Abstract
This study examines an example of the alternative conceptions and conceptual errors of students at the higher education level in a scientific context. It begins by introducing the significance and characteristics of preconceptions and alternative ideas or alternative conceptions, highlighting their impact on students’ misconceptions. Using the dissolution of a gas (oxygen) in a liquid (water) as the case study, and based on the answers to a questionnaire, this work analyzes the responses by university students which, in most cases, lack scientific rigor. The questionnaire used in this study has been designed in such a way that students provide three types of answers: the first is a yes/no/do not know question; the second is a short answer question to briefly explain the previous answer; and the third is a drawing answer question in which students are required to interpret the phenomenon at the molecular level by drawing a picture. Surprisingly, minimal differences were observed between the university students enrolled in Bachelor’s degree programs (Chemical Engineering or Industrial Engineering) and Master’s degree programs (Master’s Degree in Teacher Training), over the five years (from 2018/19 to 2022/23) covered by this study. Only about 11% of the students provided acceptable reasoning, while the rest demonstrated alternative conceptions. These alternative conceptions encompassed concepts such as the formation of oxygenated water instead of the dissolution, the belief that gases do not dissolve in liquids, confusion about atomic and molecular levels, difficulties in interpreting scientific language, and reliance on simplistic and naïve ideas, among others. After the teacher’s review, the questionnaire and students’ answers were discussed in class in order to detect and correct errors. Approximately one month later, the students were asked to repeat the same questionnaire, when it was observed that the number of correct answers, showing adequate reasoning, had increased to 75%. The results of this study, using a very simple questionnaire that only takes 10 min, could be valuable for guiding teachers to question and transform their pedagogical content knowledge in order to improve the transmission of scientific content, which may involve difficulties that, a priori, were not expected in university students.
1. Introduction
Although many studies have been conducted on student misconceptions, it is still a very active area of work in education, especially in scientific disciplines such as chemistry [1,2,3,4,5,6]. The role that preconceptions and alternative ideas or conceptions, which students construct with respect to different phenomena, play in learning scientific concepts and in generating misconceptions is widely recognized. They are an obstacle to learning new concepts because, in addition to forming an explanatory and predictive framework, they are deeply rooted [7].
According to Furió et al. [8], a conceptual error is a “wrong answer” affecting a given scientific concept, which is due to the existence of an alternative conception (representation of the concept different from the one accepted within the theoretical body of scientific knowledge in use) in a person’s mind. Furthermore, they suggest that alternative conceptions should not be considered an impediment to learning, but as the necessary starting point for students to construct new knowledge. In particular, Furió and Furió [9] pointed out that when students are asked what an ‘external reality’ means to them, a ‘naïve’ realistic view of both the direct image coming from the senses (such as “gases cannot be seen or touched”) and the microscopic level of description of matter usually emerges from their responses. Another aspect of students’ thinking, according to these authors, derives from their integration in the social and cultural environment, particularly transmitted through language. They also pointed out that a main aspect of students’ alternative conceptions are due to impulsivity or a ‘lack of methodological reflection’, following not very rigorous criteria. Thus, they distinguished several alternative conceptions that originate from different ways of reasoning, such as: the ‘methodology of superficiality or common sense’, characterized by quickly drawing conclusions and based on only a few qualitative observations (usually poorly substantiated) or on evidence assumed to be true in everyday life; ‘simple causalism’, inspired by lax criteria, such as the similarity between cause and effect; ‘functional fixation’, which is rote learning of concepts and rules that prevent both reflection and creative thinking; ‘functional reduction’, considering only a single variable to analyze a dependent variable or effect that depends on several variables or causes; and ‘linear sequence rationing’, or step by step reasoning when a holistic analysis is actually required.
Knowing the students’ alternative ideas is important for teachers to take into account when constructing curricular materials and developing appropriate strategies in educational practice. For example, Pnevmatikos et al. [10] recently pointed out that academics of higher education institutions should consider students’ pre-existing ideas before designing and implementing instructional interventions in order to facilitate the acquisition of ideas for critical thinking. Thus, didactic perspectives should create the best conditions for students to modify their previously elaborated conceptions and generate others more congruent with scientific concepts. Many authors emphasize that the study of students’ ideas is an important tool to help teachers question and transform their own pedagogical content knowledge (PCK), an essential aspect in approaching the teaching and learning process [11,12].
From a constructivist perspective, learners, active and creative, construct and reconstruct conceptions of the world around them. Through the teaching–learning process, these conceptions interact and intervene with the scientific vision provided by the teacher, with additional new information. As a result of this interaction, new meanings emerge, and scientific concepts are constructed. For this reason, the analysis of students’ alternative ideas has been one of the lines of research most addressed in the area of Science Didactics. Moreover, as Martín del Pozo et al. [12] indicate, the predisposition to consider students’ ideas helps to keep teachers away from the mere transmissive model of teaching.
Usually, studies of alternative conceptions have referred to primary and secondary education levels, as well as to the corresponding teacher trainees. One of the most studied topics in science education is related to students’ ideas of the atom, and relevant articles have explored these ideas in secondary students [13,14,15]. In fact, the conceptual errors of teachers coincide, to a large extent, with those of their students. As an example, the research conducted by Hämälä-Braskén et al. [16] indicates that 40% to 80% of primary school teachers in Finland have the same types of conceptual errors as their students (aged 5 to 12 years). Moreover, initiatives like the one presented by Dolu et al. [17], which involves providing university-level students who are aspiring teachers with a course on science misconceptions, are highly recommended.
On the other hand, Zoller [18] already noticed in the 1990s that, at university level, freshman chemistry is believed to be, probably, the most problematic traditional science discipline due to learning difficulties, misconceptions, and misunderstanding. And the Royal Society of Chemistry published the book “Beyond Appearances: Students’ misconceptions about basic chemical ideas” [19] in order to collect the research on students’ misconceptions in chemistry, describing and discussing the most significant misconceptions, together with indications about their origins and inclusion of activities.
In this vein, to share what works and to improve the teaching and learning of chemistry, Lamichhane et al. [20] identified undergraduate students’ misconceptions about energy diagrams, which are very relevant when introduce students to thermodynamics and kinetics of reactions. Another example, related to the understanding of the molecular structure at university level, is due to Karonen et al. [21], who studied heuristic hindering for the development of understanding of Lewis structures.
More related to the research of this work are the students’ alternative conceptions of the physical and chemical properties of water, which have been extensively studied, but almost always in reference to students in pre-university educational stages [22,23,24]. Since water is deeply ingrained in social culture and because of the experiences related to water, both in daily life and in schools, teaching about it becomes complex [23]. Thus, Giraldo Toro et al. [24], when analyzing students’ ideas about the physical and chemical properties of water during the secondary education stage (known as the “ESO cycle” in Spain, ages 13 to 16), reported that most of the students were aware of the influence of temperature on the dissolution processes; however, they stated that solubility in water increased with temperature for both solid and gaseous solutes.
As an example of the complexity inherent to the understanding of phenomena involving water, even for university students, the study by Smith and Villarreal is remarkable [25]. This research examined the concept of particle position in reversible physical changes, such as changes of state (the melting–freezing process of water) or dissolution of solids in an aqueous medium, with first-year university students. Despite visualizing the process at the particle level and discussing it, misconceptions persisted.
Although much information is available on alternative conceptions and misconceptions related to physical changes such as those mentioned above (the melting–freezing and dissolution of solids in liquids), the present study aims to contribute to the knowledge of higher education level students’ understanding of a specific phenomenon: the dissolution of oxygen (a gas) in water (a liquid), something that has not been so extensively studied. The idea behind this research was to examine this fundamental topic because, without a solid understanding and grasp of the subject, teachers may struggle to effectively convey important concepts (such as just the solubility of gases in water) to their students. As Taber pointed out: “When teaching a complex new topic, the teacher needs to undertake a careful conceptual analysis of the material, to work out how the different parts of the topic link together, and to determine a logical sequence for introducing material in terms of which concepts are prerequisites for others” [4].
As most of the studies on alternative conceptions focus on pre-college level students, it becomes interesting to investigate whether differences are observed when higher education students with a scientific background participate in the study. Hence, our main objective was to identify the alternative conceptions of students concerning the essential concepts necessary for understanding this phenomenon, specifically its interpretation at the atomic–molecular level. The participants in this study were either first year Bachelor’s degree students (majoring in Chemical Engineering or Industrial Engineering) or Master’s degree students (pursuing Teacher Training). At the moment, a comparison of these results with similar findings at the secondary level has not been possible since no relevant information on this specific topic was found in the existing literature.
The analysis of these conceptions at the university level constitutes a research area initiated by one of the authors [26,27] several years ago. The purpose is not merely to compile ideas for an ‘anthology of nonsense’, but to acknowledge that students’ ideas represent alternative knowledge that they often rely on throughout their lives. As they engage with new information in the educational process, their ideas can evolve and adapt. Therefore, exploring alternative student conceptions remains a priority in research, alongside the points discussed in the preceding paragraphs.
2. Methodology of the Study
2.1. Participants
The study was conducted over a span of five academic years, from 2018/19 to 2022/23, involving two distinct groups of students. The first group comprises first-year students pursuing Bachelor’s degrees in Industrial Engineering and Chemical Engineering (ranging from 17 to 18). These undergraduate students were required to take chemistry as a compulsory subject during the first semester and had a scientific background, having studied physics, chemistry and math during the pre-college stage. The second group consisted of students enrolled in the Master’s degree in Teacher Training. To become high school teachers in Spain, individuals must hold a Bachelor’s degree and complete a one-year Master’s degree program. In this case, the students in the study possessed Bachelor’s degrees in science or engineering fields, and the Master’s in Teacher Training equipped them with the necessary knowledge and tools to become physics and chemistry teachers at secondary schools. As part of their Master’s studies, these future teachers had to undergo two months of supervised internship at an educational center and present a final Master’s degree project.
2.2. Procedure
The research was organized as follows. After a week of discussing aspects related to the dissolution of gases in liquids, specifically, water, the students were given a paper questionnaire to complete. During the class sessions, various scenarios were presented to the students to help them grasp the concept. For instance, they were informed that commercial ‘ammonia’ used for cleaning is, in reality, a solution of ammonia (a gas) in water. Other examples included the impact of temperature on solubility, such as the respiration of fish and aquatic plants, or the ‘thermal pollution’ of rivers. The formation of bubbles when heating water before boiling and the influence of gas pressure on the amount of dissolved gas in a liquid were also covered. To quantitatively explain the latter case, Henry’s law was introduced, accompanied by everyday instances like ‘divers’ sickness’ due to the rapid ascent from underwater (decompression sickness) or the formation and release of carbon dioxide bubbles when opening carbonated beverages.
Additionally, the mechanism of dissolution of sodium chloride in water was detailed, complete with illustrations, to provide the students with a concrete example and help them understand the process of forming a well-known solution. Consequently, it was assumed that the students would be able to apply these fundamental concepts to interpret the microscopic processes involved in the dissolution of gases in water.
The objective of the research was to assesses whether the students grasped the concept of gas dissolution in liquid and, more importantly, to gauge their understanding and interpretation of this phenomenon at the atomic–molecular level. To achieve this, a questionnaire was designed as the primary tool for conducting the educational research. The questionnaire included the following questions:
- Does oxygen dissolve in water? (Possible answers: ‘yes’, ‘no’, or ‘do not know’).
- Briefly justify your answer by providing an example.
- Illustrate your answer to the initial question by providing a drawing that represents the phenomenon of dissolution or non-dissolution at the atomic-molecular level. If your answer to question 1 was ‘do not know’, draw both possibilities, the formation of dissolution and non-dissolution. A box has been included in the questionnaire for this purpose.
In total, 354 first-year students pursuing degrees in Industrial and Chemical Engineering, along with 118 students enrolled in the Master’s Degree in Teacher Training, participated in the questionnaire. The data were collected over the span of five years, and each participant was given 10 minutes to complete the questionnaire.
After collecting the questionnaires from the students and analyzing them, the professor returned the questionnaires to the students, along with evaluations and corrections. The general errors that were identified and the correct answers were thoroughly explained and discussed in the classroom as part of a group evaluation.
Approximately one month later, the same questionnaire was administered again to the students. This second assessment aimed to determine if there were any improvements or changes in the students’ understanding of the topic after the class discussions and corrections were provided.
3. Analysis and Discussion of Results
3.1. Students’ Answers and Justifications about the Fact That Oxygen Dissolves in Water
Based on the analysis of the answers, it can be concluded that there were no remarkable differences between students from different academic studies. Surprisingly, there were no notable distinctions between students pursuing Bachelor’s Degrees and those in the Master’s Degree in Teacher Training. Therefore, the results are presented as a whole. Initially, it was anticipated that Master’s students, already holding degrees in science or engineering and assumed to have a teaching vocation, would provide more plausible answers. However, it appears that this topic (the dissolution of gases in liquids) is not frequently covered in the physics and chemistry curricula, which might explain why many students have never considered it before. Similarly, there seems to be common confusion between evaporation and boiling.
The purpose of completing the questionnaire is to provide students with a formative learning experience. By engaging in this activity, students are encouraged to reflect on a phenomenon at the atomic–molecular level—something abstract yet essential for a proper understanding of the physicochemical process with important implications. The questionnaire serves as a valuable tool for the teacher. It enables the teacher to assess, among other aspects, the students’ grasp of abstraction concerning the structure of matter. This information is of great importance for the teacher’s understanding of the students’ comprehension and knowledge level in this subject.
Table 1 presents a summary of the students’ responses to the first question. Remarkably, even after one week of addressing the topic in class, only approximately 45% of the students provided the correct answer, acknowledging that oxygen dissolves, at least to some extent, in water. This finding highlights the need for further exploration and clarification of the concept to ensure better comprehension among the students.

Table 1.
Distribution of answers given by the 472 university students, including both Industrial and Chemical Engineering Degree students and Master’s Degree in Teacher Training students, to question 1, “Does oxygen dissolve in water?”.
Among the students who affirmed that oxygen does dissolve in water (45%)—some correctly specified that it dissolves, “but only in small quantities”—only approximately 25% of them provide logical reasoning in question 2, referring to answers such as: “fish can breathe thanks to this dissolved oxygen” or “fish can extract it in order to live”. It seems that, among the examples mentioned in class, this one in particular captured their attention and was well understood. Among the other 75% (of the original 45%), who also answered correctly that oxygen dissolves in water, their explanations were often extravagant. Some students’ mistaken explanations included the following examples: “because oxygenated water [sic.] is formed”; “in oxygenated water, the O2 molecules break their bond to join another O of H2O and form H2O2 [sic. ]”; “O2 is a component of H2O (otherwise fish in the sea could not live) [sic.]”; “oxygen is a gas (O2) and water already has O2 and H2 molecules bonded together [sic.]”; and “because O2 can form hydrogen bonds with H2O [sic.]”. Only a few students attempt to explain it using scientific terms, but with unclear explanations that lacked depth, such as “the O2 double bond zone repels the ends of the water molecule [sic.]”. Furthermore, some university students mistakenly explained the physical change in oxygen dissolving in water as a chemical reaction, assuming the formation of H2O2. Similar misconceptions have been previously observed among secondary students, where physical changes like boiling are misunderstood as chemical reactions, imagining that water splits into its component elements [19]. These misconceptions demonstrate that misunderstandings about water are deeply ingrained and persist across different education levels.
Interestingly, among the students who claimed that oxygen does not dissolve in water (48%), some applied the previous reasoning about the formation of hydrogen peroxide, but now to explain the opposite effect: “it does not dissolve, because it forms hydrogen peroxide [sic.]” (some students mention H2O2 using the alternative nomenclature, hydrogen peroxide). Furthermore, other alternative conceptions were evident among these students, when they made statements like: “oxygen is part of water itself [sic.]”; “water is already formed by oxygen [sic.]”; “because the exchange between its elements does not take place [sic.]”; “O2 is present, but does not dissolve [sic.]”; “in water salts dissolve, but gases do not because they ascend [sic.]”; “dissolution does not take place, but new bonds between atoms [sic.]”; “because oxygen is not polar [sic.]”; “because oxygen is a nonpolar molecule and water is polar [sic.]”; “as the solute is nonpolar, it is only soluble in polar solvents [sic.]”; “when breathing under water, the O2 particles that we emit, go to the surface [sic.]”; “if we blow through a straw, the CO2 expelled goes back outside, it does not dissolve [sic.]”; “the O2 that is blown into water, comes out [sic.]”; “air bubbles would form if you shake a bottle of water [sic.]”; “the O2 that comes out of a cylinder does not dissolve, but rises to the surface [sic.]”; “when you shake a bottle of water, you observe that small oxygen molecules rise trying to escape, which, in solution, would not happen [sic.]”; “O is added and forms a new molecule [sic.]”; “oxygen is a gas molecule that, for example, in a glass, tends to go to the surface and separate from those of water; it does not break its bonds to associate [sic.]”; “although water is the universal solvent, oxygen does not dissolve because it tends to form molecules, joining for example to hydrogen [sic.]”; and “water is unable to break O2 molecules [sic.]”, etc.
Finally, students who selected “I do not know” or left question 1 unanswered often skipped question 2 as well. Among the few who did respond to question 2, some reiterated the same (erroneous) reasoning mentioned earlier. Others expressed their doubts explicitly, sharing ideas like: “I don’t know, because when diving in the sea or in a swimming pool, bubbles come out and go to the surface, but fish do capture O2 through their gills, sponges obtain oxygen by filtering water, etc. These are diverse situations that make me doubt [sic.]”.
Based on the data provided, it can be concluded that only approximately 11% (25% of the initial 45%) of the students were capable of providing appropriate reasoning that oxygen does dissolve in water. In contrast, the vast majority of students, regardless of whether they answered affirmatively or negatively to question number 1, did not provide convincing explanations.
3.2. Students’ Alternative and Erroneous Conceptions about the Process of Dissolution of Oxygen in Water
From both the examples given in the previous paragraphs and the set of analyzed answers, it can be inferred that the students’ alternative or directly erroneous conceptions about the discussed phenomenon fall into the following categories:
- -
- The formation of hydrogen peroxide (H2O2) instead of oxygen dissolving in water: As mentioned earlier, some students incorrectly explained the solubility of oxygen in water by proposing the formation of H2O2. They believed there is a chemical transformation (chemical change) rather than a dissolution phenomenon (physical change). This misconception is quite common and was provided by almost 15% of students. Some even attempted to describe the reaction they think occurs, with answers such as: “oxygen does not dissolve in water, since when this gas is introduced, it would be formed, according to the reaction: H2O(l) + ½ O2(g) ⭢ H2O2(l) [sic.]”. Indeed, in this case, the use of the chemical nomenclature could contribute to the misunderstanding of the phenomenon. The term ‘oxygenated water’ can be interpreted in two ways: as water with oxygen gas dissolved in it or as the chemical compound with that name. This ambiguity could lead to confusion among students regarding whether the process involves a dissolution or a chemical reaction. At the PCK level, the teacher can discuss this alternative idea with the students, helping them differentiate between the dissolution process and the chemical reaction, in a general sense.
- -
- Approximately 13% of the students held the misconception that gases, in general, do not dissolve in liquids like water. This misconception might have been reinforced since primary education, where examples of dissolving solids (like common salt, sugar) in water are commonly considered, followed by some cases of liquids (such as ethanol) in water. However, dissolutions of gases in water are rarely presented, despite their significant importance.
- -
- Some students interpreted the phenomenon incorrectly, confusing it with the self-dissociation equilibrium of water. This confusion may have arisen from previous courses that introduced acid-base equilibria, leading them to mistakenly think the question is related to that concept.
- -
- Many students confused the atomic and molecular levels. They failed to distinguish between the atomic oxygen that is part of the H2O molecule, and the two oxygen atoms that are joined by a double bond to form the O2 molecule. Some students even mistakenly believed that dissolved oxygen reacts chemically with hydrogen (without specifying its origin) to produce water. This confusion reflects a very limited understanding of chemical reactivity and also indicates that some students struggle to differentiate between examples of reactions found in educational texts and their practical occurrence.
- -
- Students had difficulty in understanding scientific vocabulary. Apart from confusion in terminology related to concepts like atom and molecule, or substance, element, and compound, some students referred to oxygen as a ‘compound’ rather than as an element. Additionally, there was confusion between molecules (like O2) and gas ‘bubbles’. This confusion may arise from the representation of molecules in textbooks as circular shapes with a large size, aimed at adding interpretation. However, these students failed to grasp that these visual interpretations are simply meant to help in understanding the phenomenon and are not an accurate depiction of reality.
- -
- The students’ answers revealed erroneous use of scientific language. Two representative examples are as follows: “although water is the universal solvent, there are elements such as oxygen that cannot be dissolved because it is electronegative and small in size and tends to give up electrons; it tends to form molecules by joining, for example, hydrogen [sic.]” and “the water molecule cannot accept more oxygen as it has full electron shells, the oxygen is left floating around, O2 has a double bond, difficult to break”. These examples indicate that some students tried to showcase their mastery of the topic studied. They used terms such as electronegativity, electron shells, etc., as a way to demonstrate their understanding, even if their explanations were inaccurate. Many students seemed to focus on using the terms they think the teacher wants to hear, rather than reflecting on the question. In addition to terminological confusion, many students had a limited vocabulary, not only at a scientific level but also in general, which made it challenging for them to express their reasoning effectively.
- -
- Some students held simplistic or naïve ideas, for example, they might have believed that if they blow a stream of oxygen into water and bubbles come out, it means that nothing is dissolved in the water. These ideas demonstrate a lack of understanding of the complexities of gas dissolution and can lead to misconceptions about how gases behave in liquid environments.
- -
- Many students struggled with interpreting orders of magnitude. They are more accustomed to providing answers in exercises without fully grasping the significance of the data. For example, when they learn that oxygen is soluble in water at room temperature to the extent of just a few milligrams per liter, they mistakenly conclude that it is not soluble at all. Some students also believed that a substance either dissolves completely or not at all in another, failing to understand that there is a gradient of possible solubilities. In reality, solubility is not an all-or-nothing property; it varies along a continuum. Helping students grasp the concept of varying solubilities is crucial for their accurate understanding of dissolution processes.
Many students’ statements contained multiple misinterpretations. For instance, one student stated: “The O2 molecule is linear and can be dissolved because in mineral water, apart from H2O, there are other substances containing oxygen [sic.]”. Apart from showing an error in the interpretation of the geometry (diatomic molecules have no bond angle and, therefore, their geometry cannot be discussed), the student became confused thinking about mineral water and so reasoned that, as some components (perhaps thinking on silica or bicarbonate ions, which are usually present) contains oxygen (as SiO2 and HCO3− in the mentioned examples), perhaps it is the result of the dissolution of this element. Another student pointed out that oxygen does not dissolve in water because “oxygen is covalently bonded and forms a noble gas structure by sharing electrons and everything has to be in the same state of aggregation [sic.].” These examples suggest that some students attempted to include various concepts they encountered in class, even if they are not directly applicable or relevant to the given question. This approach may lead to further confusion and incorrect explanations. To address these issues, it is essential to focus on helping students develop a deep and coherent understanding of the relevant concepts, rather than relying on memorization or picking out unrelated pieces of information.
The students’ answers seem to align with the findings of Furió and Furió [9], as previously mentioned in the introduction. Furió and Furió conducted a review of the conceptual and epistemological difficulties that students face concerning fundamental concepts such as the corpuscular nature of matter, substances, and chemical compounds. Overcoming these difficulties is crucial in order for students to properly interpret chemical processes. In the context of the current study, it appears that some students doubted the materiality of gas dissolution (e.g., O2 in water) due to its limited perceptibility. This doubt may arise from the difficulty of directly observing such processes with the naked eye. Moreover, many of the erroneous generalizations exhibited by the students appeared to stem from their application of a naïve realistic view of the natural world to the microscopic level of matter. In the words of the aforementioned authors, students “do not understand that there are different levels of description of matter in close relation: the macroscopic level of substances with their properties and changes and, on the other hand, the microscopic level of those same substances that chemistry models on the basis of atoms, ions or molecules”.
3.3. Students’ Interpretation of the Dissolution of Oxygen in Water at the Molecular Level
As already mentioned, to complete the analysis of the interpretation of the students’ thinking on the topic of this work, and to facilitate their reasoning, they were invited to illustrate the phenomenon, at the atomic–molecular level, by means of a drawing at the end of the questionnaire (question 3). Even, as mentioned above, if they had not decided on a specific answer to the first question, they were asked to draw the two possibilities (yes: it dissolves, or no: it does not dissolve).
Around 5% of the students did not provide any drawings or left the box blank, or simply offered explanations like “it is difficult for me to interpret it”.
Students are usually surprised when the teacher asks them to draw a picture, as they are more accustomed to traditional methods of problem-solving and answering written questions. It is worth mentioning that three of the authors of this work (G.P., V.A.M., and C.M.C.A.) frequently use this technique with their students. They employ drawing to complement their ideas and enhance their abstract thinking skills.
The students’ drawings reflected their previous written expressions and discussions, but they also offered additional nuances and interpretations. These drawings provide valuable insights into how the students perceived and understood the phenomenon.
Figure 1 displays examples of the students’ graphical interpretations of the dissolution process of oxygen at the molecular–atomic level. They demonstrate a good understanding of intermolecular forces, including induced dipole–dipole and dispersion forces. However, a common minor error was observed is the assuming of the same orientation of water molecules around each oxygen molecule.
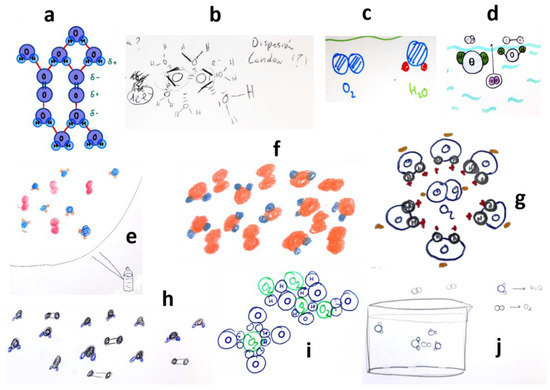
Figure 1.
Students’ drawings showing the dissolution of oxygen in water, at the molecular level, in a quite acceptable way (see text). Each subfigure (a–j) corresponds to a different student, as explained in the text.
In fact, it should be considered that, if the electron cloud of the O2 molecule is attracted by the hydrogen atom of a water molecule, a partially negative charge distribution forms on one of the atoms of oxygen and, correspondingly, a partial positive charge appears on the other end. This positive charge attracts the oxygen atom of a water molecule, as depicted in Figure 1a. While students generally understood that oxygen’s solubility in water is low (a few mg/L), the relative proportions of O2 and H2O molecules they drew are not critical since the focus is on reasoning about the phenomenon at the atomic–molecular level.
Figure 2 displays drawings made by students who mistakenly represented the composition of water as O2H instead of H2O. Some students also thought that “hydrogen” is dissolved in molecular or atomic form.
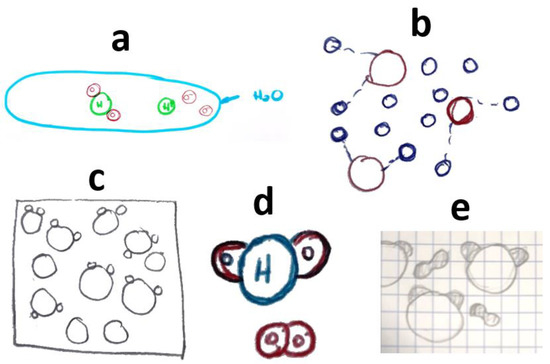
Figure 2.
Students’ drawings showing (erroneously) that water is made up of O2H molecules or that what is dissolved is “hydrogen” in molecular or atomic form. Each subfigure (a–e) corresponds to a different student, as explained in the text.
As mentioned, many students misinterpreted the phenomenon, relating it to the self-dissociation equilibrium of water (Figure 3), indicating impulsivity and a lack of methodological reflection. Others (Figure 4), suggested implausible molecular structures and that they believed that the atoms in substances can be combined in almost any way.
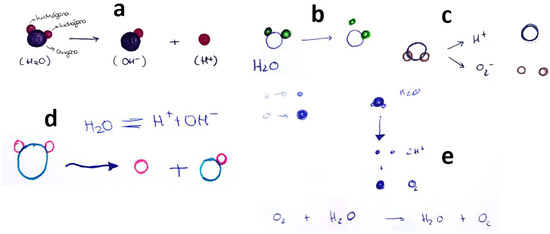
Figure 3.
Students’ drawings showing (erroneously) that the phenomenon of oxygen dissolution in water refers to the self-dissociation equilibrium of H2O. Each subfigure (a–e) corresponds to a different student, as explained in the text.
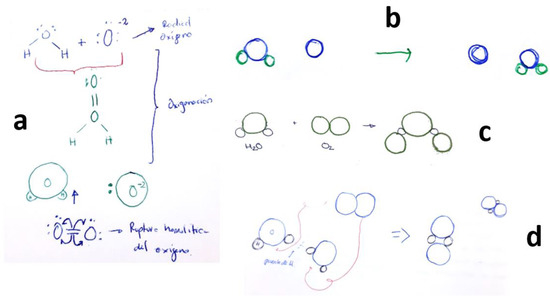
Figure 4.
Students’ drawings showing the formation of extravagant and implausible molecular structures. Each subfigure (a–d) corresponds to a different student, as explained in the text.
Indeed, as previously mentioned, the primary source of errors encountered was the misconception of students considering the formation of H2O2. This misunderstanding is depicted in Figure 5, where various structures for the hydrogen peroxide molecule are illustrated, not all of them being correct representations.
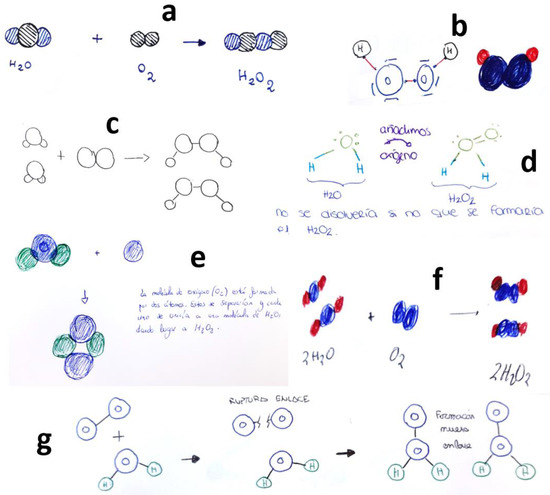
Figure 5.
Students’ drawings showing the (erroneous) concept of H2O2 formation when O2 dissolves in H2O. Each subfigure (a–g) corresponds to a different student, as explained in the text.
In Figure 6, we can observe a ‘naïve’ realistic view in some of the students’ answers. They expressed misconceptions, like believing that oxygen molecules ‘escape’ and rise in the aqueous solution (Figure 6e), or that water molecules evaporate (Figure 6c). In certain cases, when the meaning was unclear, the teacher asked students to clarify their drawings. For instance, in Figure 6f, the teacher initially thought it represented an atomic orbital p, but the student explained it was an O2 molecule with “two wings”, emphasizing the idea of oxygen ascending and escaping from the water. Figure 6d provides a simplistic and exaggerated diagram of the phenomenon, only considering it at the macroscopic level.
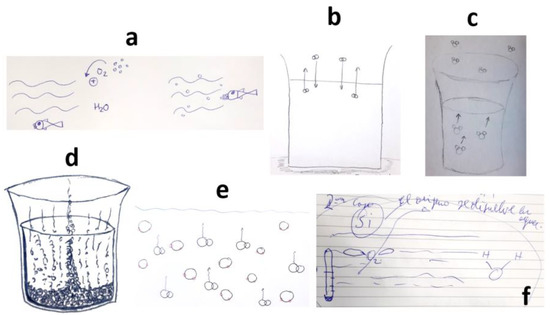
Figure 6.
Students’ drawings showing naïve explanations of the dissolution of O2 in H2O. Each subfigure (a–f) corresponds to a different student, as explained in the text.
Figure 7
illustrates some student explanations that displayed an almost complete lack of
methodological reflection on the subject. These explanations lacked a
systematic approach or thoughtful consideration.
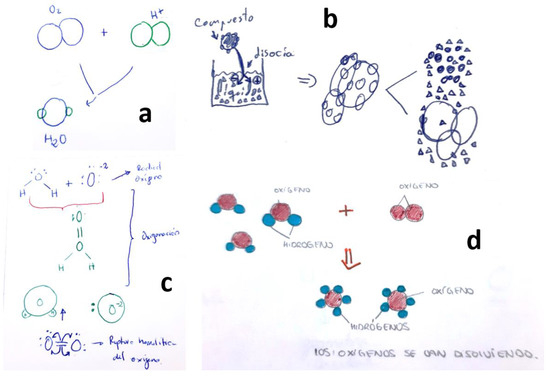
Figure 7.
Students’ drawings explaining the dissolution of O2 in H2O with a great lack of methodological reflection. Each subfigure (a–d) corresponds to a different student, as explained in the text.
Finally, Figure 8 presents examples of what was previously referred to as ‘functional fixation’ in the introduction. Some students memorized certain concepts and believed that these concepts explain everything without critical thinking or creativity. In Figure 8a and 8b, there are apparent representations of supposed atomic orbitals, which lack practical significance. Figure 8c refers to the example of the dissolution process of NaCl instead of O2, possibly because the mechanism of dissolving a solute in a solvent, particularly NaCl in water, is a common example frequently explained in secondary school.
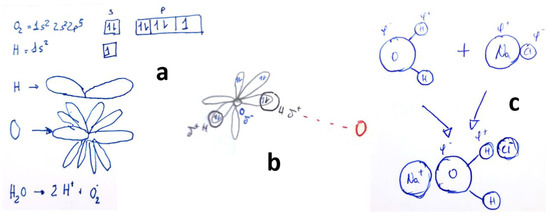
Figure 8.
Students’ drawings used to explain by ‘functional fixation’ (see text) the process of dissolution of O2 in H2O. Each subfigure (a–c) corresponds to a different student, as explained in the text.
As mentioned earlier, there were no remarkable differences found between the Industrial and Chemical Engineering Degree students and those pursuing the Master’s Degree in Teacher Training. However, the main distinction lay in Master’s students occasionally incorporating more didactic elements, not always correctly, as in the cases of Figure 1e,j and Figure 6a,c,d,f.
3.4. Results of the Improvement Strategies in the Didactics on the Students’ Understanding of the Subject
Students need to exert effort to interpret physicochemical processes like the dissolution of a gas in water at the molecular level. Instead of merely watching videos or observing drawings in slides, books or notes, active involvement in representing the process is essential.
After analyzing the students’ questionnaires, the teacher returned them with individual corrections. In the class session, both the general errors made and the correct answers were discussed through a group evaluation. The objective was to enhance the students’ comprehension of the specific phenomenon, which has practical and everyday interesting applications. Additionally, this exercise aimed to train students in reasoning and interpreting physicochemical phenomena, based on atomic–molecular structures.
The authors conducted a follow-up exercise with their students about one month after the group assessment. The purpose was to evaluate the impact on learning. The results showed that the percentage of students who correctly thought that gaseous oxygen dissolves in water increased from 45% to 90%. Moreover, almost all of them provided good examples to support their reasoning. The percentage of students who demonstrated a proper understanding of the phenomenon rose from 11% to 75%.
Regarding the nature of interactions between oxygen and water molecules, an analysis of the diagrams and drawings revealed that approximately 20% of the students retained their initial alternative conceptions even after a month. Additionally, around 5% showed new misconceptions different from their initial responses. These findings highlight the persistence of fundamental misconceptions and emphasize the importance of carefully analyzing and addressing them to enhance pedagogical content knowledge.
4. Conclusions
The study focused on analyzing a fundamental topic, the dissolution of a gas (oxygen) in a liquid (water), in order to identify university level students’ alternative conceptions related to essential concepts for understanding this phenomenon. The effectiveness of the questionnaire used here in addressing misconceptions makes it a potential model template for developing similar questionnaires in chemistry. The three-question test, including a yes/no/do not know question, a short-answer question, and a drawing answer question, efficiently detected alternative conceptions. Upon repeating the questionnaire, significant improvements were observed, indicating the elimination of misconceptions in a high percentage of students. The correct answers to question 1 (“Does oxygen dissolve in water?”) doubled, from 45% to 90%. For question 2, explaining the phenomenon and providing examples, there was a remarkable improvement from 11% to 75%. The drawing answer question in question 3 was proven to promote student reflection and understanding at the molecular level, while also enabling teachers to identify students’ mistakes and successes more easily.
Investigating students’ interpretation of physicochemical phenomena and their atomic–molecular understanding is crucial for analyzing pedagogical content knowledge and enhancing the teaching–learning process by educators. Initially, the questionnaire was completed after the concepts were presented in class, but the results were disappointing. One possible explanation is that many students believed they were already familiar with these concepts from their pre-college education due to their familiarity. Only after the students reviewed their tests, corrected by the teacher, and answers and errors were discussed in class, was the improvement evident, as could be seen in the results after repeating the test.
Although the participants in this study were all university level students (Bachelor’s and Master’s degrees), some of them exhibited conceptual errors commonly seen in high school students. These errors included confusion between physical and chemical changes, as well as misconceptions about the nature of matter, atoms, and molecules, which gives an idea of how deeply rooted some of these misconceptions are. The study revealed that some students mistakenly confused the physical change in the dissolution of a gas (oxygen) in a liquid (water) with a chemical reaction, leading to the formation of hydrogen peroxide.
Despite water and oxygen being common in our daily lives, teaching these concepts remains challenging. While many students recognized the importance of dissolved oxygen in water for fish respiration, showing them the role of dissolved O2 in electrochemical reactions (essential in industry) could further reinforce the concept. For instance, demonstrating that the oxidation of iron (typical corrosion process) does not occur when a piece of iron is immersed in water previously treated with N2 bubbling to eliminate dissolved oxygen can be a useful way to convince students of its importance.
Repeating specific examples like the dissolution of NaCl in water throughout secondary and high school courses can result in misconceptions, such as the belief that gases do not dissolve in liquids or that solubility always increases with temperature.
The similarity in results between undergraduate and graduate students in our study suggests that the topic of gas dissolution in liquids, despite its significant implications at the biological and industrial levels, has not been adequately addressed throughout their previous studies, including university-level education for Master’s students.
The results of this study, along with many other cases in the literature, highlight the importance of addressing students’ preconceptions and alternative ideas in teacher training courses. It is crucial to understand how teachers teach, in order to share effective methods and develop improvements in our teaching practices.
These conclusions underscore the potential impact on classroom teaching and future research of these topics. By addressing students’ misconceptions and enhancing pedagogical approaches, educators can create a more effective learning environment and promote a deeper understanding of the subject matter.
Some students may not show improvement in their understanding of the phenomenon, indicating the persistence of misconceptions and the challenges in changing them. However, after discussing the results and the topic in class for a month, a significant percentage of students demonstrated adequate reasoning abilities. This highlights the positive impact of classroom discussions and active engagement in enhancing students’ comprehension.
Based on the achieved results and accumulated experience, we highly recommend implementing these strategies for teachers at different educational levels. With minimal time investment (about 10 minutes of class time), these strategies offer valuable insights into students’ thinking, leading to improved knowledge and enhancing the overall teaching–learning process.
Author Contributions
Conceptualization, G.P., V.A.M. and C.M.C.-A.; methodology, G.P.; software, G.P., I.L.-H. and C.M.C.-A.; validation, G.P., V.A.M., I.L.-H. and C.M.C.-A.; formal analysis, G.P. and V.A.M.; investigation, G.P., V.A.M. and C.M.C.-A.; resources, G.P., I.L.-H. and V.A.M.; data curation, G.P.; writing—original draft preparation, G.P.; writing—review and editing, V.A.M., I.L.-H. and C.M.C.-A.; visualization, G.P.; supervision, G.P.; project administration, G.P.; funding acquisition, G.P., V.A.M. and C.M.C.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Community of Madrid under the Pluriannual Agreement with the Technical University of Madrid (UPM), within the line of action “Excellence Program for University Teaching Staff”, in the framework of the V Regional Plan for Scientific Research and Technological Innovation, PRICIT.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Taber, K. Chemical Misconceptions: Prevention, Diagnosis and Cure; Royal Society of Chemistry: Lundon, UK, 2002; Volume 1. [Google Scholar]
- Taber, K.; Pack, M.J. Chemical Misconceptions: Prevention, Diagnosis and Cure; Classroom Resources; Royal Society of Chemistry: London, UK, 2002; Volume 2. [Google Scholar]
- Barke, H.D.; Hazari, A.; Yitbarek, S. Misconceptions in Chemistry: Addressing Perceptions in Chemical Education; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Taber, K.S. Challenging misconceptions in the chemistry classroom: Resources to support teachers. Educ. Química 2009, 4, 13–20. Available online: https://raco.cat/index.php/EduQ/article/view/199238 (accessed on 11 June 2023).
- Soeharto, S.; Csapó, B. Evaluating item difficulty patterns for assessing student misconceptions in science across physics, chemistry, and biology concepts. Heliyon 2021, 7, E08352. [Google Scholar] [CrossRef]
- Üce, M.; Ceyhan, İ. Misconception in chemistry education and practices to eliminate them: Literature analysis. J. Educ. Train. Stud. 2019, 7, 202–208. [Google Scholar] [CrossRef]
- Ramos, R.; Praia, J.; Marqués, L.; Gama Pereira, L. Ideas alternativas sobre el ciclo litológico en alumnos portugueses de enseñanza secundaria. Enseñanza De Las Cienc. De La Tierra 2001, 93, 252–260. [Google Scholar]
- Furió, C.; Solbes, J.; Carrascosa, J. Las ideas alternativas sobre conceptos científicos: Tres décadas de investigación. Alambique 2006, 48, 64–77. [Google Scholar]
- Furió, C.; Furió, C. Dificultades conceptuales y epistemológicas en el aprendizaje de los procesos químicos. Educ. Química 2020, 11, 300–308. [Google Scholar] [CrossRef]
- Pnevmatikos, D.; Christodoulou, P.; Georgiadou, T.; Lithoxoidou, A. Undergraduate students’ conceptualization of critical thinking and their ideas for critical thinking acquisition. Educ. Sci. 2023, 13, 416. [Google Scholar] [CrossRef]
- Larkin, D. Misconceptions about “misconceptions”: Preservice secondary Science teachers’ views on the value and role of student ideas. Sci. Teach. Educ. 2012, 96, 927–959. [Google Scholar] [CrossRef]
- Martín del Pozo, R.; Rivero, A.; Azcárate, P. Las concepciones de los futuros maestros sobre la naturaleza, cambio y utilización didáctica de las ideas de los alumnos. Rev. Eureka Enseñ. Divulg. Cienc. 2014, 11, 348–363. [Google Scholar] [CrossRef]
- Adbo, K.; Taber, K.S. Learners’ mental models of the particle nature of matter: A study of 16-year-old Swedish science students. Int. J. Sci. Educ. 2009, 31, 757–786. [Google Scholar] [CrossRef]
- Cokelez, A. Junior High School Students’ ideas about the shape and size of the atom. Res. Sci. Educ. 2012, 42, 673–686. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Markos, A.; Zarkadis, N. Understanding the atom and relevant misconceptions: Students’ profiles in relation to three cognitive variables. Sci Educ. Int. 2016, 27, 464–488. [Google Scholar]
- Härmälä-Braskén, A.S.; Hemmi, K.; Kurtén, B. Misconceptions in chemistry among Finnish prospective primary school teachers–a long-term study. Int. J. Sci. Educ. 2020, 42, 1447–1464. [Google Scholar] [CrossRef]
- Dolu, G.; Ürek, H. Identification and elimination of several misconceptions of university level students regarding the misconceptions in science course. Croat. J. Educ. 2015, 17, 353–382. [Google Scholar]
- Zoller, U. Students’ misunderstandings and misconceptions in freshman chemistry (general and organic). J. Res. Sci. Teach. 1990, 27, 1053–1065. [Google Scholar] [CrossRef]
- Kind, V. Beyond Appearances: Students’ Misconceptions about Basic Chemical Ideas; Royal Society of Chemistry: London, UK, 2004; Available online: https://www.researchgate.net/publication/228799159 (accessed on 11 June 2023).
- Lamichhane, R.; Reck, C.; Maltese, A.V. Undergraduate chemistry students’ misconceptions about reaction coordinate diagrams. Chem. Educ. Res. Pract. 2018, 19, 834–845. [Google Scholar] [CrossRef]
- Karonen, M.; Murtonen, M.; Södervik, I.; Manninen, M.; Salomäki, M. Heuristics hindering the development of understanding of molecular structures in university level Chemistry education: The Lewis structure as an example. Educ. Sci. 2021, 11, 258. [Google Scholar] [CrossRef]
- Fries-Gaither, J. Common Misconceptions about States and Changes of Matter and the Water Cycle 2008. Available online: http://beyondpenguins.ehe.osu.edu/issue/water-ice-and-snow/common-misconceptions-about-states-and-changes-of-matter-and-the-water-cycle (accessed on 11 June 2023).
- Marcén, C.; Cuadrat, J. Argumentos educativos para enseñar-aprender el agua en la enseñanza obligatoria. Ser. Geogr. 2012, 18, 65–75. [Google Scholar]
- Giraldo Toro, M.T.; Cañada Cañada, F.; Dávila Acedo, M.A.; Melo Niño, L.V. Ideas alternativas de los alumnos de secundaria sobre las propiedades físicas y químicas del agua. Tecné Epistem. Y Didaxis TED 2015, 37, 51–70. [Google Scholar] [CrossRef]
- Smith, K.C.; Villarreal, S. Using animations in identifying general chemistry students’ misconceptions and evaluating their knowledge transfer relating to particle position in physical changes. Chem. Educ. Res. Pract. 2015, 16, 273–282. [Google Scholar] [CrossRef]
- Martín Sánchez, M.; Sánchez, M.T.; Sotres, F.; Paz, I.; Pinto, G. Reacción entre el sodio y el agua: Una demostración experimental para ilustrar fenómenos físicos y químicos. Rev. Esp. Fís. 2015, 29, 33–40. [Google Scholar]
- Pinto, G. Interpretación a nivel molecular del enlace de hidrógeno: Dificultades conceptuales en alumnado universitario. Educ. Química 2023, 34, 162–175. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).