry learning approach. Chem. Educ. Res. Pract. 2017, 18, 641–658. [Google Scholar] [CrossRef]
- Lopes, R.M.; Silva Filho, M.V.; Marsdden, M.; Alves, N.G. Problem-based learning: A teaching toxicology chemistry experience. Quim. Nova 2011, 34, 1275–1280. [Google Scholar] [CrossRef]
- Hauser-Davis, R.A.; Comarú, M.W.; Lopes, R.M. Metallothionein determination can be applied to learn about aquatic metal pollution and oxidative stress detoxification mechanisms trhough Problem-Based Learning. Biochem. Mol. Biol. Educ. 2002, 48, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Moore, S. New Approaches to Problem-Based Learning; Routledge: London, UK, 2010. [Google Scholar]
- Schwartz, P.; Mennin, S.; Webb, G. Problem-Based Learning: Case Studies, Experience and Practice; Kogan: London, UK, 2021. [Google Scholar]
- Hung, W.; Loyens, S.M. Problem-Based Learn. Interdiscip. J. Probl.-Based Learn. 2012, 6, 2. [Google Scholar] [CrossRef][Green Version]
- Savery, J.R.; Duffy, T.M. Problem Based Learning: An instructional model and its constructivist framework. Educ. Technol. 1995, 35, 31–37. [Google Scholar]
- Yew, E.H.J.; Schmidt, H.G. Evidence for constructive, self-regulatory, and collaborative processes in problem-based learning. Adv. Health Sci. Educ. 2009, 14, 251–273. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.; Gijselaers, W.H.; Moust, J.H.C.; De Grave, W.S.; Wolfhagen, I.; Van der Vleuten, C.P.M. Trends in research on the tutor in problem-based learning: Conclusions and implications for educational practice and research. Med. Teach. 2002, 24, 173–180. [Google Scholar] [CrossRef]
- Burnham, J.A.J. Developing Student Expertise in Scientific Inquiry. In Teaching Chemistry in Higher Education. A Festschrift in Honour of Professor Tina Overton; Seery, M.K., Mc Donnell, C., Eds.; Creathach Press: Dublin, Ireland, 2019; pp. 391–404. [Google Scholar]
- Hall, M.L.; Vardar-Ulu, D. An inquiry-based biochemistry laboratory structure emphasizing competency in the scientific process: A guided approach with an electronic notebook format. Biochem. Molec. Biol. Educ. 2013, 42, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Granados, L. El aprendizaje basado en problemas como estrategia didáctica en educación superior. Voces Educ. 2018, 3, 155–167. [Google Scholar]
- Cavazos-Rocha, N.; Rodríguez-Martínez, O.; Espinosa-Pedroza, A.; Waksman-Minsky, N.; Saucedo, A.L. NMR Teaching Strategies in the Instrumental Analysis Laboratory: Identification and Quantification of Caffeine in Energy Drinks. J. Chem. Educ. 2023, 100, 1934–1941. [Google Scholar] [CrossRef]
- Sruthi, D.; Dhanalakshmi, M.; Yashavantha Rao, H.C.; Parthasarathy, R.; Jayabaskaran, C. Extraction, isolation, and characterization of phytochemicals, the bioactive compounds of plants. In Recent Frontiers of Phytochemicals: Applications in Food, Pharmacy, Cosmetics, and Biotechnology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–8. [Google Scholar] [CrossRef]
- Juangsamoot, J.; Ruangviriyachai, C.; Techawongstien, S.; Chanthai, S. Determination of capsaicin and dihydrocapsaicin in some hot chilli varieties by RP-HPLC-PDA after magnetic stirring extraction and clean up with C18 cartridge. Int. Food Res. J. 2012, 19, 1217–1226. [Google Scholar]
- Yida, L.; Yulian, C.; Yuanliang, W.; Jiaxu, C.; Yuxin, H.; Yingzi, Y.; Luoming, L.; Zongjun, L.; Youhua, R.; Yu, X. Total phenolics, capsaicinoids, antioxidant activity, and α–glucosidase inhibitory activity of three varieties of pepper seeds. Int. J. Food Prop. 2020, 23, 1016–1035. [Google Scholar] [CrossRef]
- Chan, K.K.; Hamid, M.S.; Webster, R.D. Quantification of capsaicinoids in chillies by solid-phase extraction coupled with voltammetry. Food Chem. 2018, 265, 152–158. [Google Scholar]
- Maringolo, V.; Carvalho, A.Z.; Rocha, D.L. Simple multi-signal calibrations exploiting flow analysis systems. Talanta 2024, 272, 125787. [Google Scholar] [CrossRef] [PubMed]
- Karger, B.L. HPLC: Early and Recent Perspectives. J. Chem. Educ. 1997, 74, 45–48. [Google Scholar] [CrossRef]
- Gumustas, M.; Zalewski, P.; Ozkan, S.A.; Uslu, B. The History of the Core-Shell Particles and Applications in Active Pharmaceutical Ingredients Via Liquid Chromatography. Chromatographia 2019, 82, 17–48. [Google Scholar] [CrossRef]
- E-Siong, T.; Chin-Lam, L. The Analysis of Carotenoids and Retinoids: A Review. Food Chem. 1991, 41, 147–193. [Google Scholar] [CrossRef]
- Leong, W.-H.; The, S.-Y.; Moshaddeque Hossain, M.; Nadarajaw, T.; Zabidi-Hussin, Z.; Chin, S.-Y.; Lai, K.-S.; Erin Lim, S.-H. Application, monitoring and adverse effects in pesticide use: The importance of reinforcement of Good Agricultural Practices (GAPs). J. Environ. Manag. 2020, 260, 109987. [Google Scholar] [CrossRef]
- Gennaro, M.C.; Abrigo, C.; Cipolla, G. High-performance liquid chromatography of food colours and its relevance in forensic chemistry. J. Chromatogr. A 1994, 674, 281–299. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Ferreiro-González, M.; Barroso, C.G.; Palma, M.; Barbero, G.F.; Espada-Bellido, E. Optimizing and Comparing Ultrasound- and Microwave-Assisted Extraction Methods Applied to the Extraction of Antioxidant Capsinoids in Peppers. Agronomy 2019, 9, 633. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Li, Q.; Jin, W.; Chen, W.; Han, J.; Zhang, Y. Simultaneous Optimization for Ultrasound-Assisted Extraction and Antioxidant Activity of Flavonoids from Sophora flavescens Using Responss Surface Methodology. Molecules 2019, 24, 112. [Google Scholar] [CrossRef]
- Paraskevas, M.; Tilleman, T.; Eichen, Y.; Yellin, R.A. Informal Personalized Teaching (IPT): Bridging Gaps in Theoretical and Practical Knowledge during Idle Times in a Chemistry Lab. J. Chem. Educ. 2022, 99, 3984–3992. [Google Scholar] [CrossRef]
- Candas, B.; Altun, T. Investigating Flipped Laboratory Practices of Science Student Teachers. J. Turk. Sci. Educ. 2023, 20, 173–188. [Google Scholar] [CrossRef]
- Stephenson, N.S.; Duffy, E.M.; Day, E.L.; Padilla, K.; Herrington, D.G.; Cooper, M.M.; Carmel, J.H. Development and Validation of Scientific Practices Assessment Tasks for the General Chemistry Laboratory. J. Chem. Educ. 2020, 97, 884–893. [Google Scholar] [CrossRef]
- Dolmans, D.H.J.M.; Schmidt, H.G. What Do We Know About Cognitive and Motivational Effects of Small Group Tutorials in Problem-Based Learning? Adv. Health Sci. Educ. 2006, 11, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Hussen Seid, M.; Assefa, Y.; Legas Muhammed, B.; Moges, B.T.; Tsehay Birhanu, E.; Fentaw, Y.; Ahmed Tilwani, S.; Reshid Ahmed, M. Students’ amd Teachers’ Perception and Practice towards Laboratory Work in Chemistry Teaching-Learning: Evidence from Secondary Schools in North Wollo Zone, Ethiopia. Educ. Res. Int. 2022, 2022, 7254105. [Google Scholar] [CrossRef]
- García-Ponce, Á.L.; Martínez-Poveda, B.; Blanco-López, Á.; Medina, M.Á.; Quesada, A.R. Not all has been said about glucose oxidase/peroxidase: New pedagogical uses for a classical and robust undergraduate laboratory experiment. Biochem. Mol. Biol. Educ. 2019, 47, 341–347. [Google Scholar] [CrossRef]
- Hartman, R.; Helmy, R.; Al-Sayah, M.; Welch, C.J. Analytical Method Volume Intensity (AMVI): A green chemistry metric for HPLC methodology in the pharmaceutical industry. Green Chem. 2011, 13, 934–939. [Google Scholar] [CrossRef]
- Available online: https://reds-sdsn.es/wp-content/uploads/2017/02/Guia-ODS-Universidades-1800301-WEB.pdf (accessed on 22 April 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).