Abstract
This paper presents a qualitative cross-level study with a focus on prospective and in-service teachers’ pedagogical content knowledge (PCK) of redox reactions in Germany. The objective was to investigate and analyze the differences in PCK between those in pre-service teacher education and those working as teachers. The sample included four different groups: bachelor’s students, master’s students, graduate teacher trainees, and in-service teachers. Data were collected by an online questionnaire and semi-structured interviews. The online questionnaire was developed based on misconceptions and learning difficulties regarding redox reactions. Sixty-two participants answered the questionnaire and the interviews were carried out with twelve participants. The results revealed that teaching experience makes a difference. Pre-service teachers described quite traditional and content-focused approaches while experienced teachers emphasized the application of the content. Experienced teachers showed a more developed repertoire of instructional strategies. Participants differed also in their knowledge about learners and the curriculum. Concerning assessment, practices were at a quite general pedagogical knowledge level and not domain-specific. Although teacher education in Germany includes several chances for internships, it is suggested that central aspects of teachers’ PCK start to develop and settle only when they begin to work as teachers. To avoid perpetuating traditional practices, investment in continuous professional development is needed.
1. Introduction
Redox reactions are a key concept and a central issue on most chemistry curricula for secondary schools [1]. Phenomena such as photosynthesis in plants, human breathing, reactions where energy is produced like the burning of fossil fuels, and the corrosion of metals are examples of redox reactions. The examples highlight the importance of learning about redox reactions in both their natural and technical applications. Redox reactions have been, however, also identified as one of the most difficult subjects for both learning and teaching [2]. Many studies report students’ difficulties relating to this content [3,4]. Some of these difficulties have been attributed to gaps in teacher training [5]. For many decades, it was believed that to become a good teacher, it was only required to know the specific content well [6]. However, if this were the only feature that characterized a good teacher, all experts in this content would be excellent teachers, which is indeed not observed in practice [6,7]. Although experts have a well-defined body of knowledge, which distinguishes them from others and at the same time enables them to practice their profession, there is more knowledge needed for someone to be considered a “good teacher” [8]. With the aim of systematizing the necessary knowledge base for teaching, Shulman [9] differentiated teachers’ knowledge base into subject matter knowledge (SMK), pedagogical knowledge (PK) about teaching and learning in general, and domain-specific pedagogical knowledge for the subject taught. For the latter, he introduced the concept of pedagogical content knowledge (PCK) as one of the basic components in teacher knowledge for effective teaching.
PCK is thought to help reconstruct a theoretical body of knowledge on content and pedagogical practices with the main objective of making the subject comprehensible to students in certain educational settings [10]. PCK is suggested to link the subjects (where most content knowledge comes from) and the field of education (where most knowledge of teaching comes from) [9]. An essential point of Shulman’s idea was to bring the teachers’ unique province of knowledge, the knowledge to teach a certain content in a certain teaching situation, to a specific group of learners, to the center of the discussion about effective teacher education [9]. In addition, there is some consensus that pedagogical content knowledge develops throughout a teacher’s career along with teaching experience [11]. Therefore, the purpose of this study was to map the differences in teachers’ PCK between different career phases, with respect to the specific topic of redox reactions in teacher education in Germany.
2. Theoretical Framework
The term pedagogical content knowledge (PCK) was presented by Shulman, who defined it as that knowledge “…which goes beyond the knowledge of the subject itself and reaches the dimension of knowledge of the subject for teaching” [9] (p. 9). The author stated that PCK would be composed of “the most useful forms of representation …, the most powerful analogies, illustrations, examples and demonstrations” [9] (p. 9).
After Shulman’s initial proposition of PCK, the number of proposals regarding how to understand the knowledge required for the teaching profession increased [11,12] and different concepts and characteristics were suggested [13,14,15,16,17,18,19]. In 1990, Grossman [15] proposed a model of teacher knowledge that can be considered as an enhancement of Shulman’s ideas by suggesting that teacher knowledge is based on four components: subject matter knowledge; general pedagogical knowledge; knowledge of context; and pedagogical content knowledge. Gess-Newsome, in 1999 [20], considered this the most comprehensive model of teacher knowledge. In addition, the different components of the PCK defined by Grossman [15] are the main definitions used by science education researchers to conceptualize PCK in their studies [21].
Currently, PCK is considered one of the pillars of teachers’ knowledge [19]. It remains an extremely useful idea for teacher training [22] and has been connected to other constructs, such as Fachdidaktik in German-speaking countries [23]. Although for more than three decades there were various definitions and interpretations of what PCK proposed, there is, however, a consensus that it is one of the basic knowledge domains necessary for the teaching profession. It is also generally agreed upon that PCK consists of different domains. In 1999, Magnusson et al. defined PCK for science teaching as consisting of five components: (i) orientations to teaching science, (ii) knowledge of the student’s understanding of science, (iii) knowledge of science curricula, (iv) knowledge of instructional strategies, and (v) knowledge of the assessment of scientific literacy [16].
PCK models have implications for teacher training. They can help teacher educators to plan a curriculum for teacher training. Schneider [24] affirmed that it makes sense to think about PCK as a tool for teacher educators to guide their thinking about teacher learning and developing teachers’ knowledge. Rollnick et al. [19] argued that PCK must be inserted into teacher training. Similarly, Talanquer [25] believed that teacher education programs should contribute to the construction of PCK. There is some consensus that pedagogical content knowledge develops during the teacher’s education, in the context of their professional practice [11,22] and the advice of trusted colleagues [26].
Several studies have also been dedicated to researching the development of PCK. Geddis et al. [27] found that experienced teachers focused more on the development of procedural knowledge than conceptual knowledge, which was mostly adopted by novice teachers who used a transmission model of teaching. De Jong and van Driel [28] explored the development of eight pre-service teachers’ PCK during a teacher education program and found a development in student teachers’ knowledge of teaching difficulties. Justi and van Driel [29] developed a course on models and modeling for five science teachers participating in a graduate teacher education program. The course consisted of a series of institutional meetings in combination with an action research project. The authors showed that conducting and reflecting on their research projects contributed to the development of science teachers’ PCK. Even though it is the subject of many studies, PCK development is complex. Abell [22] highlighted that there is still a need to find the relationship between PCK and teacher practice in terms of quality and quantity. Nilsson and Loughran [30] (p. 701) said that “the development of PCK is clearly a complex process determined by the content to be taught, the context in which the content is taught, the context in which the content is taught and the way the teacher reflects on his/her teaching experiences”.
Concerning the teaching of contents related to the concept of redox reactions, de Jong and Treagust [3] (p. 335) emphasized the importance that teachers “develop their knowledge of students’ alternative conceptions of electrochemical phenomena and students’ difficulties in understanding these phenomena”. Many studies have focused on the difficulties encountered by students in understanding concepts related to redox reactions [4,31,32,33,34]. Some common difficulties are based on a lack of understanding of electric current, electrical conductivity in solutions, representation of oxidation–reduction reactions, reduction potential, the dependence between reduction reactions and oxidation reactions, the electron transfer process, the meaning of oxidation numbers, the identification of reagents as oxidants and reductants, and redox reaction balancing. In addition, several students are unable to differentiate reactions at the macroscopic level of the substances and at the submicroscopic level of the particles [35].
Some studies on redox reactions have shown the inaccurate and often inappropriate language presented in textbooks to explain concepts involved in redox reactions [2,3,36,37]. Another factor that has led to alternative conceptions is the existence of several models for explaining this content. Sometimes redox reactions are introduced from a historical perspective and, therefore, approached by different models that allow them to be identified. This use of several models can be disconcerting for some students because it focuses on alternative definitions, such as applying the oxygen-based definition of redox reactions to identify all redox processes.
Further studies focused on teachers’ pedagogical content knowledge (PCK) of redox reactions [38,39,40,41,42] and teaching strategies and practical activities involving redox reactions [43,44,45]. Nevertheless, it is noteworthy to say that studies on teachers’ conceptions of redox reactions are still scarce [5,39,46]. What is known is that, in general, teachers present difficulties in relation to pedagogical content knowledge, that is, how to teach redox reactions. These difficulties may come from gaps in their initial teacher education. In this sense, this study is guided by the following research questions: (1) How does the development of PCK concerning the teaching of redox reactions occur in the case of (prospective) chemistry teachers from north-western Germany? (2) Are there any similarities and/or differences in teachers’ PCK at distinct career phases (bachelor’s, master’s, post-MEd trainee program, and in-service)?
3. Materials and Methods
In accordance with the proposed objectives, the present study is characterized as qualitative research [47], which investigated PCK in a qualitative cross-level study with pre- and in-service German secondary chemistry teachers. A cross-level study can bear several hallmarks of a longitudinal study of parallel groups [48]; for example, in education it can involve measures of the different stages of teachers’ careers within a certain educational program [49]. Considering that the development of PCK is a complex process in which its components interact dynamically, two of the four main data sources were used to investigate the teachers’ PCK [21]: (1) questionnaires, (2) interviews.
3.1. Background
German teacher education consists of two phases. The first one takes place at university and is divided into two sections: the bachelor’s degree (six semesters) and the Master of Education (MEd) degree (four semesters). The second one takes place at a teacher training institution with a trainee program (Referendariat in German) (usually eighteen months) followed by options of continuous professional development when working as a teacher [50].
In the first phase, both the bachelor’s and Master of Education degrees include the content-specific study of two school subjects and additional courses in education and psychology. Currently, future teachers study both subjects to the same extent and they can decide which of them will be the subject of both their bachelor’s and master’s theses, or they can do their Master of Education thesis on general education. The post-MEd trainee program is compulsory and includes observing other teachers, teaching under the supervision of teachers, and independent teaching. In this phase, future teachers are required to teach 12 h per week in their two subjects. In addition, they attend general pedagogical and subject-specific educational seminars weekly. In most German trainee programmes, the subject seminars alternate biweekly between the two school subjects. In teaching, teachers in Germany are very free regarding how to operate their lessons. They decide which contexts, experiments, models, or activities are used. Teachers and schools are even free to choose which textbook to use. They can select from a set of textbooks from different publishers, as long as the book was previously approved by the ministry of education in the corresponding German federal state.
During the teacher education program, subject matter related to the concept of redox reactions is taught mainly during the bachelor’s courses, including general, inorganic, organic, and physical chemistry. In addition, contents related to PCK components are taught during the BSc and MEd courses, such as different courses in Chemiedidaktik. (The German term Fachdidaktik, or in its specific form Chemiedidaktik in chemistry education, is difficult to translate into English because of language and cultural differences [51]. It deals with all domains of chemistry education from a theoretical and practical perspective.) Generally, there are no self-standing courses dealing with redox reactions in teaching and learning only.
On the secondary level, content related to the concept of redox reactions is taught, in most German schools, from grade 7. First, in grades 7 and 8, redox reactions are supposed to be taught in terms of combustion, the winning of metals, and corrosion as reactions involving the transfer of oxygen. In grade 9, redox reactions are explained as reactions between electron donors and acceptors. From grade 10, the redox concept is extended to electrochemistry and redox reactions in the context of organic chemistry [52].
3.2. Participants
The participants consisted of four different groups of (prospective) teachers from different stages of German teachers’ careers: bachelor’s students, master’s students, students in a compulsory post-MEd trainee program (Referendariat), and in-service teachers. All the participants in this study were involved in a similar teacher training program, but at different moments. The in-service participants belonged to different schools.
Participants in this study were invited to answer the questionnaire online, which offered privacy, trust and the freedom to answer according to their availability and work schedule. Sixty-two participants answered the questionnaire voluntarily: 37 (60%) were females and 25 (40%) were males. Table 1 summarizes other important participant characteristics. According to Table 1, most participants (23) had less than a year of teaching experience. Fifteen had between 1 and 5 years of experience, two teachers between 6 and 10 years, and five between 11 and 20 years. Seven teachers had more than 20 years of experience. Ten had never taught before. Most participants (38) currently taught (or had taught) content related to redox reactions. From the 24 in-service teachers, only one had never taught content of redox reactions.

Table 1.
Characteristics of the participants.
3.3. Data Collection
Data were collected by means of two instruments: an online questionnaire and semi-structured interviews. The questionnaire was elaborated to access chemistry teachers’ PCK of redox reactions. It was based on a literature review of alternative conceptions and difficulties in the learning of redox reactions. The questionnaire intended to diagnose how teachers approach content of redox reactions, what domain-specific difficulties they had faced, what curriculum materials and instructional strategies they had used, and what they knew about assessment methods. The structure of the questionnaire was inspired by the idea of content representations (CoRes) by Loughran et al. [53] and the components of PCK according to Magnusson et al. [16]: (i) orientations to teaching science, (ii) knowledge of the student’s understanding of science, (iii) knowledge of science curricula, (iv) knowledge of instructional strategies, and (v) knowledge of the assessment of scientific literacy. Four experts in the area analyzed the questionnaire. The questionnaire was developed in English and then translated into German. To avoid questions that could induce bias and ambiguity, a semantic analysis was carried out by a chemistry professor and four PhD students. The final questionnaire (Appendix A) consisted of 15 questions; 6 of them were of a checkbox type, 8 were rating scales and 1 was a dichotomous question. For all questions, there was an empty space for comments and justifications of the answers. Questionnaires have previously been used as a research tool in the investigation of the pedagogical content knowledge [54,55].
In addition, semi-structured interviews were carried out with the aim of obtaining a more elaborated source of data on teachers’ PCK of redox reactions not obtained through the questionnaire. The semi-structured interview guide (Appendix B) consisted of questions that could complement and exemplify the answers given to the questionnaire. Although it contained a set of questions, the interviewer was free to ask other questions if considered appropriate, based on the previous answers that the participants gave to the questionnaire. Several studies investigating PCK have used interviews as strategies [56,57]. All participants who answered the questionnaire were invited to participate in interviews, although only 12 volunteered to do it. Thus, the semi-structured interviews were carried out with 12 German teachers in different stages of their career, comprising two bachelor’s students, two master’s students, two trainee teachers and six in-service teachers. Each interview lasted about 20 to 30 min and was audiotaped.
At the beginning of the questionnaire and the interview, participants were informed about the research objectives, how the data would be collected, and how the material would be used for analysis. In addition, participants were informed about the confidentiality of the data. Their participation was voluntary. All participants declared that they had been informed about the research objectives and agreed to participate voluntarily in this research.
3.4. Data Analysis
In this study, qualitative research methods were used [47]. The written justifications from the questionnaires and the interview transcriptions were analyzed by qualitative thematic analysis via a deductive approach (using the five components of the PCK proposed by Magnusson et al. [16]) and an inductive approach (by identifying any emergent themes from the data). First, the responses were divided into small segments of information; those segments with the same meaning were labeled with codes; then the codes were grouped into themes [47]. In addition, for the checkbox questions, frequency counting was performed for each group (bachelor’s, master’s, trainee and in-service) and for the rating scale questions, a nominal scale was associated with numerical values (not important at all: 1, of little importance: 2; of average importance: 3; very important: 4, and absolutely essential: 5), and then a weighted mean was calculated for each group. Finally, the responses of the different groups were analyzed for similarities and differences, to obtain information about the chemistry teachers’ PCK of redox reactions at different stages of their career.
4. Results and Discussion
It was observed that in-service teachers, i.e., those who had already finished their teacher training, commented and justified their answers more than prospective teachers (the groups at bachelor’s and master’s level, and graduate teacher trainees). Although each person had a different structure in his/her PCK, some trends could be observed among the four groups.
From the analyzed data, it was observed that the PCK component knowledge of assessment does not allow us to arrive at any relevant inference in the sense of analyzing differences in professional knowledge. In general, what was observed was a lack of knowledge about assessment among the different groups. In accordance with the results found by Aydin et al. [58], teachers generally do not have domain-specific PCK for assessment in the field of understanding electrochemistry. Even in the field of research much has been conducted on what teachers comprehend, but little on what they understand about assessment [59]. In relation to the other components, some differences were observed. Some were subtler, such as the case of orientation to teaching science, and others were more striking, such as the case of knowledge of strategies.
The analysis below is limited to evidence related to three of the five PCK components. The examples chosen are in the components of knowledge of science curricula, knowledge of the student’s understanding of science, and knowledge of instructional strategies. In this study, these were the components in which it was possible to identify more differences between the groups.
4.1. Knowledge of the Science Curriculum
Knowledge of the science curriculum corresponds to the teachers’ knowledge of the goals and objectives for the subject they are teaching; the knowledge that teachers have about the programs, national documents, and materials that are relevant to teaching particular science content. Thus, the teachers’ curriculum knowledge should include knowledge of the general goals of the curriculum and of the activities and materials to carry out these goals. In our study, it was possible to find some indications of the goals of teaching redox reactions. In particular, three questions (“How important do you consider the teaching of redox reactions?”, “What do you most intend students to learn about redox reactions?”, and “Why is it important for students to know the content of redox reactions?”) were the starting point to ask the teachers to describe the purposes and goals of their teaching. These questions were a “beginning point in unpacking science teachers’ understanding of what matters in a particular content area and […] why it is important to be taught” [60] (p. 17).
Regarding the importance of teaching redox reactions, it can be noted that the four groups considered teaching this content generally important (Figure 1).
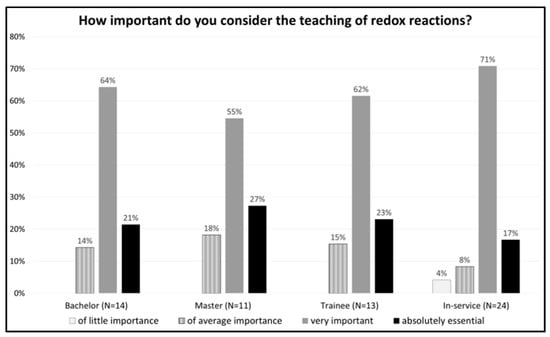
Figure 1.
Distribution of the answers to the question: How important do you consider the teaching of redox reactions?
Nobody chose the option “not important at all”. Just one in-service teacher chose the option “of little importance”, with the justification that “Everyday use is of limited importance”. This opinion differed from the others, since most of the in-service teachers justified the importance of redox reactions by referring to daily life applications.
What can be observed is that, regardless of career stage and independent of the difficulty associated with the content, the participants recognized the importance of teaching electrochemistry. When asked for justification of their answer, the participants associated “importance” with two main categories: basic concepts and everyday life applications (Figure 2). This analysis showed a shift in the predominant emphasis in teaching redox reactions, from referring to their importance within chemistry, done by the bachelor’s students, toward a focus on the applications of redox reactions, common among the in-service teachers. For example, bachelor’s students stated in the questionnaires that teaching redox reactions was important because it is a basic concept of chemistry and important to understand other concepts:
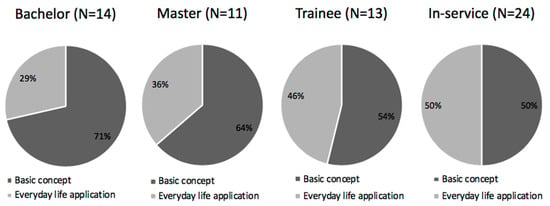
Figure 2.
Distribution of the justifications for the question: How important do you consider the teaching of redox reactions?
“They form a basic framework for many fields of chemistry.” (Bachelor’s student 11)
“Redox reactions are an important component of chemistry knowledge.” (Bachelor’s student 10)
“Many chemical reactions are redox reactions. An understanding of this is, therefore, necessary for a comprehensive understanding of chemistry in general.” (Bachelor’s student 13)
The content-focused approach gradually decreased in the case of master’s students and trainee students, and in-service teachers showed a greater emphasis on the application of this content:
“In everyday life, we are constantly confronted with redox reactions (for example, breathing and the combustion of petroleum products).” (In-service teacher 18)
“I find that teaching redox reactions is important because it can explain everyday phenomena (e.g., rusting). In addition, redox chemistry can be used to prevent rusting.” (In-service teacher 24)
This tendency can also be observed in the answers to the question “Why it is important to teach redox reactions?”. Figure 3 shows that most participants (94%) considered that teaching redox reactions is important because it is a basic chemical concept. It is interesting that despite bachelor’s students also attributing importance to the relationship of this content with daily life, they were more concerned with the balancing of chemical reaction equations (content). On the other hand, in-service teachers expected students to understand and recognize the applications of this content. For this group, it was more important for students to make relationships between the content and everyday life and discuss the potential environmental and economic impacts.
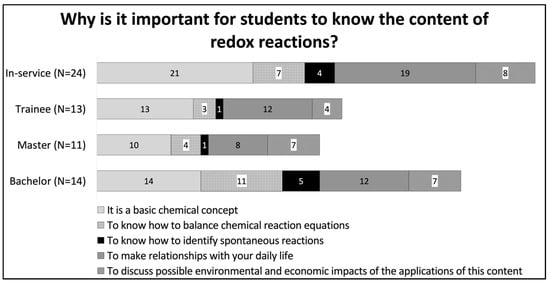
Figure 3.
Distribution of the answers to the question: Why is it important for students to know the content of redox reactions?
As observed in the justifications for the previous question, bachelor’s students considered that it is important to prioritize the learning of concepts, leaving the learning of their applications in the background. This factor can also be reinforced by analyzing the answers given to the question: “What do you most intend students to learn about redox reactions?” (Table 2).

Table 2.
Distribution of the positioning of the participants in relation to the question: What do you most intend students to learn about redox reactions?
The same trend can be observed, where professionals change their purposes and teaching objectives over time. While bachelor’s students prioritized content knowledge, with continuing training and experience, the tendency migrated toward prioritizing the application of the content. As reported by Schneider and Plasma [59] (p. 542), “preservice teachers were clearly focused on covering basic content correctly and completely,” while with years of experience, the purposes and goals for teaching are determined by phenomena in everyday life.
In Table 2, it is observed that the concept of electron transfer was the most important for all groups. The participants expected students to understand that in a redox process, there are elements that lose electrons while some others gain them.
There were no big differences between the groups. However, one fact that can be highlighted in relation to in-service teachers is that they indicated different levels of importance for each concept. For the other groups, it can be observed that the average value is repeated for different concepts, while for in-service teachers the average value is not repeated for any concept. This may indicate that less experienced teachers only know a few aspects from the theoretical domain, so they emphasize several of them. Experienced teachers, knowing the different aspects better in the context of teaching, might be better able to differentiate between the concepts and rank them from most important to least important.
Knowledge of the science curriculum also became clearer during the interviews. When the bachelor students, with their limited experience from only a few internships, were asked about the teaching process in relation to the content of redox reactions, they showed a lack of knowledge of the programs that are relevant in teaching redox reactions:
“I’m not completely sure about what they learned before. I am starting with my 8th grade. I’m starting oxidation at the easiest point where we just talk about metal reacting with oxygen. That’s what I do. I just started right now. Today I did the iron plus oxygen reaction.” (Bachelor’s student 1)
This bachelor’s student did not know the concepts that students had previously learned, and he/she did not provide further details of what the students would learn next. In the following example, similarly to the quote above, the student only reported what he/she was doing, without providing more information, even when questioned about the point during the course at which redox reactions should be taught:
“In the 8th grade, you have to teach metals, like iron and copper, and that kind of thing. And what happens when you put it in water. That is how I start redox reaction in the 8th grade, but we don’t call it redox reaction. We called it metal and oxygen.” (Bachelor’s student 2)
Master’s students and trainee teachers also did not show confidence in their interviews when they spoke about the knowledge needed for teaching redox reactions, but they listed more concepts:
“They should know the electron shell configuration. They should know the periodic system, the periodic table and the groups and families, and they should already know the octet rule.” (Master’s student 2)
“Maybe they have to understand the model of atoms because then, they know what happens during redox reactions. Maybe the concept of the words cations and anions, maybe this is important in grade 9.” (Trainee teacher 1)
In-service teachers were able to express their knowledge about the curriculum. In the questionnaire, they justified that the sequence may change according to the difficulties and learning of their students:
“The planning also includes process diagnostics, which influences the subsequent teaching.” (In-service teacher 20)
“Sometimes you have to change the planning spontaneously when learning difficulties occur.” (In-service teacher 23)
Moreover, during the interviews, they talked about this topic with more certainty and they managed to provide more details on how the content of redox reactions is taught over the years in different grades, and to point out some content that students need to grasp before they learn redox reactions:
“They have to know the structure of matter. They have to know what an oxidation reaction is because most of the time they have already heard about that, like something metal is reacting with oxygen. So, this is called an oxidation reaction, and then reduction is when a substance loses the oxygen. Thus, in the upper grades, you can go more toward the electron but in the lower grades, they don’t have the previous knowledge.” (In-service teacher 2)
It is interesting to observe that teachers could use the different models for redox reactions and explain their uses according to the level of the students. The definition of the gain and loss of the substance oxygen is usually introduced in a phenomenological context for lower secondary school students. For upper secondary students, the concept is often described in a particulate context, the loss and gain of electrons. Descriptions in terms of the loss and gain of hydrogen are presented mainly in chapters about organic reactions [3].
In-service teachers could connect the content of redox reactions with what is represented in the curriculum. They believed that there are certain topics that students should know before they learn about redox reactions to facilitate their understanding.
“In grade 8, students need to know about chemical reactions. They also need to know about the atomic model of Dalton and the notation of symbols, because they then can represent such oxidation and reduction reactions. Redox reactions should be taught at the phenomenological level in the course of combustion reactions. In this context, redox reactions can be used to extract important metals from ores. In the course of oxidations, I always teach the law of mass conservation. Before that, chemical reactions should be taught. In grade 10, students should be familiar with the notation of symbols, the periodic table, the Bohr atomic model and ions. This is important because it explains electron transfers. I teach redox reactions according to the properties of metals and metal bonding. Based on this, previously taught reactions of metals and the previous redox concept are extended. Redox reactions are the basis for electrochemistry.” (In-service teacher 4)
In addition, one teacher recognized that up to grade 10, there are students with different educational perspectives in the same classroom and it is important to think about the content and the way of teaching it for the various students.
The question “What materials do you use (or would you choose) to teach the content of redox reactions?” reveals teachers’ knowledge of the science curriculum because it is related to the teachers’ ideas about curricular resources for this topic. From the options indicated by the participants, there was generally not much difference in the use of curricular materials among the groups.
The tendency observed was a preference for the use of experiments (90%), worksheets (80%), and school textbooks (64%), and little use of scientific articles (17%) and games (24%). In general, the participants did not explain the reasons for their choices. It was observed that some future teachers were aware of their need to update themselves in relation to materials. This concern only appeared among the trainee teachers and in-service teachers. However, the participants still did not provide many details in the questionnaires about the ways in which this material was useful in helping students learn about redox reactions. In general, it was observed that in-service teachers could better explain their choices and relate them with practice. They showed more knowledge and more confidence about their choices:
“I use experiments with protocols. Some animations to clarify the processes on the particle level. Sometimes I use wrong equations to talk with the students about frequently occurring errors.” (In-service teacher 4)
This quotation indicates that the in-service teacher could explain the function of each material, for example, animations to differentiate the representational levels of chemistry and equations to alert students to frequent mistakes. This result is compatible with the findings by Schneider and Plasma [59], who reported that teachers know more about the science curriculum with years of experience.
4.2. Knowledge of Students’ Understanding of Science
This component is very important to highlight what teachers know about the knowledge of their students and how this knowledge influences their teaching. It shows teachers’ knowledge of students’ misconceptions. The question “What are the main difficulties faced by students when learning the content of redox reactions?” is related to this component and the answers to it provide understandings of alternative conceptions and potential difficulties in teaching redox reactions. According to Figure 4, in general, the participants expected the same difficulties. The main difficulty was related to conceptual difficulties, meaning that teachers considered the students to have great difficulty in differentiating the terms oxidizing and reducing agents, and reduction and oxidation, and in balancing redox reaction equations.
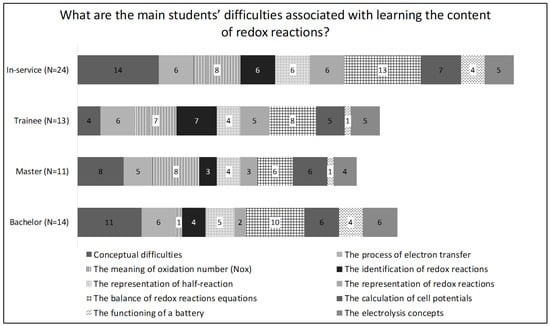
Figure 4.
Distribution of the answers to the question: What are the main difficulties faced by students when learning the content of redox reactions?
Although both pre- and in-service teachers, did not consider the teaching of the balancing of redox reactions as a priority, this was one of the main potential difficulties mentioned by them. This difficulty may be due to the strategy and material used by teachers, who often prescribe the use of procedures to balance redox reactions as provided in books, procedures that are difficult for students to understand [61]. Another point to be highlighted is the difficulty pointed out in relation to the transfer of electrons and the oxidation number. Although the two are considered basic concepts, that is, crucial in the teaching of redox reactions, students may have great difficulty in understanding and adopting these terms.
In the same way as knowledge of the science curriculum, a small difference was observed in relation to the in-service teachers, who signaled two main difficulties. From Figure 4, for the bachelor’s, master’s and trainee students, there were several difficulties that were suggested at above 35%, while for experienced teachers this only occurred for two of the difficulties.
Both bachelor’s and master’s students did not comment on their answers regarding students’ difficulties in the questionnaire, but during the interviews, they were able to give more details about common obstacles related to redox reactions. All of the four groups mentioned the same source of problems: the comprehension of the idea that a redox reaction is defined as a loss and gain of oxygen first, a concept that later needs to be revised [2]:
“We start in 8th grade with the reaction of iron or copper with oxygen. I don’t agree with our school. Because that is the start of a mis-thinking or something. I don’t agree with it, but I have to do this because of the school.” (Bachelor student 2)
“Misconceptions about oxygen theory in our planning. So, we discuss this and it’s interesting because we are about five chemistry teachers here. Everybody does it in their own way and there are some teachers that say: “I’m teaching this in the old way, so I’m saying redox reactions happen with oxygen” and one year later I have to say: oh, there’s new theory and electrons.” (In-service teacher 1)
The participants agreed that this was not a good approach. They did not, however, change their way of teaching, because they felt the need to teach according to the official syllabus and according to what teachers from other schools were generally doing.
In relation to difficulties and misconceptions, the participants were asked if they were aware of students’ alternative conceptions and misunderstandings about redox reactions. For this, they were asked to position themselves between strongly disagree, disagree, undecided, agree, and strongly agree (Table 3).

Table 3.
Distribution of the positioning of the participants in relation to the proposition: I am aware of the students’ alternative conceptions and misunderstandings about redox reactions.
According to Table 3, most of the participants agreed in the questionnaire that they were aware of students’ difficulties, something that is generally covered in pre-service chemistry education courses. However, there was a difference in the justifications between those with experience and those without. While in-service teachers explained that they knew the difficulties and affirmed that they use them in the classroom, the students in the trainee phase explained that they were still learning about this concept.
The previous question was about the main difficulties faced by students. In this one, it was asked if teachers were aware of students’ conceptions, a more self-evaluative question. Analyzing these two questions, it was observed that although the trainee teachers had selected many topics in the previous question, in this question they were not able to self-evaluate about why they knew the difficulties or not. Perhaps this shows that they had marked many topics of difficulty because, in fact, they had no clear idea of what a difficulty is. This fact may indicate that experience also influences the knowledge of students’ difficulties; in other words, practice can provide opportunities for teachers to understand what the real difficulties are. This result is compatible with the findings by Girotto Júnior and Fernandez [62], who reported that knowledge about students’ difficulties in relation to a given topic is acquired through practice in the classroom.
4.3. Knowledge of Instructional Strategies
Knowledge of instructional strategies refers to the teachers’ ideas about the methods or specific activities to make a subject comprehensible to their students. It also includes how to use them and when. The question “What strategies do you use (or would you choose) to teach the content of redox reactions?” revealed teachers’ knowledge of specific strategies for teaching.
Figure 5 shows that the most suggested strategy was practical work (91%). This is consistent with the statement that the type of material they used most was experiments. In addition, it can be observed that in-service teachers’ answers did not vary; all options were chosen by more than 50% of the teachers. On the other hand, for the other groups, their answers were divided among the different strategies. This may indicate that, like the results shown by van Driel et al. [11], the lack of experience causes a poorer repertoire of instructional strategies.
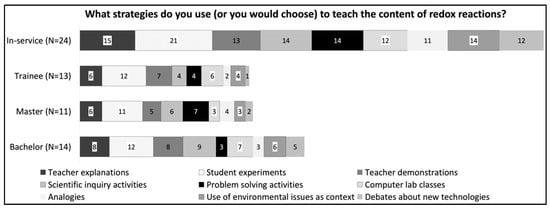
Figure 5.
Distribution of the answers to the question: What strategies do you use (or would you choose) to teach the content of redox reactions?
Knowledge of materials and knowledge of strategies are related to each other. The material used depends on the strategy adopted. In this way, if the teacher does not know which strategy is better, he/she will not know how to choose the best material. In fact, in this study, it cannot be said that teachers did not have this knowledge, but like with the knowledge of the science curriculum, from the justifications given, it was possible to identify how they claimed to use the strategies and if they thought that the strategy adopted helped students to learn the redox reactions concept.
The bachelor’s students believed that one appropriate strategy was to work with experiments to motivate students. The master’s students and trainee teachers provided other examples beyond practical work:
“I would start with a small experiment to motivate the students. For example, burning some metals, then you have redox reactions, then you can explain and describe what they saw. If sometimes an experiment doesn’t work, you can use video to show it to students. But I don’t like YouTube videos because most times they don’t explain the concept in the right way. Animations are also very nice. We have tablet PCs at school. And then students can work with this technique and visualize what is going on during redox reactions. The transfer of electrons can be shown during the animations. If you only explain with two pictures, for example, maybe the students don’t know what happens. I think this strategy can help students understand it.” (Trainee teacher 1)
It can be seen in the statement above that the trainee teacher used several strategies (experiment, videos, animations, pictures) and had established objectives for each one. They also used experimentation for motivation, as did bachelor’s and master’s students. In-service teachers also suggested using practical work, but with a more investigative approach:
“I prefer experimentation. A demonstration experiment. I show this experiment, or I give an experiment that we can discuss for the hypothesis: what will happen in this experiment and then we will make the conclusions. I sometimes use small movies to show it or sometimes we work with some texts. We do not work every time with a chemistry book; in our school, not every pupil has their own chemistry book. We don’t have so many books, so we can just use them sometimes in the class, then we do some exercises.” (In-service teacher 1)
In addition to recognizing the importance of using experiments, in-service teachers also demonstrated knowledge of other strategies for the teaching of redox reactions: they used exercises, experiments, videos, and texts, as in the following statement:
“I show an experiment where it is possible to show that both reactions happen together. I try to use as many different things as possible. Textbook, experiments, see what happens through videos or videos made by themselves. As much as possible. If you just read the textbook, sometimes they don’t understand and when you use different things they say “Yes! Easy! Now I understand.” Most different things also don’t just only work with the textbook. You can work in groups, and then they can explain for each other. Sometimes they are afraid to ask questions to the teacher, when working in groups they can ask their classmates.” (In-service teacher 3)
According to the above excerpt, it is possible to observe that teachers try to use different strategies: affirming that it is better to vary methods than to use only the textbook, for example. And the teachers can be seen to always pay attention to the students’ difficulties. If the students do not understand a certain explanation of a text from a textbook, they change the strategy. This is a very important point. If the teacher has a good knowledge of strategies for teaching redox reactions, he/she can alternate their use according to the dynamics of each classroom.
Although teachers claimed to use experimentation, they understood that this is not the only way to teach redox reactions:
“I use everything. I am a friend of experiments. Not only demonstration experiments, but also experiments that are conducted by students. Otherwise, it is too boring even for me when you only use the board. Nevertheless, we cannot only do experiments. But also, we use multimedia, movies for example, especially if you cannot conduct the experiment because you don’t have the reagents or if it is too dangerous to do it here. Also, movies about how it reacts at an atomic level. I think the combination of everything. People are different, most students like experiments but there are students that don’t like them. Some students can learn a little bit faster if they read, some can learn faster if they see. So if you combine different strategies, you have different offers for everybody.” (In-service teacher 5)
Once again, it is possible to notice that teachers believed that knowledge of varied teaching strategies helps to gain a better understanding on the part of students. One of the important aspects here was the concern of this teacher for using videos that address the submicroscopic level, making it possible to deal with the three representational levels in chemistry: the macroscopic, submicroscopic, and symbolic levels.
There were three statements regarding the strategies in the questionnaire, also a more self-evaluative question. Table 4 shows the positioning of each group.

Table 4.
Distribution of the positioning of the participants in relation to the propositions: I select certain teaching strategies that aim to facilitate student learning about redox reactions; I use different strategies to teach redox reactions, and I try to use different strategies each time I teach the topic of redox reaction.
According to Table 4, it can be noted that this self-assessment varies among different career levels. Most participants agreed that they selected strategies thinking about the students’ learning and tried to use these strategies in a different way during the class, but hardly changed these strategies later. This can indicate that there is a lack of reflection. Through the interviews, it was possible to better understand why the participants did not use different strategies each time they taught the topic of redox reactions.
When bachelor’s and master’s students suggested using different strategies in the questionnaire, they justified their answers as follows:
“Depending on the student’s knowledge, different strategies can be applied.” (Bachelor’s student 11)
“There are not general teaching strategies; I would choose different teaching strategies depending on the learning group.” (Master’s student 6)
They were very straightforward in their justifications and just stated that the choice of strategies was due to the students’ knowledge. In the case of in-service teachers, these justifications were more detailed:
“Since my traineeship, I have been dealing with the problems of teaching the redox concept. I have already written a corresponding publication, in which my point of view becomes clear. In teacher training and at school, I apply my strategies for mediating the redox concept. In the different learning groups, I use different strategies: In class 9 electron transfer is mediated for the first time; I use analogies like the transfer of a scale. In upper secondary education, I use considerations of energy to convey the redox concept. The terms donator and acceptor, however, are technically inconsistent because it is not electron transfer but rather the theft of electrons.” (In-service teacher 8)
It can be observed that the in-service teacher justified his/her answer by relating the choice of strategy to the knowledge of students and the knowledge of the curriculum for redox reactions. In addition, in-service teachers were able to justify when and why they did not use certain strategies:
“If it has been shown before that there is a problem, I change my approach. But I’m not just trying to make something new, with each group something new, that does not make sense to me.” (In-service teacher 4)
What is observed here is that the in-service teacher did not see the need to modify his/her strategy for each class, since he/she had already made a self-assessment and found that the strategy was efficient for that context. From this example, it can be seen that in-service teachers reflect on their actions and are able to adapt according to the needs of each context.
According to the excerpts above, there seems to be an improvement in the knowledge of strategies from bachelor’s to in-service level. Van Driel et al. [63] also found gains in the growth of knowledge of strategies with teaching experience.
In addition to the analysis of the components of PCK, along with the justifications and comments to the questionnaire, it was observed that pre- and in-service teachers recognized the role of experience:
“I’ve never taught them properly, but I think with experience this would improve my teaching.” (Bachelor student 11)
“In my opinion, the experiences of the past have triggered a process of thinking which has led to the improvement of the teaching by means of educational literature and continuing education events.” (In-service teacher 11)
From these excerpts, it can be suggested that pre-service teachers felt the need for more experience in teaching to answer the questions properly, while the in-service teachers justified their answers with their respective years of experience, stressing that their knowledge increased with practical experience.
5. Conclusions
This cross-level study investigated how German teachers’ PCK varies among the different phases of teacher education: from the beginning of their university studies to the in-service phase of their career. An online questionnaire was answered by 62 participants and 12 (prospective) teachers agreed to participate in an interview.
From the analyzed data, it was observed that the PCK components’ orientation toward science teaching and knowledge of assessment do not allow us to arrive at any relevant inference in the sense of analyzing the differences in professional knowledge. However, in relation to the other three components, some differences were observed. Some were subtler, as in the case of knowledge of the curriculum, and others were more striking, as in the case of the knowledge of strategies.
In relation to knowledge of the curriculum, it was observed that less experienced teachers knew several aspects of the theoretical plane, but experienced teachers could differentiate and rank them from most important to least important. Besides that, in-service teachers could better explain the relationship of redox reactions to the other concepts present in the curriculum.
It was possible to identify that among the different levels of the teacher career, they knew some of the students’ conceptions and difficulties in relation to the redox reactions concept. The difference observed was in the ability of in-service teachers to use them in the classroom.
As pointed out in the literature, experienced teachers showed more knowledge about instructional strategies. In-service teachers’ justifications of the strategies they used were more detailed and they were able to justify when and why they did not use different strategies. Experimentation was the most frequently mentioned strategy by all groups; however, in-service teachers suggested using experimentation with a more investigative approach.
This study was important to reinforce the idea that experience may influence the development of PCK. Nevertheless, with only this study, it cannot be said with certainty that it is only experience that influences PCK, because the differences found in this study may be due to other factors. In a broader analysis, when the complexity of PCK is considered, we can establish that when one component of knowledge develops, it can influence others. More research is necessary to find out what those other components are and how they influence each other. For example, if a teacher develops their knowledge of the curriculum, this can influence other categories such as knowledge of students’ understanding.
The first phase of teacher education seems to play a particularly important role in the development of the content, and experience seems to contribute to the development of PCK. Thus, initial teacher education should offer more opportunities to gain practical experience. Future teachers may benefit from opportunities to practice instructional processes in the actual lessons that they plan, conduct, and evaluate. Student teachers in Germany, however, do undertake school internships and teach. But, these are single experiences on certain topics. The question of whether to intensify internships must be discussed. It must, however, also be asked whether this in turn would lead to a loss in preparation of sufficient content knowledge as a base upon which to develop PCK [64]. Thus, investment in continuous professional development programs focusing on redox reactions might be a solution to accompany the development of PCK based on practical experience. Assessment seems to be a necessary focus in that regard.
This study has some limitations. The results are based on a cross-level study, so causal interpretations about individual changes in teacher knowledge are not warranted. Future research should be based on a longitudinal study approach to address these limitations and better understand how PCK development occurs. However, a longitudinal design would not have been feasible here, as it would have required spending decades to reflect the development over the different phases of the teaching career. In the study, we analyzed differences and similarities between pre- and in-service teachers. This study investigated only 62 pre- and in-service chemistry teachers. Larger-scale studies can produce results that are more generalizable. Finally, since this study has revealed that PCK relating to redox reactions can be developed with teaching experience, the tool for identifying PCK development may be altered for other science topics. With this knowledge, further trends or relationships in aspects of PCK development or improvement according to certain topics can be explored.
Author Contributions
Conceptualization, L.F.G., C.F. and I.E.; Formal analysis, L.F.G., C.F. and I.E.; Funding acquisition, C.F.; Investigation, L.F.G., C.F. and I.E.; Methodology, L.F.G., C.F. and I.E.; Supervision, C.F. and I.E.; Writing—original draft, L.F.G.; Writing—review and editing, L.F.G., C.F., and I.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by São Paulo Research Foundation (FAPESP), grant numbers #2013/07937-8; #2014/14356-4 and #2016/16354-4.
Acknowledgments
The authors would like to acknowledge the São Paulo Research Foundation (FAPESP) for funding this research and the participants for their generous participation.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Research about teaching the subject of reduction–oxidation (redox) reactions
This questionnaire is part of a research study about the teaching of redox reaction content. I would like to ask for your cooperation in answering it. The answers are anonymous and the estimated time to answer it is approximately 20 min. Your collaboration will provide valuable information for planning teaching sequences that enhance the learning of redox reaction content.
Initially, I would like to know your opinion about teaching the oxidation–reduction (redox) reaction content.
(1) How important do you consider the teaching of redox reactions?
( ) not important at all
( ) of little importance
( ) of average importance
( ) very important
( ) absolutely essential
Please justify your answer (For each question/item there was a blank space for justifications.)
(2) Do you teach (or have you ever taught) content related to redox reactions?
( ) Yes ( ) No
In case, at which grade level(s) do you regularly teach redox reactions?
Please specify the content in the corresponding grade level(s).
(3) What do you most intend the students to learn about redox reactions? (For each item, participants should position themselves from not important at all to absolutely essential)
( ) not important at all ( ) of little importance ( ) of average importance
( ) very important ( ) absolutely essential
(a) The process of electron transfer
(b) The functioning of a galvanic cell
(c) The corrosion process
(d) The concept of oxidation potential/reduction potential
(e) The calculation of the oxidation number (Nox)
(f) The representation of half-reactions
(g) The identification of redox reactions
(h) The identification of the applications linked to the redox reaction content
(i) Others
(4) Why is it important for students to know the content of redox reactions?
[ ] It is a basic chemical concept
[ ] To know how to balance chemical reaction equations
[ ] To know how to identify spontaneous reactions
[ ] To make connections with their daily life
[ ] To discuss possible environmental and economic impacts of the applications of this content
[ ] Others _____________________________________________
(5) According to your experience, what are the students’ main difficulties associated with learning the content of redox reactions?
[ ] Conceptual difficulties (reduction, oxidation, reducing agent, oxidizing agent)
[ ] The process of electron transfer
[ ] The meaning of oxidation number (Nox)
[ ] The identification of redox reactions
[ ] The representation of half-reaction
[ ] The representation of redox reactions
[ ] The balance of redox reaction equations
[ ] The calculation of cell potentials
[ ] The functioning of a battery
[ ] Electrolysis concepts
[ ] Students generally do not have difficulties
[ ] Others _____________________________________________
(6) What are your difficulties connected with teaching the content of redox reactions?
[ ] Difficulties in basic contents of chemistry
[ ] Lack of time to adequately teach the content
[ ] Lack of equipment to conduct experiments related to redox reactions
[ ] Difficulties in integrating the redox concepts with other areas of chemistry
[ ] Difficulties in integrate the redox concepts with technological applications and environmental issues
[ ] I have no difficulties
[ ] Others _____________________________________________
(7) What materials do you use (or would you choose) to teach the content of redox reactions?
[ ] School textbook [ ] Scientific articles [ ] Videos
[ ] Worksheets [ ] Simulations [ ] Games
[ ] Self-developed teaching materials
[ ] Others _____________________________________________
(8) What strategies do you use (or would you choose) to teach the content of redox reactions?
[ ] Teacher explanations [ ] Student experiments
[ ] Teacher demonstrations [ ] Scientific inquiry activities
[ ] Problem solving activities [ ] Computer lab classes
[ ] Analogies [ ] Use of environmental issues as context
[ ] Debates about new technologies
[ ] Others _____________________________________________
(9) What ways of assessing students’ understanding about the content of redox reactions do you use?
[ ] Oral tests [ ] Written tests
[ ] Multiple choice tests [ ] Essays
[ ] Group projects [ ] Oral communication
[ ] Classroom observations [ ] Lab report
[ ] Others _____________________________________________
In this part, I would like to know your level of agreement with the following statements.
( ) strongly disagree ( ) disagree ( ) undecided ( ) agree ( ) strongly agree
(10) I am aware of the students’ alternative conceptions and misunderstandings about redox reactions.
(11) I use different strategies to teach redox reactions.
(12) I select certain teaching strategies that aim to facilitate student learning about redox reactions.
(13) I know how to evaluate students’ learning about redox reactions.
(14) I know how to evaluate students’ learning by adopting different strategies.
(15) I try to use different strategies each time I teach the topic of redox reaction.
In conclusion, I would like to ask some questions to better characterize the participants of this research. Remember that your data will be treated anonymously, but we can simultaneously assign it to other questionnaires at a later time.
(16) Please enter the first and third letters of the first name of your mother and the day of her birth. (E.g., Luise Müller, 14.01.1948 = LI14) __ __ __ __
(17) Gender
( ) Female ( ) Male
(18) Age
( ) Under 21 ( ) 21–25 ( ) 26–30 ( ) 31–35 ( ) 36–45 ( ) 46–55 ( ) Over 55
(19) Level of Training
( ) Bachelor: ____ semesters
( ) Master of Education: ____ semesters
( ) Traineeship: ____ months
( ) Already finished my teacher training studies
(20) Content-specific subjects studied during teacher training
( ) Chemistry ( ) Biology ( ) Mathematics ( ) Physics ( ) Other_____________
(21) Disciplines you currently teach
( ) Chemistry ( ) Biology ( ) Mathematics ( ) Physics ( ) Science ( ) None ( ) Other___________
(22) Level of education you teach or want to teach
( ) Hauptschule ( ) Realschule ( ) Gymnasium
( ) Gesamt-/Oberschule ( ) Berufsschule
(23) Type of institution in which you teach
( ) Private ( ) Public
(24) How long have you been teaching?
( ) I have never taught before ( ) Less than a year ( ) 1–5 years
( ) 6–10 years ( ) 11–20 years ( ) More than 20 years
(25) Do you teach (or have you ever taught) content related to redox reactions?
( ) Yes ( ) No
In case, at which grade level(s) do you regularly teach redox reactions?
Please, specify the content in the corresponding grade level(s)
Appendix B
Semi-structured interview guide
The research asked the following questions based on the answers given in the online questionnaire.
- (1)
- Can you describe for me your professional career from graduation until today?
- (2)
- Do you teach (or have you ever taught) redox reactions?
- (a)
- At which grade level(s) do you regularly teach redox reactions?
- (3)
- Can you describe for me how you start teaching redox reactions? Perhaps providing some examples for clarification.
- (4)
- What is the most important thing that you want to teach about redox reactions?
- (a)
- Why do you think it is important?
- (b)
- What content do you think students need before they learn redox reactions?
- (c)
- How does redox reactions connect to other content?
- (5)
- What do you consider when you plan a lesson about redox reactions?
- (a)
- Are there things related to redox reactions that you purposefully leave out?
- (i)
- What are they and why?
- (6)
- What are some difficulties with teaching this topic?
- (a)
- Are there other factors that influence how you teach this topic?
- (7)
- What materials do you use to teach the content of redox reactions?
- (a)
- You use (an animation, picture, analogy, etc.) to help students learn redox reactions. Why? Are there any particular reasons for the use of this (strategy) for teaching?
- (b)
- In what way is this strategy particularly useful in helping students to learn the redox reaction concept(s) you want them to understand?
- (8)
- How do you know that students understand the ideas or concepts about redox reactions that you teach?
- (a)
- What strategies do you use to understand students’ understanding of redox reactions?
- (9)
- If you were to teach content again, would there be changes?
- (a)
- Which?
- (b)
- Why?
Do you want to comment on your answers?
Do you have something to add to your answers?
References
- Soudani, M.; Sivade, A.; Cros, D.; Mèdimagh, M.S. Transferring knowledge from the classroom to the real world: Redox concepts. Sch. Sci. Rev. 2000, 82, 65–92. [Google Scholar]
- Österlund, L.-L.; Berg, A.; Ekborg, M. Redox models in chemistry textbooks for the upper secondary school: Friend or foe? Chem. Educ. Res. Pract. 2010, 11, 182–192. [Google Scholar] [CrossRef]
- De Jong, O.; Treagust, D.F. The teaching and learning of electrochemistry. In Chemical Education: Towards Research-Based Practice; Gilbert, J.K., De Jong, O., Justi, R., Treagust, D.F., Van Driel, J.H., Eds.; Kluwer: Dordrecht, The Netherlands, 2002; pp. 317–337. [Google Scholar]
- Österlund, L.-L.; Ekborg, M. Students’ understanding of redox reactions in three situations. Nordic Stud. Sci. Educ. 2009, 5, 115–127. [Google Scholar] [CrossRef]
- Rollnick, M.; Mavhunga, E. PCK of teaching electrochemistry in chemistry teachers: A case in Johannesburg, Gauteng Province, South Africa. Educación Química 2014, 25, 354–362. [Google Scholar] [CrossRef]
- Kind, V. Pedagogical content knowledge in science education: Perspectives and potential for progress. Stud. Sci. Educ. 2009, 45, 169–204. [Google Scholar] [CrossRef]
- Kirschner, S.; Borowski, A.; Fischer, H.E.; Gess-Newsome, J.; von Aufschnaiter, C. Developing and evaluating a paper-and-pencil test to assess components of physics teachers’ pedagogical content knowledge. Int. J. Sci. Educ. 2016, 38, 1343–1372. [Google Scholar] [CrossRef]
- Fernandez, C. Knowledge base for teaching and Pedagogical Content Knowledge (PCK): Some useful models and implications for teachers’ training. Probl. Educ. 21st Century 2014, 60, 79–100. [Google Scholar]
- Shulman, L.S. Those who understand: Knowledge growth in teaching. Educ. Res. 1986, 15, 4–14. [Google Scholar] [CrossRef]
- Shulman, L.S. Knowledge and teaching: Foundations of the new reform. Harv. Educ. Rev. 1987, 57, 1–22. [Google Scholar] [CrossRef]
- van Driel, J.H.; Verloop, N.; de Vos, W. Developing science teachers’ pedagogical content knowledge. J. Res. Sci. Teach. 1998, 35, 673–695. [Google Scholar] [CrossRef]
- Fernandez, C.; Goes, L.F. Conhecimento pedagógico do conteúdo: Estado da arte no ensino de ciências e matemática. In Conocimiento Didáctico del Contenido. Una Perspectiva Iberoamericana; Garritz, A., Rosales, S.F.D., Lorenzo, M.G., Eds.; Editorial Académica Española: Saarbrücken, Germany, 2014; Volume 1, pp. 65–99. [Google Scholar]
- Carlson, J.; Daehler, K.R. The Refined Consensus Model of Pedagogical Content Knowledge in Science Education. In Repositioning Pedagogical Content Knowledge in Teachers’ Knowledge for Teaching Science; Hume, A., Cooper, R., Borowski, A., Eds.; Springer: Singapore, 2019; pp. 77–92. [Google Scholar]
- Gess-Newsome, J. A model of teacher professional knowledge and skill including PCK: Results of the thinking from the PCK Summit. In Re-examining Pedagogical Content Knowledge in Science Education; Berry, A., Friedrichsen, P., Loughran, J., Eds.; Routledge: New York, NY, USA, 2015; pp. 22–42. [Google Scholar]
- Grossman, P.L. The Making of a Teacher: Teacher and Teacher Education; Teachers College Press: New York, NY, USA, 1990. [Google Scholar]
- Magnusson, S.; Krajcik, L.; Borko, H. Nature, sources and development of pedagogical content knowledge. In Examining Pedagogical Content Knowledge: The Construct and Its Implications for Science Education; Gess-Newsome, J., Lederman, N.G., Eds.; Kluwer Academics: Dordrecht, The Netherlands, 1999; pp. 95–132. [Google Scholar]
- Park, S.; Oliver, J.S. Revisiting the conceptualisation of pedagogical content knowledge (pck): Pck as a conceptual tool to understand teachers as professionals. Res. Sci. Educ. 2008, 38, 261–284. [Google Scholar] [CrossRef]
- Park, S.; Oliver, J.S. National Board Certification (NBC) as a Catalyst for Teachers’ Learning about Teaching: The Effects of the NBC Process on Candidate Teachers’ PCK Development. J. Res. Sci. Teach. 2008, 45, 812–834. [Google Scholar] [CrossRef]
- Rollnick, M.; Bennett, J.; Rhemtula, M.; Dharsey, N.; Ndlovu, T. The Place of Subject Matter Knowledge in Pedagogical Content Knowledge: A case study of South African teachers teaching the amount of substance and chemical equilibrium. Int. J. Sci. Educ. 2008, 30, 1365–1387. [Google Scholar] [CrossRef]
- Gess-Newsome, J. Secondary Teachers’ Knowledge and Beliefs about Subject Matter and their Impact on Instruction. In Examining Pedagogical Content Knowledge: The Construct and Its Implications for Science Education; Gess-Newsome, J., Lederman, N.G., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 51–94. [Google Scholar]
- Chan, K.K.H.; Hume, A. Towards a Consensus Model: Literature Review of How Science Teachers’ Pedagogical Content Knowledge Is Investigated in Empirical Studies. In Repositioning Pedagogical Content Knowledge in Teachers’ Knowledge for Teaching Science; Hume, A., Cooper, R., Borowski, A., Eds.; Springer: Singapore, 2019; pp. 3–76. [Google Scholar]
- Abell, S.K. Twenty Years Later: Does pedagogical content knowledge remain a useful idea? Int. J. Sci. Educ. 2008, 30, 1405–1416. [Google Scholar] [CrossRef]
- Kansanen, P. Subject-matter didactics as a central knowledge base for teachers, or should it be called pedagogical content knowledge? Ped. Cult. Soc. 2009, 17, 29–39. [Google Scholar] [CrossRef]
- Schneider, R.M. Pedagogical Content Knowledge Reconsidered: A Teacher Educator’s Perspective. In Re-Examining Pedagogical Content Knowledge in Science Education; Berry, A., Friedrichsen, P., Loughran, J., Eds.; Routledge: London, UK, 2015; pp. 162–177. [Google Scholar]
- Talanquer, V. Qué conocimiento distingue a los Buenos maestros de química? Educación Química 2004, 15, 52–58. [Google Scholar] [CrossRef]
- Appleton, K. How Do Beginning Primary School Teachers Cope with Science? Toward an Understanding of Science Teaching Practice. Res. Sci. Educ. 2003, 33, 1–25. [Google Scholar] [CrossRef]
- Geddis, A.N.; Onslow, B.; Beynon, C.; Oesch, J. Transforming Content Knowledge: Learning to Teach about Isotopes. Sci. Educ. 1993, 77, 575–591. [Google Scholar] [CrossRef]
- De Jong, O.; van Driel, J.H. Exploring the Development of Student Teachers’ PCK of the Multiple Meanings of Chemistry Topics. Int. J. Sci. Math. Educ. 2005, 2, 477. [Google Scholar] [CrossRef]
- Justi, R.; van Driel, J. The development of science teachers’ knowledge on models and modelling: Promoting, characterizing, and understanding the process. Int. J. Sci. Educ. 2005, 27, 549–573. [Google Scholar] [CrossRef]
- Nilsson, P.; Loughran, J. Exploring the Development of Pre-Service Science Elementary Teachers’ Pedagogical Content Knowledge. J. Sci. Teach. Educ. 2012, 23, 699–721. [Google Scholar] [CrossRef]
- Garnett, P.J.; Treagust, D.F. Conceptual difficulties experienced by senior high school students of electrochemistry: Electric circuits and oxidationreduction equations. J. Res. Sci. Teach. 1992, 29, 121–142. [Google Scholar] [CrossRef]
- Garnett, P.J.; Treagust, D.F. Conceptual difficulties experienced by senior high school students of electrochemistry: Electrochemical (galvanic) and electrolytic cells. J. Res. Sci. Teach. 1992, 29, 1079–1099. [Google Scholar] [CrossRef]
- Sanger, M.J.; Greenbowe, T.J. Common student misconceptions in electrochemistry: Galvanic, electrolytic, and concentration cells. J. Res. Sci. Teach. 1997, 34, 377–398. [Google Scholar] [CrossRef]
- Sanger, M.J.; Greenbowe, T.J. Students’ Misconceptions in Electrochemistry Regarding Current Flow in Electrolyte Solutions and the Salt Bridge. J. Chem. Educ. 1997, 74, 819–823. [Google Scholar] [CrossRef]
- Barke, H.D.; Hazari, A.; Yitbarek, S. Misconceptions in Chemistry; Springer: Berlin, Germany, 2009. [Google Scholar]
- Ogude, N.A.; Bradley, J.D. Ionic conduction and electrical neutrality in operating electrochemical cells. J. Chem. Educ. 1994, 71, 29–34. [Google Scholar] [CrossRef]
- Sanger, M.J.; Greenbowe, T.J. An analysis of college chemistry textbooks as sources of misconceptions and errors in electrochemistry. J. Chem. Educ. 1999, 76, 853–860. [Google Scholar] [CrossRef]
- Aydin, S.; Boz, Y. The nature of integration among PCK components: A case study of two experienced chemistry teachers. Chem. Educ. Res. Pract. 2013, 14, 10. [Google Scholar] [CrossRef]
- Freire, L.I.F.; Fernandez, C. Professores novatos de química e o desenvolvimento do PCK de oxidorredução: Influências da formação inicial. Educación Química 2014, 25, 312–324. [Google Scholar] [CrossRef][Green Version]
- Goes, L.F.; Fernandez, C. Using CoRes for capturing Pedagogical Content Knowledge of redox reactions. In Science Education Research: Engaging Learners for a Sustainable Future, Electronic Proceedings of the ESERA 2015 Conference, Helsinki, Finland, 31 August–4 September 2015; Lavonen, J., Juuti, K., Lampiselkä, J., Uitto, A., Hahl, K., Eds.; University of Helsinki: Helsinki, Finland, 2016; pp. 2163–2173. [Google Scholar]
- Hume, A.; Berry, A. Constructing CoRes—A strategy for building PCK in pre-service science: Teacher education. Res. Sci. Educ. 2011, 41, 341–355. [Google Scholar] [CrossRef]
- Hume, A.; Berry, A. Enhancing the practicum experience for pre-service chemistry teachers through collaborative CoRe design with mentor teachers. Res. Sci. Educ. 2013, 43, 2107–2136. [Google Scholar] [CrossRef]
- Huddle, P.A.; White, M.D.; Rogers, F. Using a teaching model to correct known misconceptions in electrochemistry. J. Chem. Educ. 2000, 77, 104–110. [Google Scholar]
- Niaz, M.; Chacón, E. A conceptual change teaching strategy to facilitate high school students’ understanding of electrochemistry. J. Sci. Educ. Technol. 2003, 12, 129–134. [Google Scholar] [CrossRef]
- Sesen, B.A.; Tarhan, L. Inquiry-based laboratory activities in electrochemistry: High school students’ achievements and attitudes. Res. Sci. Educ. 2013, 43, 413–435. [Google Scholar] [CrossRef]
- Özkaya, A.R. Conceptual difficulties experienced by prospective teachers in electrochemistry: Half-cell potential, cell potential, and chemical and electrochemical equilibrium in galvanic cells. J. Chem. Educ. 2002, 79, 735–738. [Google Scholar] [CrossRef]
- Creswell, J.W. Educational Research: Planning, Conducting and Evaluating Quantitative and Qualitative Research; Pearson Education: Boston, MA, USA, 2012. [Google Scholar]
- Cohen, L.; Manion, L.; Morrison, K. Research Methods in Education; Taylor & Francis: New York, NY, USA, 2007. [Google Scholar]
- Markic, S.; Eilks, I. Potential changes in prospective chemistry teachers’ beliefs about teaching and learning—A cross-level study. Int. J. Sci. Math. Educ. 2013, 11, 979–998. [Google Scholar] [CrossRef]
- Cortina, K.S.; Thames, M.H. Teacher Education in Germany. In Cognitive Activation in the Mathematics Classroom and Professional Competence of Teachers: Results from the COACTIV Project; Kunter, M., Baumert, J., Blum, W., Klusmann, U., Krauss, S., Neubrand, M., Eds.; Springer: Boston, MA, USA, 2013; pp. 49–62. [Google Scholar]
- Van Dijk, E.M.; Kattmann, U. A research model for the study of science teachers’ PCK and improving teacher education. Teach. Teach. Educ. 2007, 23, 885–897. [Google Scholar] [CrossRef]
- Landesinstitut für Schule Naturwissenschaften, Biologie—Chemie—Physik: Bildungsplan für das Gymnasium Jahrgangsstufe 5–10. Available online: http://www.lis.bremen.de/ (accessed on 26 November 2019).
- Loughran, J.; Mulhall, P.; Berry, A. In search of pedagogical content knowledge in science: Developing ways of articulating and documenting professional practice. J. Res. Sci. Teach. 2004, 41, 370–391. [Google Scholar] [CrossRef]
- Stender, A.; Brückmann, M.; Neumann, K. Transformation of topic-specific professional knowledge into personal pedagogical content knowledge through lesson planning. Int. J. Sci. Educ. 2017, 39, 1690–1714. [Google Scholar] [CrossRef]
- Sorge, S.; Kröger, J.; Petersen, S.; Neumann, K. Structure and development of pre-service physics teachers’ professional knowledge. Int. J. Sci. Educ. 2019, 41, 862–889. [Google Scholar] [CrossRef]
- Henze, I.; van Driel, J.H.; Verloop, N. Development of experienced science teachers’ pedagogical content knowledge of models of the solar system and the universe. Int. J. Sci. Educ. 2008, 30, 1321–1342. [Google Scholar] [CrossRef]
- Luft, J.A.; Firestone, J.B.; Wong, S.S.; Ortega, I.; Adams, K.; Bang, E. Beginning secondary science teacher induction: A two-year mixed methods study. J. Res. Sci. Teach. 2011, 48, 1199–1224. [Google Scholar] [CrossRef]
- Aydin, S.; Friedrichsen, P.M.; Boz, Y.; Hanuscin, D.L. Examination of the topic-specific nature of pedagogical content knowledge in teaching electrochemical cells and nuclear reactions. Chem. Educ. Res. Pract. 2014, 15, 658–674. [Google Scholar] [CrossRef]
- Schneider, R.M.; Plasman, K. Science teacher learning progressions: A review of science teachers’ pedagogical content knowledge development. Rev. Educ. Res. 2011, 81, 530–565. [Google Scholar] [CrossRef]
- Loughran, J.; Berry, A.; Mulhall, P. Understanding and Developing Science Teachers’ Pedagogical Content Knowledge; Sense: Dordrecht, The Netherlands, 2006. [Google Scholar]
- De Jong, O.; Acampo, J.; Verdonk, A. Problems in teaching the topic of redox reactions: Actions and conceptions of chemistry teachers. J. Res. Sci. Teach. 1995, 32, 1097–1110. [Google Scholar] [CrossRef]
- Girotto Júnior, G.; Fernandez, C. Following early career chemistry teachers: The development of Pedagogical Content Knowledge from pre-service to a professional teacher. Probl. Educ. 21st Century 2013, 55, 57–73. [Google Scholar]
- Van Driel, J.H.; De Jong, O.; Verloop, N. The development of preservice chemistry teachers’ pedagogical content knowledge. Sci. Educ. 2002, 86, 572–590. [Google Scholar] [CrossRef]
- Mamlok-Naaman, R.; Eilks, I.; Bodner, G.M.; Hofstein, A. The Professional Development of Chemistry Teachers; Royal Society of Chemistry: Cambridge, UK, 2018. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).