Abstract
Silanes, and organically modified silanes in particular, are commercially used to protect the built environment from deterioration and, in indoor applications, to minimize water vapor condensation and microbiological contamination. Increasing their uptake, we argue in this study, includes the need to adopt a systems-thinking view of this green chemistry technology. After identifying the key advantages of these coatings, we highlight important educational consequences to undergraduate courses and doctoral programs in chemistry and materials science which are common in many research topics, well beyond nanocoating science and technology.
1. Introduction
Preventing microbiological contamination due to accidental introduction of hazardous microbes onto surfaces is of the uttermost importance in a number of working, healing, learning, and recreational environments, including food and beverage production facilities [1], hospitals [2], and school kitchens [3].
The condensation of water vapor contained in the air when the temperature of the surface reaches the dew point temperature creates favorable conditions for growth and biofilm formation of pathogens such as fungi (mold) or dangerous bacteria such as Salmonella enterica and Listeria monocytogenes [4]. Once biofouling occurs, pathogens form a biofilm exhibiting a high level of resistance to various chemical and physical sanitation processes [5]. If condensation takes place on a food-contact surface, the risk associated with cross-contamination of food products increases many times due to the possibility of localized pathogen growth and spot contamination of food products.
Similarly, the protection of buildings from water penetration due to capillary rise [6] and consequent corrosion of reinforcing steel bars in concrete structures due to chloride ingress, efflorescence, salt burst, freeze-thaw damage from deicing salt and biofouling including algae, moss and mold formation, is required to prolong the lifespan of buildings, lower maintenance costs, and preserve the function and aesthetic appearance.
Both water vapor condensation and water penetration can be minimized using chemical coatings, most of which are comprised of polymer resins. Condensation-resistant coatings are “a controversial alternative” [4] which “may or may not work, depending on many physical and environmental factors in the facility” [4], with few being appropriate for application in food-processing facilities since they require approval for food-contact or non-food-contact surfaces.
Amid said coatings, easily applied waterborne silica-based hybrid sol-gel coatings [7] provide an environmentally friendly, versatile, and safe alternative, suitable for different working and recreational indoor environments.
In the case of building protection, silane-based paints in the form of silicates, and of organically modified silica (ORMOSIL), in particular, are successfully employed to protect the built environment, from contemporary and historic buildings to marine structures, due to their exceptional versatility, long duration, and their unique ability to preserve the aesthetic of the treated surfaces [8].
Increasing their uptake, we argue in this study, includes the need to adopt a systems-thinking view of this green chemistry technology. After identifying the key advantages of these coatings, we highlight important educational consequences to undergraduate courses and doctoral programs in chemistry and chemical engineering which are common in many research topics, well beyond nanocoating science and technology.
2. Silane-Based Hydrophobic Coatings
Silane paints use liquid monomeric alkylsilanes to create hydrophobic coatings within the inner porosity of a substrate. Contrary to nanosols using prehydrolized organosilica coatings obtained via partial hydrolytic polycondensation of silanes of the general formula Si(OR)4 and R’nSi(OR)4-n, monomeric silane molecules can penetrate 1-nm pores and chemically bind with the building material through the OH groups at the material outer surface, eventually polymerizing within the pores to effectively prevent water from penetrating into the building envelope (Figure 1).
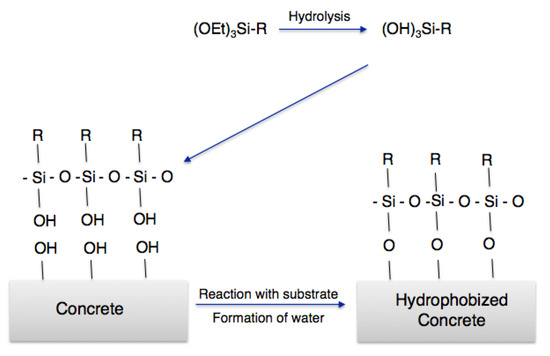
Figure 1.
Reaction of an organofunctional trialkoxysilane with the concrete surface.
On the other hand, waterborne ORMOSIL nanosols make use of a water-alcohol (typically, ethanol or isopropanol) mixture in the presence of a catalytic amount of acid to promote hydrolysis to afford storage-stable nanosol containing plentiful silanols [9].
The nanosol, stabilized against thermodynamically favored aggregation by the presence of alcohol or another organic solvent such as a glycol or glycerol, is easily deposited on widely different substrates forming a lyogel (from the Greek “lyo” for solvent) which dries at relatively low temperature to form a porous, thin (several microns thick), homogeneous, and dense xerogel film comprised of polymeric organosilica tightly bound to the substrate [7,9].
The coating of the condensation-reducing coating composition can be applied by a variety of coating methods. Examples of suitable coating methods include spray coating, brush coating, or spread coating with a roller. The application is best done at temperatures above 10 °C. After application, formatting of the xerogel typically requires between 24 h and 48 h.
Hydrophobic and superhydrophobic coatings, namely with the wettability measured by static water-contact angle between 90° and 150° and greater than 150°, respectively, are easily obtained by varying the nature, the amount, and the number of organically modified silanes employed in the precursor mixture [7,9,10].
Contrary to silicones, which are also Si polymers comprised of a Si–O backbone, ORMOSIL nanosols easily bind through the silanol groups to a large variety of surfaces, including wood, plastics, glass, metal, concrete, natural stone, sandstone, marble, and granite.
The ultraviolet light reaching Earth’s surface is mostly (99%) comprised of UV-A (315–400 nm) radiation, the energy of which is between 380 and 300 kJ/mol and is lower than the binding energy of both Si–O and Si–C bonds (444 kJ/mol and 385 kJ/mol, respectively) [11]. Accordingly, ORMOSIL coatings show exceptional stability against light-driven degradation in outdoor applications.
For example, scholars in Belgium, regularly monitoring the effectiveness of a commercial ORMOSIL paint based on isobutyltriethoxysilane (Dynasylan BHN, later trade-named Protectosil BHN) in protecting a quay wall of the container terminal in the harbor of Zeebrugge over a period of 12 years since 1993, found a significantly reduced chloride penetration of the wall section treated with the commercial silane paint, both for the splash and tidal zones of the treated wall, at each monitoring campaign (1996–1998–2005) in comparison to the untreated part of the same wall (Figure 2) [12].
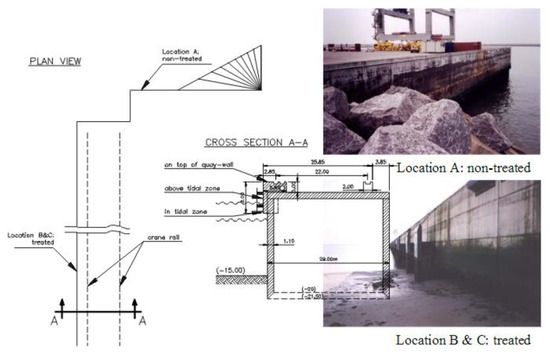
Figure 2.
Plan view and cross-section of the quay wall at Zeebrugge Harbor, Belgium. (Reproduced from [12] with kind permission.)
One remarkable feature noted by the scholars in 1993 was that the waterproofing coating applied by means of airless spraying at low pressures required consumption of 0.35 L per m2 (300 g/m2), in full agreement with the laboratory tests on concrete cubes [12].
Fifteen years later, a similar commercial hydrophobic coating based on different silanes applied with a foam roller on walls to prevent condensation required consumption of 0.04 L per m2 (coverage of 25 m2/L). The 35 to 50 m thick (depending on the application method) resulting xerogel film, formed upon drying at room temperature, reduces the size of condensation droplets formed on cold surfaces where the water droplets form small spheres, decreasing dripping and delaying formation of biofilms [13].
These droplets, eventually moving off the surface, carry particles of dirt with them, resulting in the self-cleaning effect for which sol-gel coatings are increasingly used and the functionalizing of the glass surface of photovoltaic modules and solar thermal collectors.
3. Structural Insight
Understanding the performance of ORMOSIL coatings requires a unified understanding of their unique molecular and nanoscale structure, as well as of their chemistry [14].
ORMOSILs are glasses, namely optically transparent amorphous materials of low surface energy (20 to 55 mN m−1) which impart desirable surface roughness/topography by a deliberate selection of their chemical compositions.
For example, a comparison of a xerogel surface derived from a 50:50 mol% of n-octyltriethoxysilane (C8) and tetraethylorthosilicate (TEOS) with a xerogel coating obtained from a 1:49:50 mol% of n-octadecyltriethoxysilane (C18), C8, and TEOS precursor might suggest little or no surface energy and nanoscale topography differences.
To the contrary, the 50:50 C8:TEOS xerogel has a homogeneous distribution of the hydrocarbon groups at the xerogel surface and a very smooth surface on the nanoscale measured by a 0.24 nm roughness value, whereas the xerogel surface from the 1:49:50 mol% C18:C8:TEOS precursor mixture has the more hydrophobic component C18 and the silanol (Si-OH) groups segregated at the surface with a surface roughness value of 1.15 nm [15].
We remind that to ensure the formation of a homogeneous ORMOSIL with a homogeneous distribution of organic groups throughout the oxide network, especially for high degrees of organic modification at the silicon atom, a two-step acid/base synthesis is generally used to promote the co-polymerization of tetramethylorthosilicate (TMOS) or TEOS with the organically modified silanes included in the hydrolyzed sol over self-condensation [16].
Contrary to what happens with organic polymers, the structure of hybrid ORMOSILs is highly porous and open to external molecules, ensuring that coated substrates can literally “breath.” For example, the hydrophobized quay wall at Zeebrugge Harbor (Figure 2) treated with iso-butyltriethoxysilane is impermeable to water but highly accessible to the diffusion of CO2 molecules causing the carbonation of concrete as shown by the carbonation depth of the treated location which varied from 4 to 6 mm in the tidal zone, towards 8–12 mm above the tidal zone, and up to 12–16 mm on top of the quay wall [12].
To understand why ORMOSIL structures show high physical and chemical stability, it is instructive to review the structure of methyl-modified ORMOSILs prepared by increasing the degree of organic modification of the silica structure and by mixing methyltriethoxysilane (MTEOS) and TEOS up to obtain xerogels from MTEOS only.
In the absence of MTEOS, the silica structure is dominated by four-member rings (the percentage of six-member units in SiO2 obtained from TEOS only is just 15%, but a dramatic increase in the fraction of six-member rings to 20%, 56%, 84%, and 97% occurs as the MTMS content increases to 25%, 50%, 75%, and 100%) (Figure 3) [17].
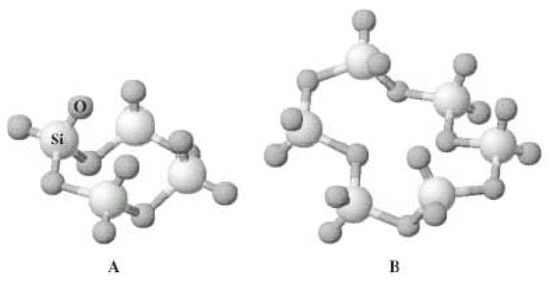
Figure 3.
Schematic diagram of the more common types of primary cyclic arrangements of the structural units SiO4 in xerogels: (A) four-member siloxane ring (SiO)4 and (B) six-member siloxane ring (SiO)6. (Reproduced from [17] with kind permission.)
Eventually, the structure obtained when the precursor is only MTMS is almost entirely formed by the larger, less tensioned, six-member rings, more able to accommodate the unreactive methyl groups. Interestingly, this correlates with the enhancement of the relative degree of crystallinity (and thus long-range order) that is normally observed in ORMOSIL upon increasing the degree of alkylation of the ceramic matrix, when a new peak is observed at 2θ~7° to 10° centered at the Bragg d1-spacing 8.7 Å for fully methylated ORMOSIL. The intensity of the latter peak is triple, relative to the intensity of the signal centered at 2θ~23°, corresponding to Bragg d1-spacing 3.88 Å associated with the spacing between silicon atoms connected by means of an oxygen bridge [18].
The former signal is due to the presence of the six-member siloxane ring [17], whereas the modest shift in the peak that appeared at the larger angle towards lower values from 2θ = 23.2° to 2θ = 22.4° for similar ORMOSILs prepared, respectively from TEOS and from 100% methyltriethoxysilane (MTEOS), only points to slight elongation of the aforementioned spacing of Si atoms connected by the siloxane bond from 3.9 Å to 4.05 Å [19].
However, the latter increasing distance does not point to reduced bond strength with the addition of the methyl groups leading to less stable structures [19] but rather to the aforementioned less tensioned molecular structure of the ORMOSIL corresponding to increased physical stability [17].
Changing the alkyl group length, going from methyl to ethyl to propyl groups, does not change the basic structure of the ORMOSIL matrices in terms of siloxane structural rings since, for instance, all 75%-alkyl modified glasses contain a large predominance of six-member rings (above 84%) [17].
The presence of the methyl groups at the outer surface of these units during the alcogel drying reduces the magnitude of capillary stress during drying, promoting an increase in the xerogel porosity and surface area, while at the same time the organic groups act as network disrupters decreasing the stiffness of the organosilica network.
The outcome of this structural conflict is that ORMOSILs of increasing methylation have a larger surface area and total porosity and reduced average pore size [20]. Moreover, the increasing lack of reactive silanol groups at the sol-gel cage surface with increasing methylation decreases the tendency of adjacent surface Si-OH groups in the shrinking gel to undergo further condensation reactions forming Si-O-Si bonds [21].
Finally, the thermogravimetric analysis unveils that whereas in fully methylated sol-gel xerogels only one type of methyl groups is present, namely those at the surface which are lost due to oxidation in the air at 500 °C, for mixed ORMOSILs with methylation degrees between 10% and 90% two types of methyl groups are present: those at the outer surface and those occluded in the xerogel matrix, as shown by different peaks between 400 °C and 800 °C [19].
From the surface viewpoint of external reactants approaching the xerogel surface, furthermore, ORMOSILs are surface fractals whose surface fractality correlates linearly with their interfacial polarity sensed by entrapped Reichardt’s dye [10]. In detail, the surface fractal dimension, Ds, observed in the narrow range of dimensions (1–2 orders of magnitudes) increases linearly with the relative amount of methyl-modification of the sol-gel glass, following the narrowing of the average pore size.
Organosilica-based nanosols generally adhere to substrate surfaces via formation of siloxane bonds with hydroxyl groups at the substrate surface. The surfaces of materials such as glass, fiberglass, polycarbonate, wood, cotton, stone, concrete, marble, iron, and aluminum (where plentiful OH groups are present due to partial metal oxidation) are therefore easily coated requiring simple curing at room temperature.
This means that the surface of most buildings, ship decks, cold storage rooms, bathrooms, underground parking lots, water tanks, grain silos, and food-processing plants is ideally suited to be treated with anticorrosion and water-resistant sol-gel ORMOSIL coatings.
It also explains why, prior to coating, surfaces are freed from previous polymeric coatings and cleaned (for example with non-toxic and disinfectant isopropanol) to remove dirt, dust, oil, wax, grease, and all other contaminants that might interfere with the adhesion of the coating, after which the coating is applied either via brushing, rolling, or via spray gun under modest pressure.
The coating of plastic surfaces dominated by C-H bonds requires prior prolonged treatment of the plastic surface in order to promote the development of interlocked ceramic and polymeric networks through the diffusion of the Si alkoxide precursors [22].
4. The Need for a Systems Thinking Approach
Protection of the built environment from moisture and deterioration directly impacts worker and consumer health and safety, economic maintenance costs, and the quality and reliability of the services or products supplied to customers.
Sol-gel hydrophobic and breathable coatings comprise an excellent and now affordable chemical technology to protect buildings, outdoor and indoor, from the hazards posed by condensation and other forms of environmental degradation.
They should, however, be used in synergy with other low-cost and environmentally friendly technologies such as ventilation with high-volume, low-speed fans which, by consuming low amounts of electricity and emitting no noise, dramatically reduce the relative humidity in the air in indoor working environments [23].
The latter ventilation technology also destratifies air, increasing its temperature at lower heights [23]. Similarly, these coatings are ideally used in conjunction with natural ventilation using air heated by low-cost solar air collectors, easily integrated on the façades exposed to the South and West [24].
In other words, the use of chemical, environmentally friendly coatings such as ORMOSILs should be part of a systems thinking approach for the protection of the built environment that encompasses worker and supplier (the construction industry, coating suppliers, chemistry professionals, and chemistry and engineering educators) education to develop new knowledge and skills that effectively support working and living in safe and healthy buildings, promoting well-being.
Similar concepts, for which the advantages offered by innovation in materials, construction techniques, and technologies in building construction and refurbishment are often lost during the in-use performance in terms of energy consumed and indoor environmental quality, are now increasingly shared by practitioners of construction science scholars [25].
Very often, scholars and PhD graduates publishing research reports in the field of sol-gel science and technology have little knowledge of the practical applications of sol-gel coatings. A quick test is as follows. Ask her/him the following two questions: “What do you know about the practical utilization of ORMOSIL coatings as anti-condensation and building protection coatings?” and “Can you give the name of one sol-gel coating product or one manufacturer?”
Doubts also surround fundamental aspects. For instance, most respondents in the aforementioned scholarly community asked whether a hydrophobic sol-gel coating applied on a façade would result in a permeable or impermeable surface to water vapor will answer that the façade would become impermeable.
The contrary is true as water vapor permeability must be preserved to effectively protect the building material. Commercial ORMOSIL coatings applied on building facades since the early 1990s ensure that no liquid moisture is trapped in the substrate after treatment, allowing water vapor to escape from the interior [26].
For instance, a field trial at Stroudsburg’s (Pennsylvania) Monroe County >30 years old parking garage treated with a commercial alkyl-modified silane coating (Protectosil CIT) applied via an airless spray gun in two coats (approximately 0.203 L/m2 per coat) for hydrophobization of steel-reinforced concrete and protection of steel from corrosion due to chloride and water penetration showed the stable performance between 1996 and 2002 [27]. The corrosion was found to remain at very low values (<0.1 μA/cm2) from the high of >0.6 μA/cm2 measured in 1996, prior to treatment.
Several other silane-based building protection products based on the same concept are currently available on the marketplace. One example is Silres BS class of silane-based formulations, available both as a solvent-based or water-based mixture of silane, siloxane, and fluoropolymer (<C8) to impart surfaces in natural or synthetic stone (including cotto, marble, travertine, and granite) with water-repellent and oil-repellent (oleophobic) properties.
Besides protection from water and chloride penetration, the multifunctional coating makes it easy to remove oil, grease, and similar oleophilic substances from the treated surfaces [28]. Again, the manufacturer emphasizes how the fact that pores of the treated substrate remain open ensures excellent water vapor diffusion, making the building façade breathable.
One of the main reasons that explain the relatively modest uptake of silanes as protective coatings, regardless of their exceptional performance, greenness, and versatility, is their high cost when compared to polymers. The high cost, in its turn, is chiefly due to (i) a highly concentrated market, and (ii) obsolete silane-production technology.
Both situations, we show in the following section, are due to rapid change unleashing the large potential of silane-based protective coatings.
5. Perspective and Educational Consequences
A report from a reputable market intelligence company, published in 2018, found that the global silanes market had significantly expanded in the previous decade (2008–2017), leading suppliers to expand their production capacities to increasingly supply customers in paint and coating sectors as well as directly in competition with the latter, complete coating formulations to automotive and construction companies [29].
Driven by further increasing demand from the latter sectors, the market was expected to continue to grow at 5% compound annual growth rate until 2022 and add another $433 million to the 2017 global revenues, leading to further expansion of production capacities and to the development of new products. The industry, however, was found to be highly concentrated, with a few chemical companies specializing in silane manufacturing owning a large share of the market [29].
One key reason explaining the limited number of players was the high capital and operational cost of silane production based on the reaction of ethanol with silicon tetrachloride (SiCl4), prepared from expensive metallic silicon in its turn derived from the energy-consuming reduction of silica-rock with carbon.
Today, manufacturing of the key TEOS precursor may directly start from low-cost SiO2 through a quick and green route discovered by Japanese scholars in 2017 [25] based on the simple reaction between silica and ethanol at 260 °C in the presence of 3 Å molecular sieves as dehydrating agents and 10 mol% KOH as base catalyst (Equation (1)) [30].
SiO2 + 4EtOH ⇄ Si(OEt)4 + 2H2O
TEOS is obtained in 70% yield and the molecular sieves are fully recyclable upon simple thermal treatment at 300 °C under vacuum for 15 h. In this way, TEOS may originate at very low cost from rice hull ash, an abundant agricultural by-product.
Similarly, the production of organotrialkoxysilane needed to produce ORMOSILs can now efficiently take place via the heterogeneously catalyzed hydrosilylation of readily available and cheap olefins over a recyclable solid catalyst in a process that can be easily made continuous [31].
Given these technical advances and driven by the true megatrend of sustainability, it is likely that the chemical industry currently undergoing a major reshape [32] will shortly feature several new silane producers. The outcome will be lower prices, enhanced availability, and the introduction of several new silane-based formulations for the protection of the built environment, indoor and outdoor.
In this context of accelerated change, it is necessary to provide chemistry professionals with updated education of practical value on silane-based protective coatings through which they may explain the advantages of these coatings to customers in the industry seeking their advice. This implies the need to reshape today’s undergraduate courses in materials chemistry to encompass those practical aspects of sol-gel science and technology whose current lack creates the aforementioned common situation of scientific (chemistry and chemical engineering) experts’ knowledge and skills having little practical value.
As graduates are unaware that “sizeable proportions of scientific research now occur outside the academy” [32] and are often given a doctorate without ever gaining “research experience in a site of scientific knowledge production other than the university during his or her training” [32], doctoral and undergraduate chemistry education should be reformed and enhanced to actually include contemporary research outcomes, connectivity tools, and visualization resources with immediate benefits for society at large [33].
It is no longer acceptable that a doctor in chemistry or materials science specializing in sol-gel science and technology is unable to give the name of a commercial formulation or suggest a practical application of a silane-based protective coating.
A systems view of education in the university considered as a system whose purpose is defined from the perspective of students—the users and customers of university teaching—(“provide me with all the facilities and help I need to achieve a positive outcome from my time at your university”) [34] purposefully suggests reshaping educational programs and teaching methodologies around what matters to students, and not to professors.
Waterborne or alcohol-based silane-based nanocoatings are among the most important achievements of fundamental and applied research in materials chemistry of the last three decades (1990–2020). They are safe and green replacements of conventional polymeric coatings, showing significantly enhanced performance, along with a uniquely excellent health and safety profile.
Adopting a systems view such as that proposed in this study, adding to several others in contemporary chemistry education research [35], has substantial benefits for students, educators universities, and society at large that will greatly benefit from their mass-scale uptake for the protection of the built environment and of the well-being of its main users: all of us.
Author Contributions
Conceptualization, R.C., Y.A., A.F., L.I. and M.P.; Methodology, R.C., Y.A., A.F. and L.I.; Writing—review & editing, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The picture of the Hangzhou Bay Bridge, protected with a silane-based coating, was kindly retrieved from Wikipedia under the Creative Commons Attribution-Share Alike 3.0 Unported license at the following URL: https://en.wikipedia.org/wiki/Hangzhou_Bay_Bridge#/media/File:Hangzhou_Bay_Bridge_South.JPG.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chatterjee, A.; Abraham, J. Microbial contamination, prevention, and early detection in food industry. In Handbook of Food Bioengineering: Microbial Contamination and Food Degradation; Holban, A.M., Mihai Grumezescu, A., Eds.; Academic Press: Amsterdam, The Netherlands, 2018; Volume 10, pp. 21–47. [Google Scholar]
- Claro, T.; O’ Reilly, M.; Daniels, S.; Humphreys, H. Surface microbial contamination in hospitals: A pilot study on methods of sampling and the use of proposed microbiologic standards. Am. J. Infect. Control. 2015, 43, 1000. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.M.; Beilke, L.; Barreto, J.F. Microbial contamination and good manufacturing practices in school kitchen. J. Food Saf. 2018, 38, e12417. [Google Scholar] [CrossRef]
- Bowser, T.; Jadeja, R. Strategies to Reduce Moisture Condensation in Food Facilities: Food Technology Fact Sheet FAPC-203. Oklahoma State University. 2016. Available online: http://pods.dasnr.okstate.edu/docushare/dsweb/Get/Document-10432/FAPC-203web.pdf (accessed on 23 June 2020).
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, N.; Karoglou, M.; Bakolas, A.; Moropoulou, A. Building materials capillary rise coefficient: Concepts, determination and parameters involved. In New Approaches to Building Pathology and Durability—Building Pathology and Rehabilitation; Delgado, J.M.P.Q., Ed.; Springer: Singapore, 2016; Volume 6, pp. 27–44. [Google Scholar]
- Pagliaro, M.; Ciriminna, R.; Palmisano, G. Silica-Based Hybrid Coatings. J. Mater. Chem. 2009, 19, 3116. [Google Scholar] [CrossRef]
- Sano, K.; Kanematsu, H.; Tanaka, T. Overview of silane-based polymer coatings and their applications. In Industrial Applications for Intelligent Polymers and Coating; Hosseini, M., Makhlouf, A., Eds.; Springer: New York, NY, USA, 2016; pp. 493–509. [Google Scholar]
- Ciriminna, R.; Fidalgo, A.; Palmisano, G.; Ilharco, L.M.; Pagliaro, M. Silica-based sol-gel coatings: A critical perspective from a practical viewpoint. In Biobased and Environmental Benign Coating; Tiwari, A., Galanis, A., Soucek, M.D., Eds.; Wiley: New York, NY, USA, 2016. [Google Scholar]
- Rottman, C.; Grader, G.; Avnir, D. Polarities of sol-gel-derived Ormosils and of their interfaces with solvents. Chem. Mater. 2001, 13, 3631. [Google Scholar] [CrossRef]
- Walsh, R. Bond dissociation energies in organosilicon compounds. In Silicon Compounds: Silanes and Silicones, 3rd ed.; Arkles, B., Larson, G., Eds.; Gelest: Morrisville, PA, USA, 2013; pp. 163–166. [Google Scholar]
- Schueremans, L.; van Gemert, D.; Giessle, S. Deterioration in quay-wall structures and effect of hydrophobic treatment. In Proceedings of the International Forum on Engineering Decision Making Second IFED Forum, Lake Louise, AL, Canada, 26–29 April 2006. [Google Scholar]
- Mirapakon. FC105—Imperium Aqua. 2018. Available online: https://drive.google.com/file/d/1dtlOG9TF9TpPiDXc_fx3SlsYnsIytgHE/view (accessed on 23 June 2020).
- Pagliaro, M.; Ciriminna, R.; Man, M.W.C.; Campestrini, S. Better chemistry through ceramics: The physical bases of the outstanding chemistry of ORMOSIL. J. Phys. Chem. B 2006, 110, 1976. [Google Scholar] [CrossRef]
- Gunari, N.; Brewer, L.H.; Bennett, S.M.; Sokolova, A.; Kraut, N.D.; Finlay, J.A.; Meyer, A.E.; Walker, G.C.; Wendt, D.E.; Callow, M.E.; et al. The control of marine biofouling on xerogel surfaces with nanometer-scale topography. Biofouling 2011, 27, 137. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.C.; Jitianu, A. Organic-inorganic hybrid melting gels. J. Sol Gel Sci. Technol. 2010, 55, 86. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ciriminna, R.; Ilharco, L.M.; Pagliaro, M. Role of the alkyl-alkoxide precursor on the structure and catalytic properties of hybrid sol-gel catalysts. Chem. Mater. 2005, 17, 6686. [Google Scholar] [CrossRef]
- Lana, S.L.B.; Seddon, A.B. X-Ray diffraction studies of sol-gel derived ORMOSILs based on combinations of tetramethoxysilane and trimethoxysilane. J. Sol Gel Sci. Technol. 1998, 13, 461. [Google Scholar] [CrossRef]
- Rios, X.; Moriones, P.; Echeverría, J.C.; Luquín, A.; Laguna, M.; Garrido, J.J. Characterisation of hybrid xerogels synthesised in acid media using methyltriethoxysilane (MTEOS) and tetraethoxysilane (TEOS) as precursors. Adsorption 2011, 17, 583. [Google Scholar] [CrossRef]
- Harreld, J.; Ebina, T.; Tsubo, N.; Stucky, G. Manipulation of pore size distributions in silica and ormosil gels dried under ambient pressure conditions. J. Non Cryst. Solids 2002, 298, 241. [Google Scholar] [CrossRef]
- Deshpande, R.; Hua, D.-W.; Smith, D.M.; Brinker, C.J. Pore structure evolution in silica gel during aging/drying. III. Effects of surface tension. J. Non Cryst. Solids 1992, 144, 32. [Google Scholar] [CrossRef]
- Chou, T.P.; Cao, G. Adhesion of Sol-Gel-derived organic-inorganic hybrid coatings on polyester. J. Sol Gel Sci. Technol. 2003, 27, 31. [Google Scholar] [CrossRef]
- Chastain, J. Air Quality Improves Food Quality. Food, Quality & Safety. 21 November 2018. Available online: http://www.foodqualityandsafety.com/article/air-quality-improves-food-quality (accessed on 23 June 2020).
- Ciriminna, R.; Pecoraino, M.; Meneguzzo, F.; Pagliaro, M. Solar air heating and ventilation in buildings: A key component in the forthcoming renewable-only energy mix. Energy Technol. 2017, 5, 1165. [Google Scholar] [CrossRef]
- Shrubsole, C.; Hamilton, I.G.; Zimmermann, N.; Papachristos, G.; Broyd, T.; Burman, E.; Mumovic, D.; Zhu, Y.; Lin, B.; Davies, M. Bridging the gap: The need for a systems thinking approach in understanding and addressing energy and environmental performance in buildings. Indoor Built Environ. 2018, 28, 100. [Google Scholar] [CrossRef]
- Evonik. Protectosil—Your Partner in Building Protection. 2020. Available online: http://www.protectosil.com/product/dynasylan/downloads/protectosil-building-protection-en.pdf (accessed on 23 June 2020).
- Giessler, S.; Standke, B.; Büchler, M. A new silane system for corrosion reduction of steel reinforced concrete. In Hydrophobe IV 4th International Conference on Water Repellent Treatment of Building Materials; Aedificatio Publishers: Unterengstringen, Switzerland, 2005; pp. 17–26. [Google Scholar]
- Wacker Chemie, Silres BS—Preserving and Enhancing Natural Beauty. 2020. Available online: https://www.wacker.com/h/en-us/medias/7417-EN.pdf (accessed on 23 June 2020).
- Technavio. Global Silanes Market 2018–2022, London: 2018. Available online: http://www.technavio.com/report/global-silanes-market-analysis-share-2018 (accessed on 23 June 2020).
- Fukaya, N.; Choi, S.J.; Horikoshi, T.; Kataoka, S.; Endo, A.; Kumai, H.; Hasegawa, M.; Sato, K.; Cho, J.-C. Direct synthesis of tetraalkoxysilane from silica and alcohol. New J. Chem. 2017, 41, 2224. [Google Scholar] [CrossRef]
- Pandarus, V.; Ciriminna, R.; Gingras, G.; Béland, F.; Pagliaro, M.; Kaliaguine, S. Waste-free and efficient hydrosilylation of olefins. Green Chem. 2019, 21, 129. [Google Scholar] [CrossRef]
- Hancok, S.; Walsh, E. Beyond knowledge and skills: Rethinking the development of professional identity during the STEM doctorate. Stud. High. Educ. 2016, 41, 37. [Google Scholar] [CrossRef]
- Pagliaro, M. Chemistry education fostering creativity in the digital era. Isr. J. Chem. 2019, 59, 565. [Google Scholar] [CrossRef]
- Dunnion, J.; O’Donovan, B. Systems thinking and higher education: The vanguard method. Syst. Pract. Action Res. 2014, 27, 23. [Google Scholar] [CrossRef]
- Hurst, G.A. Systems thinking approaches for international green chemistry education. Curr. Opin. Green Sustain. Chem. 2020, 21, 93. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).