Photodegradation of 1-Butyl-3-methylimidazolium Chloride [Bmim]Cl via Synergistic Effect of Adsorption–Photodegradation of Fe-TiO2/AC
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Characterization of the Photocatalyst
2.4. Photodegradation Study
3. Results and Discussion
3.1. Characterization of the Photocatalysts
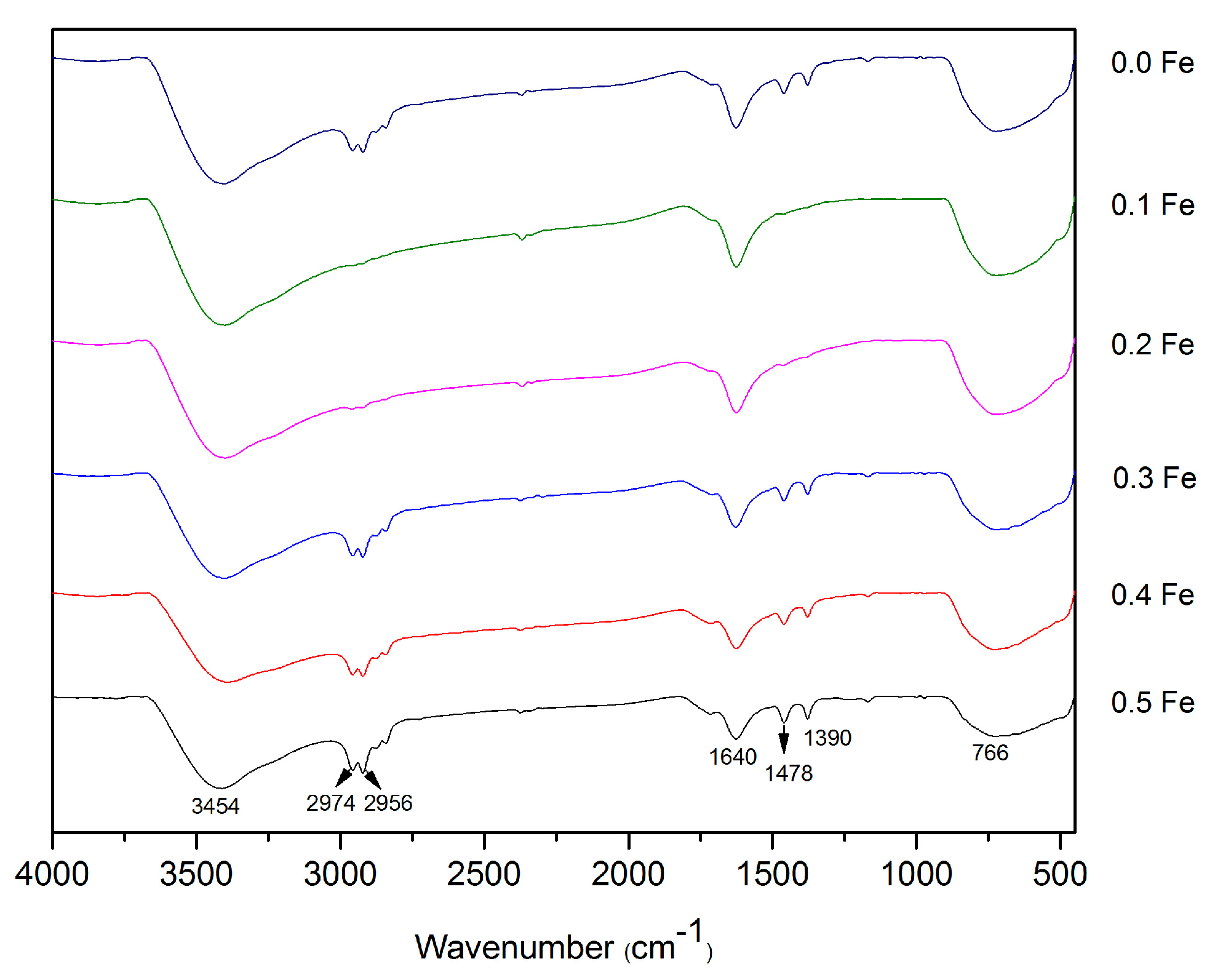
3.1.1. Fourier Transform Infrared (FTIR)
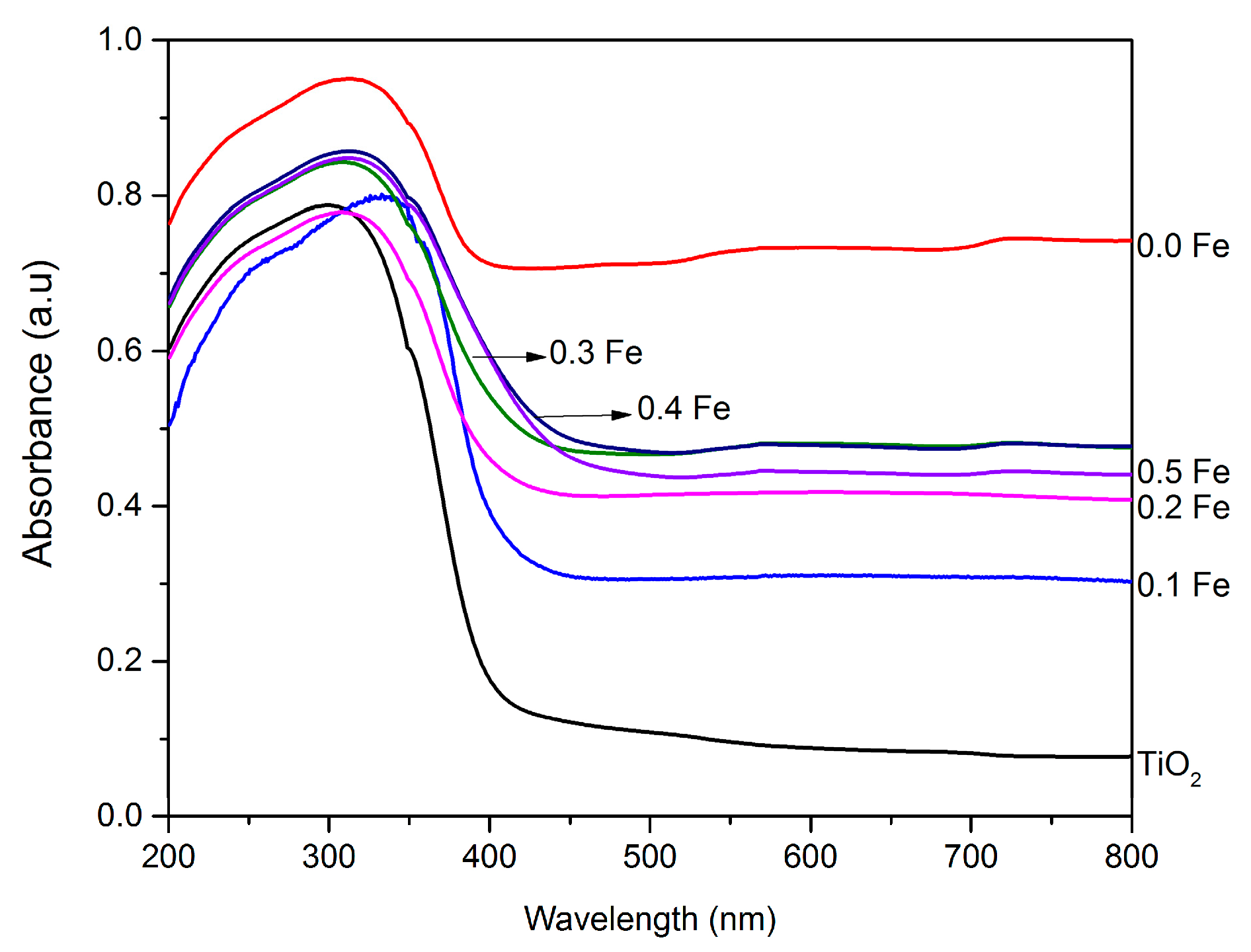
3.1.2. Diffuse Reflectance UV-Visible (DRUV-Vis) Analysis
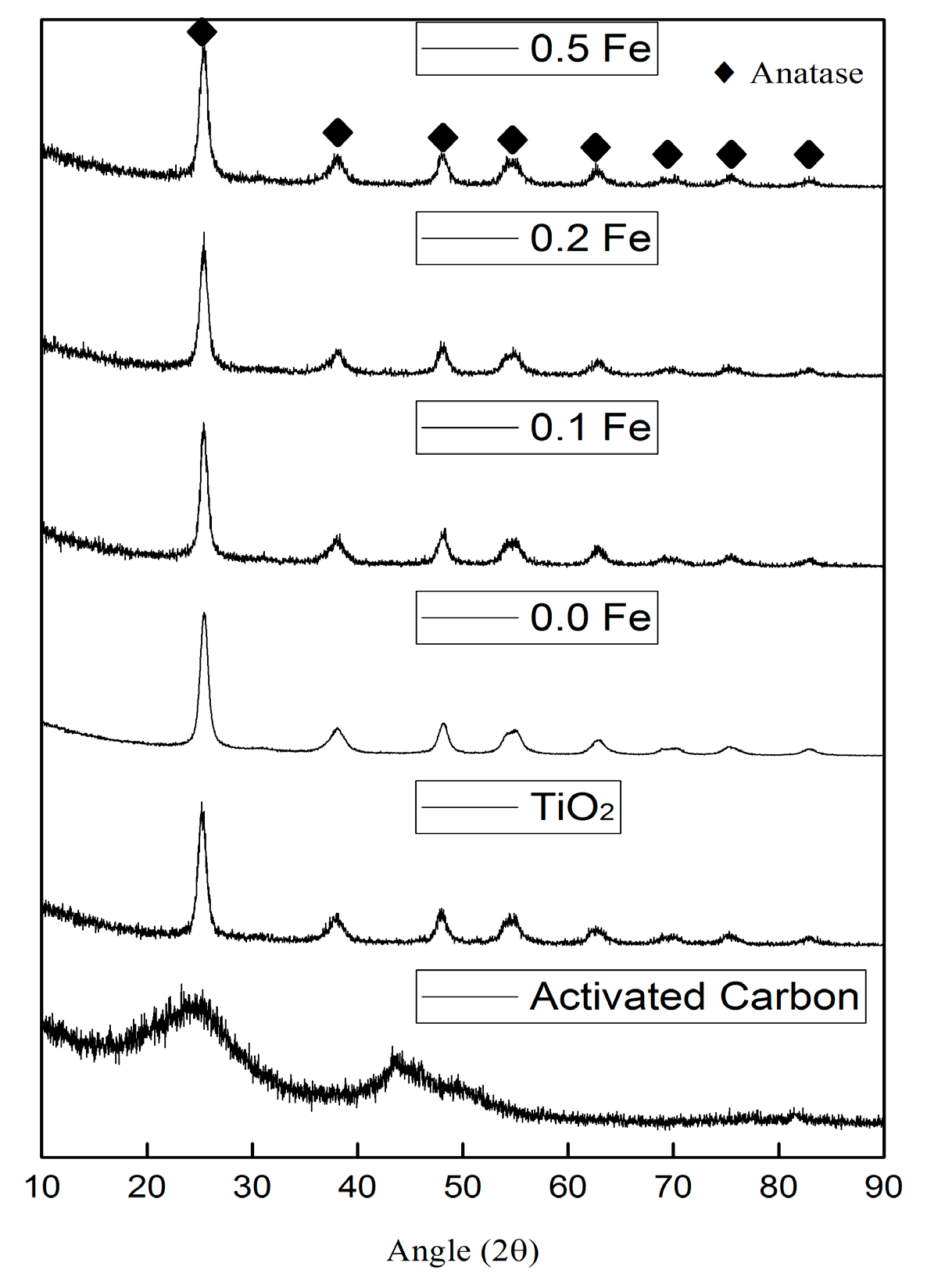
3.1.3. X-ray Diffraction (XRD)
3.2. Photodegradation Study
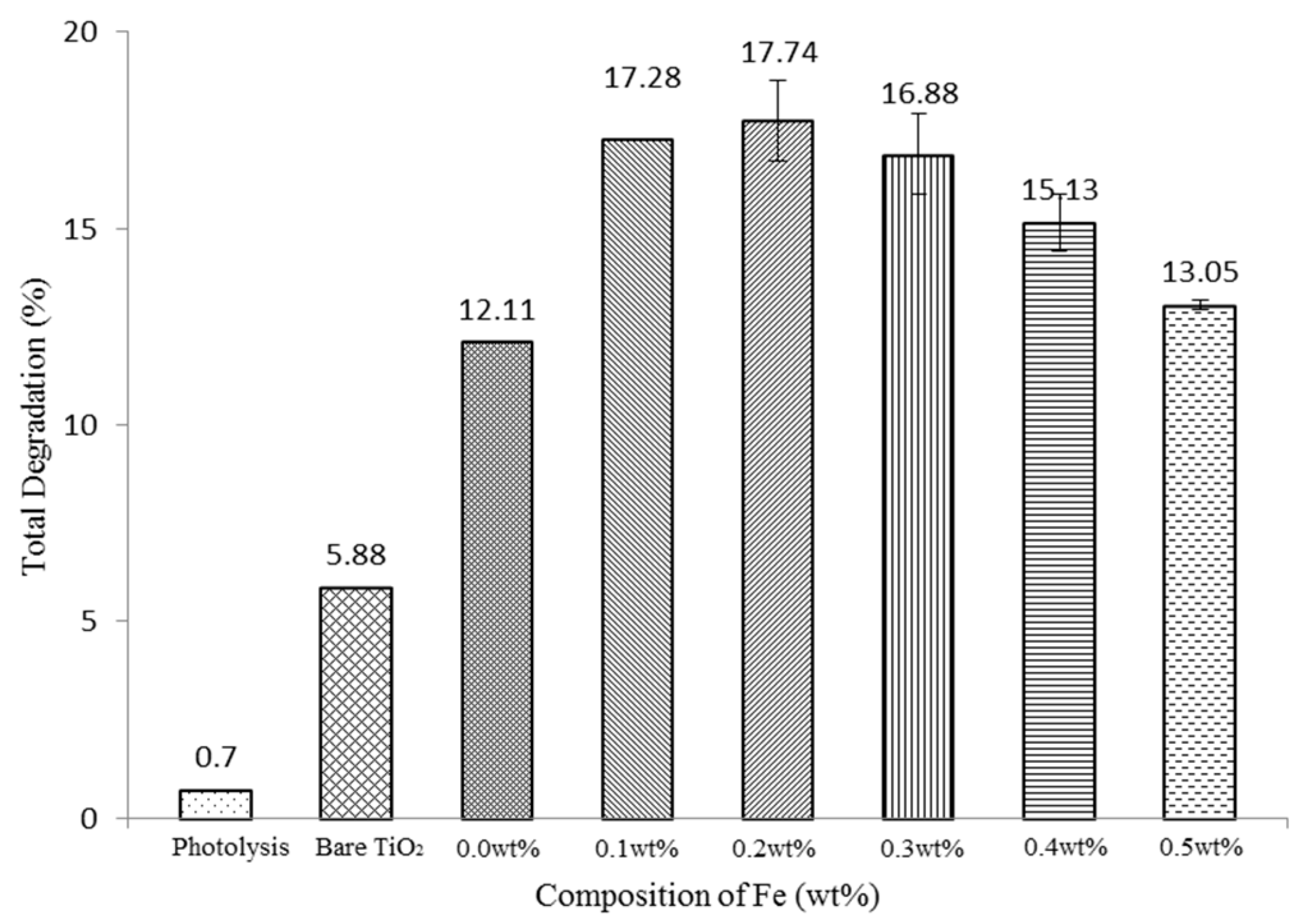
3.2.1. Effect of Fe Composition
3.2.2. Effect of Extrinsic Factor: Initial Concentration of [bmim]Cl (ILo)
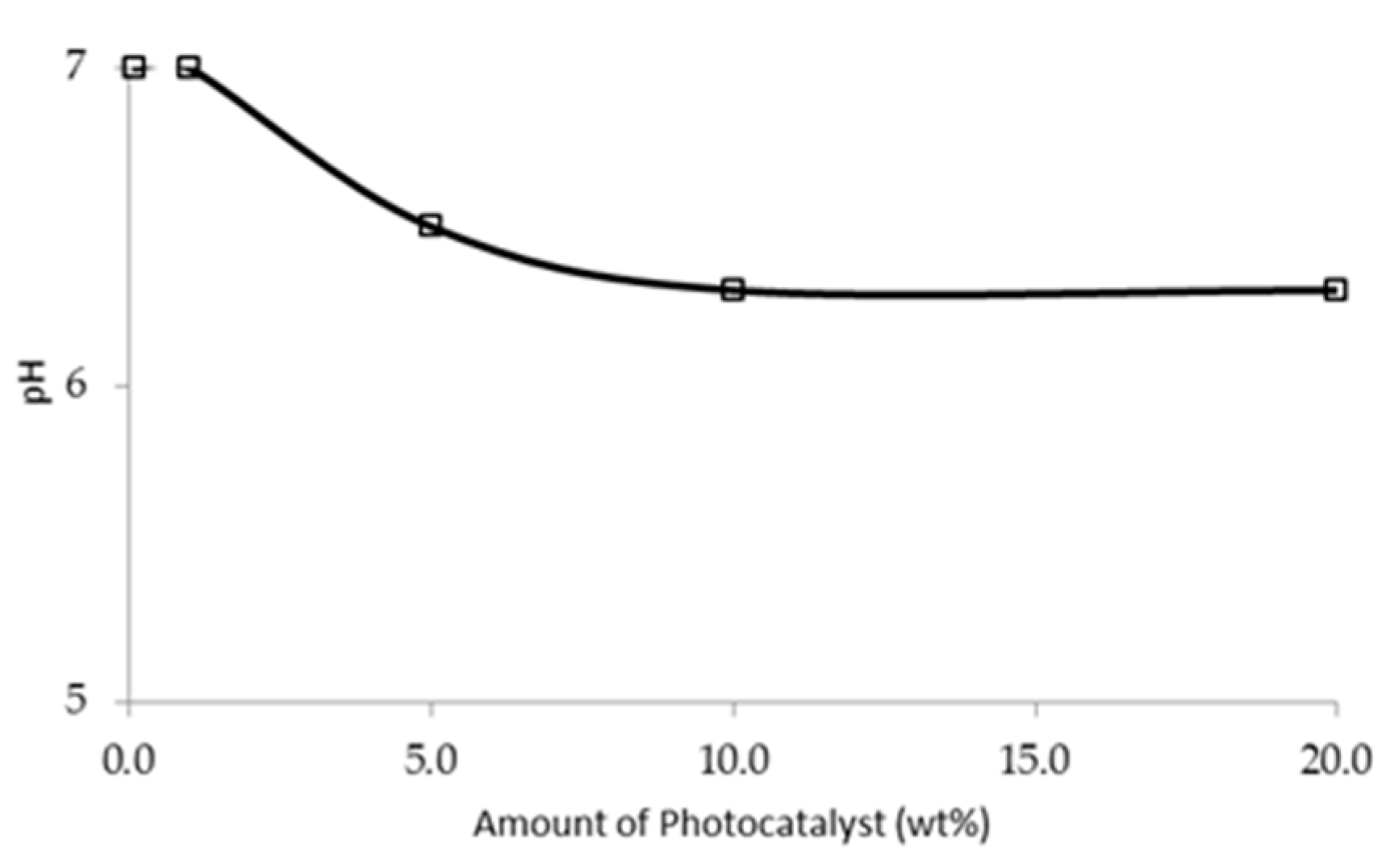
3.2.3. Effect of Extrinsic Factor: Initial pH of the [bmim]Cl Solution
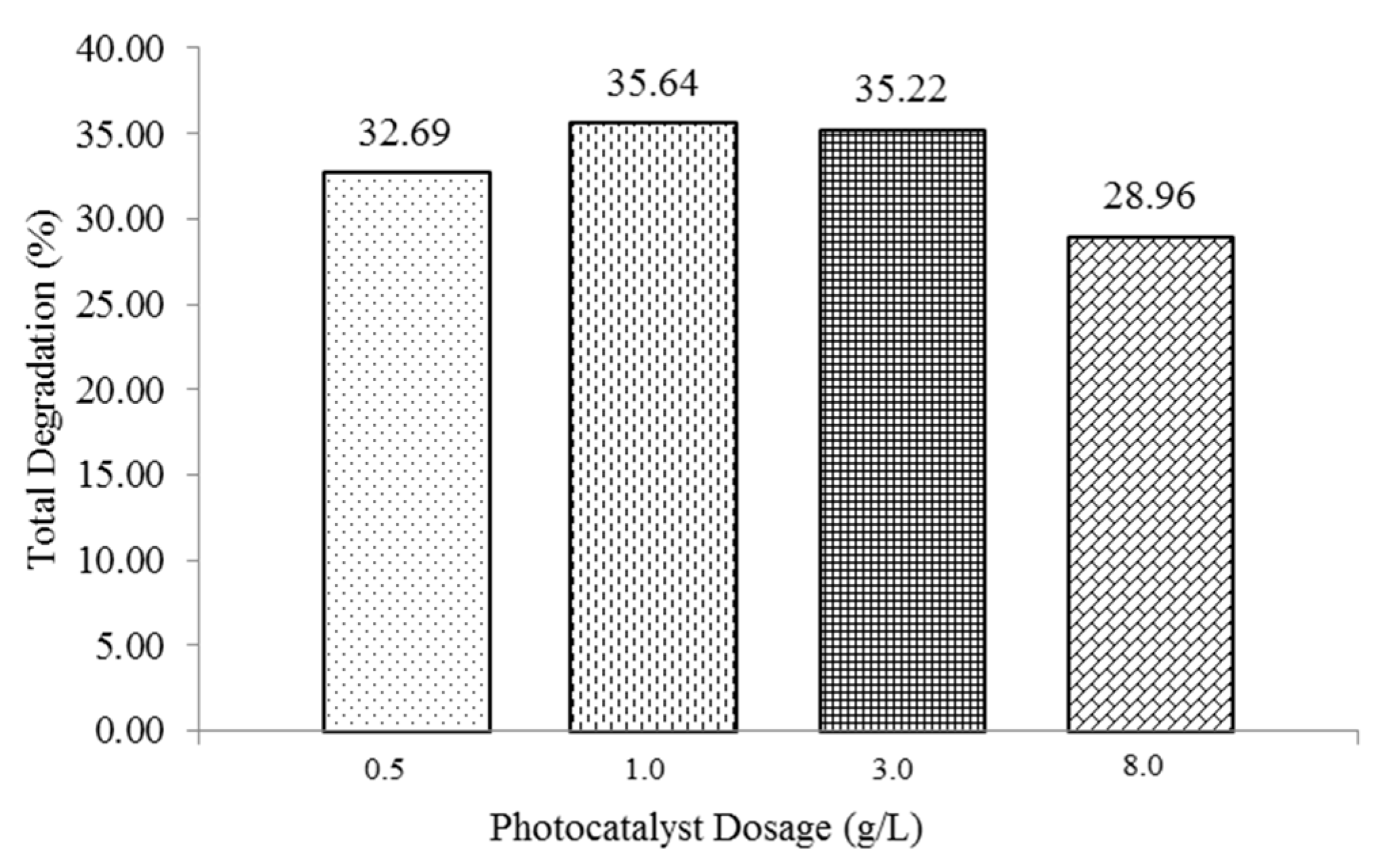
3.2.4. Effect of Extrinsic Factor: Dosage of Photocatalyst [Photocatalyst]
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Ionic liquids and catalysis: Recent progress from knowledge to applications. Appl. Catal. A Gen. 2010, 373, 1–56. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Siedlecka, E.M.; Fabiańska, A.; Stolte, S.; Nienstedt, A.; Ossowski, T.; Stepnowski, P.; Thöming, J. Electrocatalytic Oxidation of 1-Butyl-3-Methylimidazolium Chloride: Effect of the Electrode Material. Int. J. Electrochem. Sci. 2013, 8, 5560–5574. [Google Scholar]
- Munoz, M.; Domínguez, C.M.; de Pedro, Z.M.; Quintanilla, A.; Casas, J.A.; Rodriguez, J.J. Ionic liquids breakdown by Fenton oxidation. Catal. Today 2015, 240, 16–21. [Google Scholar] [CrossRef]
- Zhou, H.; Shen, Y.; Lv, P.; Wang, J.; Li, P. Degradation pathway and kinetics of 1-alkyl-3-methylimidazolium bromides oxidation in an ultrasonic nanoscale zero-valent iron/hydrogen peroxide system. J. Hazard. Mater. 2015, 284, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Buthiyappan, A.; Abdul Aziz, A.R.; Wan Daud, W.M.A. Recent advances and prospects of catalytic advanced oxidation process in treating textile effluents. Rev. Chem. Eng. 2016, 32, 1–47. [Google Scholar] [CrossRef]
- Ramli, R.M.; Chong, F.K.; Omar, A.A. Visible-Light Photodegradation of Diisopropanolamine Using Bimetallic Cu-Fe/TiO2 Photocatalyst. Adv. Mater. Res. 2013, 845, 421–425. [Google Scholar] [CrossRef]
- Ramli, R.M.; Kait, C.F.; Omar, A.A. Remediation of DIPA contaminated wastewater using visible light active bimetallic Cu-Fe/TiO2 Photocatalyst. Procedia Eng. 2016, 148, 508–515. [Google Scholar] [CrossRef]
- Saepurahman; Abdullah, M.A.; Chong, F.K. Preparation and characterization of tungsten-loaded titanium dioxide photocatalyst for enhanced dye degradation. J. Hazard. Mater. 2010, 176, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Ramli, R.M.; Kait, C.F.; Omar, A.A. Photodegradation of aqueous diisopropanolamine using Cu/TiO2: Effect of calcination temperature and duration. Appl. Mech. Mater. 2014, 625, 847–850. [Google Scholar] [CrossRef]
- Orha, C.; Pode, R.; Manea, F.; Lazau, C.; Bandas, C. Titanium dioxide-modified activated carbon for advanced drinking water treatment. Process Saf. Environ. Prot. 2016, 108, 26–33. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Pastrana-Martinez, L.M.; Silva, A.M.T.; Buijnsters, J.G.; Han, C.; Silva, C.G.; Carabineiro, S.A.C.; Dionysiou, D.D.; Faria, J.L. Nanodiamond-TiO2 composites for photocatalytic degradation of microcystin-LA in aqueous solutions under simulated solar light. RSC Adv. 2015, 5, 58363–58370. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Carabineiro, S.A.C.; Buijnsters, J.G.; Faria, J.L.; Figueiredo, J.L.; Silva, A.M.T. Nanodiamond–TiO2 Composites for Heterogeneous Photocatalysis. ChemPlusChem 2013, 78, 801–807. [Google Scholar] [CrossRef]
- Zhang, D. Enhanced photocatalytic activity for titanium dioxide by co-modification with copper and iron. Transit. Met. Chem. 2010, 35, 933–938. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, P.; Liu, W. Anatase TiO2 Nanospindle/ActivatedCarbon (AC) Composite Photocatalysts with Enhanced Activity in Removal of Organic Contaminant. Int. J. Photoenergy 2012. [Google Scholar] [CrossRef]
- Ozkal, C.B.; Koruyucu, A.; Meric, S. Heterogeneous photocatalytic degradation, mineralization and detoxification of ampicillin under varying pH and incident photon flux conditions. Desalin. Water Treat. 2016, 57, 18391–18397. [Google Scholar] [CrossRef]
- Zawawi, A.; Ramli, R.M.; Harun, N.Y. Synergistic Effect Of Adsorption-Photodegradation Of Composite TiO2/AC For Degradation Of 1-Butyl-3-Methylimidazolium Chloride. Malays. J. Anal. Sci. 2017, in press. [Google Scholar]
- Di Paola, A.; Garcı́a-López, E.; Marcı̀, G.; Martı́n, C.; Palmisano, L.; Rives, V.; Maria Venezia, A. Surface characterisation of metal ions loaded TiO2 photocatalysts: structure–activity relationship. Appl. Catal. B Environ. 2004, 48, 223–233. [Google Scholar] [CrossRef]
- Alalm, M.G.; Tawfik, A.; Ookawara, S. Enhancement of photocatalytic activity of TiO2 by immobilization on activated carbon for degradation of pharmaceuticals. J. Environ. Chem. Eng. 2016, 4, 1929–1937. [Google Scholar] [CrossRef]
- Muhamma, A.S.; Naser, J.T.; Kirm, I.; Jibril, B.Y. Photocatalytic Degradation of Methylene Blue and Phenol Using TiO2/Activated-Carbon Composite Catalysts. Asian J. Chem. 2015, 1, 343–348. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Agarwal, K.; Singh, A.K.; Polke, B.G.; Raha, K.C. Characterization of γ- and α-Fe2O3 nano powders synthesized by emulsion precipitation-calcination route and rheological behaviour of α-Fe2O3. Int. J. Eng. Sci. Technol. 2010, 2, 118–126. [Google Scholar]
- Wang, Y.; Muramatsu, A.; Sugimoto, T. FTIR analysis of well-defined α-Fe2O3 particles. Colloids Surf. A Physicochem. Eng. Asp. 1998, 134, 281–297. [Google Scholar] [CrossRef]
- Oh, W.C.; Chen, M.L. Formation of TiO2 composites on activated carbon modified by nitric acid and their photocatalytic activity. J. Ceram. Process. Res. 2007, 8, 316–323. [Google Scholar]
- Wang, X.; Liu, Y.; Hu, Z.; Chen, Y.; Liu, W.; Zhao, G. Degradation of methyl orange by composite photocatalysts nano-TiO2 immobilized on activated carbons of different porosities. J. Hazard. Mater. 2009, 169, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, Z.; Tian, F.; Ye, B.-C.; Tong, Y. Synthesis of N and La co-doped TiO2/AC photocatalyst by microwave irradiation for the photocatalytic degradation of naphthalene. J. Alloys Compd. 2016, 676, 489–498. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Y.; Ma, X.; Li, F.; Liu, D.; Chen, Z.; Zhang, F.; Dionysiou, D.D. Microwave degradation of methyl orange dye in aqueous solution in the presence of nano-TiO2-supported activated carbon (supported-TiO2/AC/MW). J. Hazard. Mater. 2012, 209–210, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Andronic, L.; Enesca, A.; Cazan, C.; Visa, M. TiO2–active carbon composites for wastewater photocatalysis. J. Sol–Gel Sci. Technol. 2014, 71, 396–405. [Google Scholar] [CrossRef]
- Lemus, J.; Neves, C.M.; Marques, C.F.; Freire, M.G.; Coutinho, J.A.; Palomar, J. Composition and structural effects on the adsorption of ionic liquids onto activated carbon. Environ. Sci. Process Impacts 2013, 15, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Lemus, J.; Palomar, J.; Heras, F.; Gilarranz, M.A.; Rodriguez, J.J. Developing criteria for the recovery of ionic liquids from aqueous phase by adsorption with activated carbon. Sep. Purif. Technol. 2012, 97, 11–19. [Google Scholar] [CrossRef]
- Huang, D.; Miyamoto, Y.; Matsumoto, T.; Tojo, T.; Fan, T.; Ding, J.; Guo, Q.; Zhang, D. Preparation and characterization of high-surface-area TiO2/activated carbon by low-temperature impregnation. Sep. Purif. Technol. 2011, 78, 9–15. [Google Scholar] [CrossRef]
- Xing, B.; Shi, C.; Zhang, C.; Yi, G.; Chen, L.; Guo, H.; Huang, G.; Cao, J. Preparation of TiO2/Activated Carbon Composites for Photocatalytic Degradation of RhB under UV Light Irradiation. J. Nanomater. 2016. [Google Scholar] [CrossRef]
- Qamar, M.; Merzougui, B.; Anjum, D.; Hakeem, A.S.; Yamani, Z.H.; Bahnemann, D. Synthesis and photocatalytic activity of mesoporous nanocrystalline Fe-doped titanium dioxide. Catal. Today 2014, 230, 158–165. [Google Scholar] [CrossRef]
- Nasralla, N.; Yeganeh, M.; Astuti, Y.; Piticharoenphun, S.; Shahtahmasebi, N.; Kompany, A.; Karimipour, M.; Mendis, B.G.; Poolton, N.R.J.; Šiller, L. Structural and spectroscopic study of Fe-doped TiO2 nanoparticles prepared by sol–gel method. Sci. Iran. 2013, 20, 1018–1022. [Google Scholar]
- Yan, K.; Wu, G.; Jarvis, C.; Wen, J.; Chen, A. Facile synthesis of porous microspheres composed of TiO2 nanorods with high photocatalytic activity for hydrogen production. Appl. Catal. B Environ. 2014, 148, 281–287. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, X.; Chen, W.; Li, L.; Zen, M.; Qin, S.; Sun, S. Photodecolorization of Rhodamine B on tungsten-doped TiO2/activated carbon under visible-light irradiation. J. Hazard. Mater. 2012, 227, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Wu, G. Titanium Dioxide Microsphere-Derived Materials for Solar Fuel Hydrogen Generation. ACS Sustain. Chem. Eng. 2015, 3, 779–791. [Google Scholar] [CrossRef]
- Choi, H.-J.; Kang, M. Hydrogen production from methanol/water decomposition in a liquid photosystem using the anatase structure of Cu loaded TiO2. Int. J. Hydrogen Energy 2007, 32, 3841–3848. [Google Scholar] [CrossRef]
- Obregón, S.; Lee, S.W.; Rodríguez-González, V. Loading effects of silver nanoparticles on hydrogen photoproduction using a Cu-TiO2 photocatalyst. Mater. Lett. 2016, 173, 174–177. [Google Scholar] [CrossRef]
- Siedlecka, E.M.; Mrozik, W.; Kaczynski, Z.; Stepnowski, P. Degradation of 1-butyl-3-methylimidazolium chloride ionic liquid in a Fenton-like system. J. Hazard. Mater. 2008, 154, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.N.; Wong, C.L.; Mohamed, A.R. An Overview on the Photocatalytic Activity of Nano-Doped-TiO2 in the Degradation of Organic Pollutants. ISRN Mater. Sci. 2011, 1–18. [Google Scholar] [CrossRef]
- Melcher, J.; Feroz, S.; Bahnemann, D. Comparing photocatalytic activities of commercially available iron-doped and iron-undoped aeroxide TiO2 P25 powders. J. Mater. Sci. 2017, 52, 6341–6348. [Google Scholar] [CrossRef]
- Senee, K.; Ratchaneekorn, W. Studies on preparation and characterization of Fe/TiO2 catalyst in photocatalysis applications. Mater. Res. Express 2017, 4, 076507. [Google Scholar]
- Hassena, H. Photocatalytic Degradation of Methylene Blue by Using Al2O3/Fe2O3 Nano Composite under Visible Light. Mod. Chem. Appl. 2016, 176. [Google Scholar] [CrossRef]
- Alkaim, A.F.; Kandiel, T.A.; Hussein, F.H.; Dillert, R.; Bahnemann, D.W. Enhancing the photocatalytic activity of TiO2 by pH control: a case study for the degradation of EDTA. Catal. Sci. Technol. 2013, 3, 3216–3222. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: a review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Andriantsiferana, C.; Mohamed, E.F.; Delmas, H. Photocatalytic degradation of an azo-dye on TiO2/activated carbon composite material. Environ. Technol. 2013, 35, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Belver, C.; Bedia, J.; Álvarez-Montero, M.A.; Rodriguez, J.J. Solar photocatalytic purification of water with Ce-doped TiO2/clay heterostructures. Catal. Today 2016, 266, 36–45. [Google Scholar] [CrossRef]


| Photocatalyst | Band-Gap Energy, Eg (eV) |
|---|---|
| TiO2 | 3.10 |
| 0.0Fe | 2.48 |
| 0.1Fe | 2.88 |
| 0.2Fe | 2.64 |
| 0.3Fe | 2.61 |
| 0.4Fe | 2.39 |
| 0.5Fe | 2.36 |
| Photocatalyst | Particle Size (nm) | Crystallographic Constant (Å) |
|---|---|---|
| TiO2 | 8.30 | a = 3.8000, b = 3.8000, c = 9.4600 |
| AC | 29.30 | a = 2.4700, b = 2.4700, c = 6.9300 |
| 0.0Fe | 24.20 | a = 3.8000, b = 3.8000, c = 9.5020 |
| 0.1Fe | 10.30 | a = 3.7850, b = 3.7850, c = 9.5010 |
| 0.2Fe | 10.70 | a = 3.7850, b = 3.7850, c = 9.4440 |
| 0.5Fe | 11.20 | a = 3.7850, b = 3.7850, c = 9.4820 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawawi, A.; Ramli, R.M.; Yub Harun, N. Photodegradation of 1-Butyl-3-methylimidazolium Chloride [Bmim]Cl via Synergistic Effect of Adsorption–Photodegradation of Fe-TiO2/AC. Technologies 2017, 5, 82. https://doi.org/10.3390/technologies5040082
Zawawi A, Ramli RM, Yub Harun N. Photodegradation of 1-Butyl-3-methylimidazolium Chloride [Bmim]Cl via Synergistic Effect of Adsorption–Photodegradation of Fe-TiO2/AC. Technologies. 2017; 5(4):82. https://doi.org/10.3390/technologies5040082
Chicago/Turabian StyleZawawi, Azhar, Raihan Mahirah Ramli, and Noorfidza Yub Harun. 2017. "Photodegradation of 1-Butyl-3-methylimidazolium Chloride [Bmim]Cl via Synergistic Effect of Adsorption–Photodegradation of Fe-TiO2/AC" Technologies 5, no. 4: 82. https://doi.org/10.3390/technologies5040082
APA StyleZawawi, A., Ramli, R. M., & Yub Harun, N. (2017). Photodegradation of 1-Butyl-3-methylimidazolium Chloride [Bmim]Cl via Synergistic Effect of Adsorption–Photodegradation of Fe-TiO2/AC. Technologies, 5(4), 82. https://doi.org/10.3390/technologies5040082






