Abstract
In the realm of scaffold-free cell therapies, there is a questto develop organotypic three-dimensional (3D) tissue surrogates in vitro, capitalizing on the inherent ability of cells to create tissues with an efficiency and sophistication that still remains unmatched by human-made devices. In this study, we explored the properties of scaffolds obtained by the electrospinning of a thermosensitive copolymer, poly(N-isopropylacrylamide-co-N-tert-butylacrylamide) (P(NIPAM-co-NtBA)), intended for use in such therapies. Two copolymers with molecular weights of 123 and 137 kDa and a content of N-tert-butylacrylamide of ca. 15 mol% were utilized to generate 3D scaffolds via electrospinning. We examined the morphology, solution viscosity, porosity, and thickness of the spun matrices as well as the mechanical properties and hydrophobic–hydrophilic characteristics of the scaffolds. Particular attention was paid to studying the influence of the thermosensitive polymer’s molecular weight and dispersity on the resultant scaffolds’ properties and the role of electroforming parameters on the morphology and mechanical characteristics of the scaffolds. The cytotoxicity of the copolymers and interaction of cells with the scaffolds were also studied. Our findings provide significant insight into approaches to optimizing scaffolds for specific cell cultures, thereby offering new opportunities for scaffold-free cell therapies.
1. Introduction
Cell-based therapies represent a promising path for regenerative medicine, aiming to harness the body’s inherent capacity to restore tissues [1,2,3]. As such, the development of scaffold-free three-dimensional (3D) tissue surrogates has become a critical field of study. Thermoresponsive polymers have gained significant interest in the scientific community due to their unique behavior, specifically their reversible coil-to-globule transition at the upper or lower critical solution temperature (UCST or LCST, respectively) [4,5,6]. This feature makes them particularly attractive for various biomedical and pharmaceutical applications, such as drug and gene delivery, tissue engineering, sensors, microarrays, and imaging devices [7,8,9,10,11,12].
The central mechanism that governs the phase transition in water-soluble thermoresponsive polymers with an LCST is the interplay of molecular interactions, specifically the hydrogen bonding between the solvent molecules and the hydrophilic segments of the polymer chains, as well as hydrophobic hydration [13,14]. At low temperatures, these groups form hydrogen bonds with water molecules, while hydrophobic groups prompt the ordering of water molecules in the hydration shell, allowing the polymers to be highly soluble in aqueous solutions. As the temperature rises, the hydrogen bonds and ordered water aggregates start to break down, leading to increased intramolecular interactions within the polymer chains and the subsequent collapse into a globular state [7].
PNIPAM, with a physiologically relevant LCST of approximately 32 °C in aqueous solutions, is one of the most extensively researched thermoresponsive polymers [4,15,16]. Its LCST allows for intriguing behavior changes around the human body temperature, making it an attractive candidate for various biomedical applications. These potential applications include drug delivery systems, theranostic devices, wound dressings, and, pertinent to our study, tissue engineering [17,18,19,20].
In the field of cell culture and tissue engineering, PNIPAM and its copolymers operate based on the following sequence of events: cell adhesion when the temperature is above the LCST, cell growth forming a 2D confluent layer, and detachment of the entire cell layer when the temperature is lowered below the LCST [21,22,23,24]. This thermoresponsive behavior has proven itself to be less harmful to cells compared to conventional cell detachment methods, such as mechanical scraping or enzymatic treatment, and provides a compelling argument for using these polymers as scaffolds in tissue engineering.
In light of these benefits, the use of thermoresponsive polymers has extended into composite material development [9,25,26,27]. For instance, a composition with PNIPAM and silk sericin has been used as a support for thermoregulated cell attachment and detachment [28,29,30] and also as a multi-polymer coating on cotton fabric for tissue engineering and sustained drug release [31]. Notably, throughout the composition of PNIPAM–silk sericin, almost no changes in the transition temperature were found, despite the high hydrophilicity of silk sericin.
Moreover, nanofiber/hydrogel composites with high mechanical strengths prepared using these polymers have been widely used for cartilage tissue engineering. For example, a poly(ethylene glycol)-poly(N-isopropyl acrylamide)/poly(caprolactone) (PEG-PNIPAAm)/PCL nanofiber/hydrogel scaffold promoted the chondrogenic differentiation of human mesenchymal stem cells (hMSCs), had improved mechanical properties, enabled the 3D growth of cells in nanofibrous hydrogel structures, and promoted cartilage repair [32]. In a similar vein, Kim et al. developed a novel HA nanofibrous hydrogel scaffold as a platform for MSCs to be used in cartilage regeneration [33].
Our research focuses on using thermoresponsive polymers, specifically copolymers of N-isopropylacrylamide (NIPAM) and N-tert-butylacrylamide (NtBA), as a basis for developing advanced scaffolds for tissue engineering. The addition of hydrophobic fragments to a PNIPAM chain was shown to lower the LCST [34]—which simplifies the handling of the copolymer at ambient temperatures—and also to improve cell adhesion and further detachment [35]. Recently, temperature-responsive electrospun scaffolds based on poly(N-isopropylacrylamide-co-N-tert-butylacrylamide (P(NIPAM-co-NtBA)) demonstrated good applicability in the production of cell sheets [36]. In the present work, we studied the influence of the thermosensitive polymer’s molecular weight and dispersity (Đ) on the resultant scaffolds and the role of electroforming parameters on the morphology and mechanical characteristics of the scaffolds. Leveraging the thermoresponsive properties of the polymers, we suggest that the produced scaffolds could offer an optimized solution that would promote efficient cell attachment and growth followed by a gentle detachment process.
Our overarching aim is to contribute to the advancement of tissue engineering, making the creation of 3D tissue substitutes more accessible and readily integrated into the body and thereby minimizing the reliance on external support materials. This progress could represent a significant leap in regenerative medicine, edging us closer to the reality of creating functional tissues for therapeutic purposes.
2. Materials and Methods
2.1. Synthesis of Thermoresponsive Copolymers
Copolymers of N-isopropylacrylamide (NIPAM) with N-tert-butylacrylamide (NtBA) were synthesized by free radical polymerization using azobisisobutyronitrile (AIBN) as an initiator in benzene at 60 °C for 24 h according to the procedure described in [37]. Then, the reaction mixture was precipitated in n-hexane. The obtained copolymers were then purified by dissolving in acetone followed by precipitation in n-hexane at least three times, and the product was dried at 45 °C in a vacuum oven. The composition of prepared poly(N-isopropylacrylamide-co-N-tert-butylacrylamide) was calculated based on 1H NMR spectroscopy according to the following equation:
where Ia and Ic are the intensities of the protons of methyl group in N-isopropylacrylamide (a) and N-tert-butylacrylamide (c) units in the copolymer chain (Figure S1).
2.2. Analysis of the Synthesized Copolymers
Gel permeation chromatography (GPC): The number-average molecular weight (Mn), weight-average molecular weight (Mw), and dispersity (Đ = Mw/Mn, also known as the polydispersity index) of the synthesized copolymers were determined using gel permeation chromatography (GPC) with an Ultimate 3000 Thermo Scientific instrument equipped with a PLgel pre-column (Agilent, dimensions 7.5 × 50 mm, particle size 5 μm) and a PLgel MIXED-C column (Agilent, dimensions 7.5 × 300 mm, particle size 5 μm). The column and pre-column were thermostated at 50 °C. The flow rate of the eluent, dimethylformamide containing 0.1 M LiBr, was set to 1.0 mL/min. Molecular weights were calculated using the Chromeleon 7.0 software (Thermo Scientific Dionex, Waltham, MA, USA) and poly(methyl methacrylate) standards (ReadyCal Kit, PSS GmbH) with Mw/Mn ≤ 1.05.
1H NMR Spectroscopy: 1H NMR spectra of copolymer solutions in CDCl3 were recorded with a Bruker AC-500 instrument operating at a frequency of 500 MHz at 25 °C.
Differential scanning calorimetry (DSC): The LCSTs of 1% aqueous solutions of copolymers were determined using a NETZSCH STA 449 F3 Jupiter® instrument. Samples were heated and cooled at a rate of 2.0 °C/min from 0 to 60 °C. The thermal properties of the copolymer solutions were calculated using NETZSCH Proteus® 8.0 software (NETZSCH, Selb, Germany).
Turbidimetry: The transmittance of 1% aqueous solutions of copolymers was measured using a Victor Nivo multimode microplate reader (Perkin Elmer, Waltham, MA, USA) at a wavelength of 405 nm. The solution temperature was increased at a rate of 0.5 °C/min from 4 °C to 40 °C. The lower critical solution temperature was determined as the temperature at which the transmittance decreased by 50%.
2.3. Fabrication and Characterization of Scaffolds
In order to obtain defect-free fibrous nonwoven materials based on synthesized thermoresponsive P(NIPAM-co-NtBA) copolymers (NIPAM:NtBA = 85/15 and 86/14 mol%), the concentrations of the solutions for electrospinning were selected and their rheological properties were studied. For this purpose, the copolymers were dissolved in absolute ethanol, being stirred using an orbital shaker (Biosan, Rīga, Latvia) at 200–300 rpm for 5–8 h at a temperature of 4 °C. The viscosity (η, mPa*s) of the molding solutions based on P(NIPAM-co-NtBA) was measured using a Physica MCR 302 rheometer (Anton Paar, Graz, Austria) with a built-in temperature and gap control at a temperature of 25 °C. Viscosity dependencies on the shear rate were obtained, and the value at 300 s−1 is presented for the comparison between samples.
Fibrous non-woven materials were obtained with an ESR100D NanoNC electroforming setup (Seoul, South Korea) according to the following procedure: solutions of the copolymers, for which rheological properties were studied, were loaded into a 12 mL plastic syringe equipped with a 20 G diameter nozzle. Depending on the specific trial, the syringe was filled with either 1 mL or 3 mL of the solution. The distance between the needle and the dynamic collector coated with aluminum foil was set at either 15 cm (the average distance) or 23 cm (the maximum allowable distance within the chamber). Depending on the experimental conditions, the rotation speed of the dynamic collector was adjusted to 600, 1800, or 2400 rpm. Electroforming processes were conducted at room temperature and humidity not exceeding 50%. Electrospinning was performed at a voltage of 25.5 kV with a feed rate of 1.5 mL/h. Depending on the specific conditions of the experiment, the drawing chamber fan (forced airflow activation, FAA) was either activated or deactivated. The nozzle was oriented at an inclination of 30 degrees to the dynamic collector. This specific angle was empirically found to facilitate a more controlled and stable jet flow during the electrospinning process, optimizing the uniformity and homogeneity of the resultant scaffolds.
The investigation of scaffold properties after their detachment from the foil is an essential part of analyzing the electrospinning process. The study of scaffolds after their detachment from the foil substrate revealed an increase in their thickness. This may be associated with the scaffold material becoming looser and more flexible, leading to a slight “inflation.” The thickness is presented for the detached or attached scaffolds as noted further in the text. The areas deformed by tweezers or other tools during the separation of the scaffold from the foil were avoided during the analysis.
The mechanical properties were analyzed with a Mach-1 v500csst micromechanical testing system (Biomomentum Inc., Laval, QC, Canada). The tensile strength, elongation at break and elasticity modulus (Young’s modulus) were measured upon the uniaxial tension of at least three dog bone-shaped samples (12 × 5 mm, transverse section, the thickness was taken from the SEM analysis) and were reported as mean ± standard deviation values.
2.4. Characteristics of the Prepared Scaffolds
The morphology of scaffolds made from P(NIPAM-co-NtBA) copolymers was analyzed using an Hitachi TM4000Plus scanning electron microscope (Hitachi High-Technologies Corporation, Tokyo, Japan). The calculation of the fiber thickness of the prepared non-woven materials was carried out in the ImageJ program.
The hydrophilicity and hydrophobicity of the surface of non-woven materials were studied by measuring contact angles, which was carried out by the sessile drop method using an Acam-MSC01 device (Apex Instruments, Kolkata, India), with distilled water being used as a liquid. The temperatures of the instrument table and the liquid were 40 °C, since thermoresponsive polymers exhibit hydrophobic properties above the LCST (25–32 °C). The measurements were taken at least 5 times for each sample.
For the porosity analysis, the scaffolds of a size of 2 × 2 cm were cut and weighed. The porosity was calculated using the following Equation (1):
where ρscaffold is the density of the scaffold derived from its geometrical parameters and weight, and ρpolymer is the approximate density of the P(NIPAM-co-NtBA) copolymer, assumed equal to the density of PNIPAM, 1.1 g∙cm−3.
2.5. Analysis of the Copolymer Cytotoxicity
The synthesized P(NIPAM-co-NtBA) (85:15) copolymer was tested for cytotoxic properties. For this study, a culture of multipotent mesenchymal stromal cells from human bone marrow (hMSCs, 5 passage) in the exponential growth phase was used. The polymer was sterilized under UV radiation in a laminar cabinet for 2 h, dissolved in a cold Hank’s solution (BioLot, Moscow, Russia) at a concentration of 50 mg/mL, and left overnight at +4 °C until completely dissolved.
Cells were seeded in the wells of 24-well plates (Corning, Corning, NY, USA) in three replicates for each polymer concentration at a density of 25 × 103 cells/cm2 and cultured in complete growth medium for 24 h under standard conditions (37 °C, 5% CO2). After 24 h, the growth medium was replaced with a mixture of the medium and polymer solutions in a 4:1 ratio. Four concentrations of the polymer were used: 0.5, 1, 5, and 10 mg/mL. Cells in complete growth medium without polymer were used as controls. Cells were cultivated in the presence of the polymer for 24 h, then thoroughly washed three times with warm Hank’s solution to remove the residual polymer. After that, the following tests were conducted: quantitative determination of total DNA using the dsDNA Quant-iT™ PicoGreen™ kit (Invitrogen, Carlsbad, CA, USA) and assessment of metabolic activity using the Alamar Blue™ test (Invitrogen, Carlsbad, CA, USA).
The PicoGreen cytotoxicity test was conducted as follows: 250 µL of ddH2O were added to all wells with cells, and three freeze–thaw cycles were carried out (20 min at −80°, 30 min at room temperature) for cell membrane lysis. Then, 100 µL of cell lysates from each well were placed in duplicates into 96-well plates (Corning) and mixed with 100 µL of PicoGreen™ dye diluted in TE buffer provided by the manufacturer (Invitrogen). After 5 min of incubation in the dark, the fluorescence intensity in all wells was measured with a Victor Nivo multimode microplate reader (Perkin Elmer, Waltham, MA, USA) at an excitation wavelength of 480 nm, emission wavelength of 520 nm in three replicates for all samples. The obtained fluorescence intensity data were converted into the concentration of double-stranded DNA using a calibration curve (fluorescence intensities plotted against DNA concentrations in the range from 0 to 1000 ng/mL), obtained using DNA standards provided in the kit.
For the Alamar Blue cytotoxicity test, wells without cells were additionally used as a negative standard for the 0% metabolic activity. A total of 300 µL of 1 × Alamar Blue™ in Hank’s solution was added to each well. Plates were incubated under standard culturing conditions (37 °C, 5% CO2) for 2 h. Then, 100 µL of the dye solution from each well was transferred in duplicates to 96-well plates (Corning), and the fluorescence intensities in all wells were measured with a Victor Nivo multimode microplate reader (Perkin Elmer, Waltham, MA, USA) at an excitation wavelength of 560 nm and an emission wavelength of 590 nm in three replicates for all samples.
2.6. Analysis of the Cell Behavior and Viability on Thermoresponsive Scaffolds
In order to study the cell behavior on the matrices, fragments of fibrous matrices sized 2 × 2 cm were wrapped around a silicone ring with an inner diameter of 9.6 mm. This allowed the matrices to be held undeformed in warm solution as well as to create a “well” a few millimeters high, convenient for cell seeding. For this type of experiment and further visualization, 0.1% of Rhodamin C (25 mg/mL in DMSO, LenReactive, Saint Petersburg, Russia) was added to the P(NIPAM-co-NtBA) solution prior to electrospinning. The obtained constructs were placed in the wells of 24-well plates and stored in a dark dry place. Then, 24 h before seeding, the constructs were sterilized under UV light, were filled with warm (37 °C) growth medium with 10% FBS, and were incubated under standard conditions (37 °C, 5% CO2). All further procedures were carried out strictly using warm solutions over short periods of time in order to avoid a preliminary disruption of the matrix’s integrity.
REF52 cells were seeded on the obtained fibrous matrices. A cell suspension was obtained using Versene solution and 0.25% trypsin (BioLot), transferred to 15 mL tubes and centrifuged (7 min, 400× g). The resulting pellet was diluted in complete growth medium. The matrices were seeded with cells at a concentration of 1.5 × 105 cells/cm2—the volume of cell suspension for one construct was 200 µL—after which the plates were left in standard cultivation conditions (37 °C, 5% CO2) overnight. The next day, 1 mL of full growth medium was added to each well, then the medium was changed every 2–3 days.
After one week of cultivation, the cells were stained with CalceinAM (Sigma, Burlington, MA, USA) for 30 min for visualization of viable cells. The matrices were carefully removed from the silicone rings, inverted, and visualized with a CellInsight CX7 high-content screening system (ThermoFisher Scientific, Waltham, MA, USA) equipped with an incubator module. The images were acquired in a confocal mode (2 µm Z-step) with a 20× objective, in the green channel for cells (excitation: 485 nm; emission: 517 nm) and in the red channel for the matrix fibers (excitation: 549 nm; emission: 615 nm). The images are presented as the maximum intensity Z-projections created with ImageJ (NIH, Bethesda, MD, USA).
For analysis of the long-term viability of cells on scaffolds, the ARPE-19 cells were seeded on the unstained matrices for 3 weeks using the same procedure. Then, Live/Dead assay was performed using 30-min staining with Calcein-AM (Sigma, Burlington, MA, USA) and propidium iodide (ThermoFisher Scientific, Waltham, MA, USA) for live and dead cells, respectively. At least five fields were recorded with the CellInsight CX7 high-content screening system, and the number of live and dead cells was counted on the maximum intensity Z-projections.
2.7. Statistical Analysis
The statistical analysis of the cytotoxicity test results and graph plotting were performed using the Prism 8.0 GraphPad software package. The normality of the distribution of the obtained data was assessed using the Shapiro–Wilk criterion. One-way ANOVA was used to compare the obtained experimental data with the control for normally distributed data, and the Kruskal–Wallis criterion was used in the case of a distribution different from normal. The data in the graphs are presented as the mean ± standard deviation. The level of significance for all of the statistical criteria was set at 0.05.
3. Results and Discussion
3.1. Characteristics of P(NIPAM-co-NtBA) Solutions
Two copolymers of N-isopropylacrylamide (NIPAM) and N-tert-butylacrylamide (NtBA) of a similar composition, but with a different number-average molecular weight (Mn), weight-average molecular weight (Mw) and dispersity (Mw/Mn), were synthesized (see Figure S1 for the NMR spectrum and Figure S2 for SEC traces of synthesized copolymers). The rheological studies (Table 1) showed that, as anticipated, the viscosity of a 15 wt% polymer solution in absolute ethanol was higher for the copolymer with a higher molecular weight and dispersity: 580 ± 100 mPa·s and 460 ± 50 mPa·s for Copolymer 1 and copolymer 2, respectively (Table 1). The dependencies of viscosity on the shear rate for both P(NIPAM-co-NtBA) copolymers and for different concentrations of copolymer 2 are shown in Figure 1. The viscosity vs. shear rate plots demonstrated a shear-thinning behavior typical for polymer solutions, related to chain disentanglement with the increasing deformation (shear rate). Note, a slight increase of the copolymer 2 solution concentration resulted in a significant increase in its viscosity due to a higher degree of chain entanglement (Figure 1 and Table 1). This finding correlates well with the significant increase of viscosity at a low shear rate for the more concentrated polymer solution (Figure 1).

Table 1.
Characteristics of the P(NIPAM–co-NtBA) copolymers and their solutions in absolute ethanol.
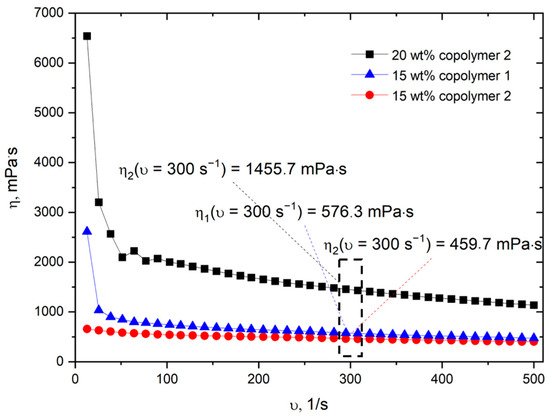
Figure 1.
Dependencies of the viscosity of P(NIPAM-co-NtBA) copolymers solutions on the shear rate.
Furthermore, a study was conducted to investigate the influence of molecular weight, dispersity and the concentration of copolymer solution on the formation of electrospun fibers.
3.2. Influence of the Molecular Weight and Dispersity of Thermoresponsive P(NIPAM-co-NtBA) Copolymers on the Structural and Hydrophobic–Hydrophilic Characteristics of Scaffolds
The SEM micrographs from Figure 2 illustrate fibers with a flat morphology, deviating from the conventionally expected cylindrical morphology. This unique morphology can be attributed to the specific constitution of the P(NIPAM-co-NtBA) copolymer. The distinctive interplay between the polymer’s components and the solvent might influence the final fiber shape during the drying process. There is a discernible difference in the thickness of fibers produced from these copolymers, with the values being 2.0 ± 0.5 µm and 2.2 ± 0.4 µm, respectively. This accentuates the sensitivity of the electrospinning process to the molecular characteristics of the copolymer.
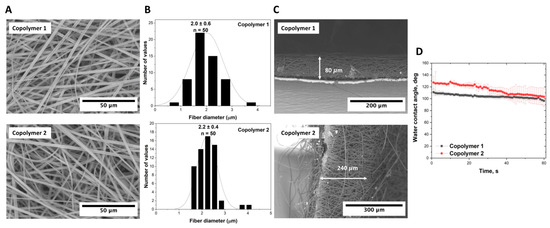
Figure 2.
Electrospun P(NIPAM-co-NtBA) scaffolds from 15% solutions of Copolymer 1 and copolymer 2: (A) SEM images of scaffold surfaces capturing the nuances of the utilized copolymers; (B) histograms detailing the fiber diameter distributions; (C) SEM images emphasizing scaffold thickness variations (scaffold attached to the foil); (D) contact angle measurements showcasing the wettability properties. All scaffolds were electrospun with 1 mL of the solution using a dynamic collector rotating at 600 RPM and FAA.
Scaffolds synthesized from the two different P(NIPAM-co-NtBA) copolymers exhibited a disparity in their thickness which was registered at 80 µm and 240 µm for copolymers 1 and 2, respectively (Figure 2C). We believe that there are two basic reasons for this phenomenon. The first reason is the lower viscosity of the copolymer 2 solution, which facilitates the jet flow from the nozzle. The second reason is the copolymers’ rather notable dispersity. As seen from the SEC profiles (Figure S2), copolymer 2 contains a visibly higher fraction of low-molecular components than Copolymer 1 does. This low-molecular fraction has a lower degree of polymer chains’ entanglement, leading to lower viscosity and higher volatility under the conditions of electrospinning. Therefore, copolymer 2 is deposited more rapidly, forming a thicker film. Furthermore, the contact angle measurements portrayed in Figure 2D also indicated differences in the hydrophobic properties of the scaffolds, with the initial values at around 110 and 130 degrees shifting to 100 and 105 degrees after 60 s, respectively. In addition to the aforementioned characteristics, the scaffolds displayed comparable porosities. The porosity of the scaffold from Copolymer 1 was measured at 94.9 ± 1.4%, while the scaffold synthesized from Copolymer 2 exhibited a porosity of 95.3 ± 1.6%. This study underscores that, despite the closeness in their porosity values, even subtle alterations in the molecular weight attributes of the P(NIPAM-co-NtBA) copolymer can lead to variations in other properties and the morphology of the synthesized materials. This emphasizes the importance of comprehensive research and meticulous methodology implementation to ensure reproducibility and to achieve the desired scientific outcomes.
3.3. Impact of the Collector’s Rotation Speed and of the Solution Viscosity on the Scaffold Fiber Thickness, Morphology, Hydrophilic–Hydrophobic Properties, and Mechanical Characteristics
According to the results displayed in Figure 3, the collector’s rotation speed had a significant effect on the scaffold parameters of Copolymer 2. Considering the 15% solution with a viscosity of 460 mPa·s, when the rotation speed was increased from 600 rpm to 2400 rpm, the fiber thickness decreased from 2.9 μm to 1.7 μm, corresponding to a 41% reduction. Note that the fourfold increase in the rotation speed resulted in a reduction in the fiber thickness by almost half. For the 20% solution with a viscosity of 1460 mPa·s, a similar, albeit much less pronounced, trend was observed. With an increase in the rotation speed from 600 rpm to 2400 rpm, the fiber thickness decreased from 5.1 μm to 4.8 μm, representing a reduction of 6%. Here we see that, despite the fourfold increase in rotation speed, the reduction in the fiber thickness for the more viscous solution was significantly lower than that for the less viscous one. An increase in the collector’s rotation speed led to a denser and more oriented arrangement of fibers, resulting here in finer fibers and increased porosity of the scaffold. It should be noted that there is an intricate relationship between collector rotation speed, fiber diameter, and scaffold porosity, which is also affected [38,39,40,41]. For both solutions (460 mPa·s and 1460 mPa·s), at a rotation speed of 2400 rpm, fibers were aligned parallel to each other. However, when the speed was reduced to 600 rpm, the arrangement of fibers became more chaotic and multilayered, without a pronounced orientation. The porosity of the scaffolds was influenced both by the rotation speed of the dynamic collector and by the solution viscosity. This is consistent with the fact that the rotation speed affects the fiber width and alignment, and, hence, influences the porosity of the resulting scaffolds. The thinner and more aligned the fibers, the higher the porosity of the scaffolds was. For scaffolds derived from the solution with a viscosity of 460 mPa·s, the porosity data were as follows: 79.6 ± 3.1% at 600 rpm, 83.6 ± 7.5% at 1800 rpm, and 85.3 ± 3.1% at 2400 rpm. Meanwhile, scaffolds from the more viscous solution of 1460 mPa·s showed different porosity patterns: 52.7 ± 0.5% at 600 rpm and 63.6 ± 0.1% at 2400 rpm. The variation in the porosity patterns between the solutions of different viscosities highlights the nuanced interplay between the solution properties, mechanical parameters, and the electrospinning process in determining the scaffold porosity. Notably, measurements at 1800 rpm were impossible for this solution due to the scaffold adhering too firmly to the foil, making detachment for the assessment unfeasible. The increase in the porosity with the ascending rotation speed can be associated with a reduction in the fiber thickness. Moreover, the variations in the solution viscosity also played a pivotal role in determining the scaffold porosity, suggesting a nuanced interplay between the solution properties and the mechanical parameters. It is important to consider these dynamic relationships when optimizing the electrospinning parameters for the desired scaffold characteristics.
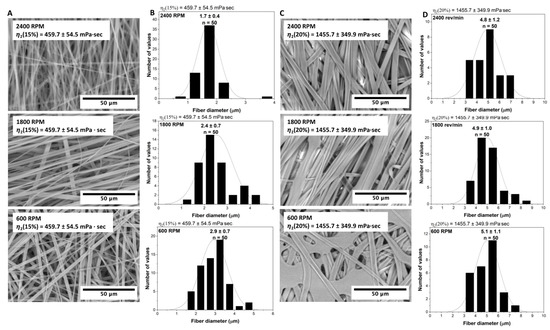
Figure 3.
Electrospun P(NIPAM-co-NtBA) scaffolds from the Copolymer 2 solutions with different concentrations and collector speeds: (A) SEM images of scaffolds from the solution with 460 ± 50 mPa·s viscosity, showcasing the effects of the collector speeds (right to left: 2400, 1800, 600 RPM); (B) histograms detailing fiber diameter distributions corresponding to (A); (C) SEM images for scaffolds from the solution with 1460 ± 350 mPa·s viscosity, emphasizing the outcomes of varying collector speeds; (D) histograms elaborating the fiber diameter variations related to (C). All scaffolds were electrospun with 1 mL of a solution, a 15 cm nozzle-to-collector distance, and without FAA.
In particular, in the scaffolds from the solution with a viscosity of 1460 mPa·s, fibers with defects in the form of droplets were found. This finding indicates that an increase in the solution viscosity may lead to an increased likelihood of defects in the scaffold structure. It is important to note that such defects were not found in the scaffolds from the solution with a viscosity of 460 mPa·s. If we consider the data for a rotation speed of 1800 rpm, we also observe a variation in the fiber sizes depending on the solution viscosity. For the solution with a viscosity of 460 mPa·s, the fiber thickness was about 2.4 μm, whereas for the solution with a viscosity of 1460 mPa·s, it increased to 4.9 μm. This result confirms the fact that a more viscous solution leads to the formation of thicker fibers, even at the same rotation speeds of the collector. Obviously, the solution viscosity plays a significant role in the formation of the scaffold structure. It affects not only the sizes of fibers but also their morphology and the degree of defectiveness. A more viscous solution typically leads to the formation of thicker and potentially defective fibers. On the other hand, a solution with a lower viscosity promotes the formation of thin, smooth, and defect-free fibers. Thus, the viscosity of the solution and the rotation speed of the collector are the two key parameters that determine the characteristics of scaffolds, and they must be carefully managed when designing electrospinning processes.
The contact angle measurements allowed us to obtain additional information about the hydrophilic–hydrophobic properties of scaffolds (Figure 4).
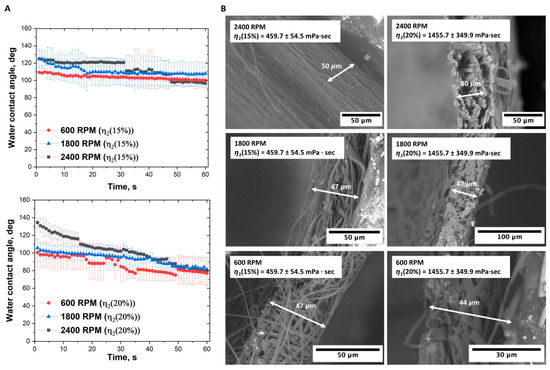
Figure 4.
Comparative analysis of electrospun P(NIPAM-co-NtBA) scaffolds from 15 wt% and 20 wt% solutions of Copolymer 2: (A) Contact angle measurements across the collector speeds of 600, 1800, and 2400 RPM; (B) SEM micrographs illustrating the thickness’s variation at the respective speeds. Electrospinning was conducted with 1 mL of the solution loading, 15 cm nozzle-to-collector distance, and without FAA.
For scaffolds fabricated from a solution with a viscosity of 1460 mPa·s at a collector rotation speed of 2400 rpm, the initial contact angle was 135 degrees, decreasing to 80 degrees over a time of 60 s. This dynamic indicates that the material becomes less hydrophobic, which may be significant for specific biomedical applications. When the rotation speed was reduced to 1800 rpm, the scaffold demonstrated a different dynamic wettability profile, with a contact angle commencing at 105 degrees and decreasing to 80 degrees. This trend confirms the influence of fabrication parameters on the material’s wettability properties. At a rotation speed of 600 rpm, the contact angle initiates at 100 degrees and converges to 78 degrees by the 60 s mark.
For scaffolds made from a solution with a viscosity of 460 mPa·s at 2400 rpm, the dynamic wetting angle started at 125 degrees and diminished to 98 degrees. At 1800 rpm, the angle oscillates from 125 to 110 degrees, suggesting that the material becomes less hydrophobic during this time. At a rotation speed of 600 rpm, the contact angle reduced slightly from 110 to 100 degrees within a 60 s timeframe. Such observations emphasize the intertwined effects of solution viscosity and rotation speed on shaping the dynamic wettability of the scaffold surface.
Delving into the mechanical test results showcased in Table 2, it is evident that the solution viscosity during electrospinning plays a pivotal role in determining the mechanical properties of scaffolds. When taking a closer look at the less viscous solution, 460 mPa·s, a discernible pattern emerges: as the collector rotation speed is reduced from 2400 rpm to 600 rpm, the Young’s modulus plummets by approximately 96%, going from 98.5 MPa down to 3.4 MPa. Correspondingly, the maximum stress also sees a significant decrease, moving from 2.7 MPa down to 0.09 MPa. Yet, the elongation at break shows an inverse relationship with a substantial increase, surging from 3.6% at 2400 rpm to 20.5% at 600 rpm. For the more viscous solution—1460 mPa·s—at 2400 rpm, the Young’s modulus stands at 330.3 MPa. With a decrease in the rotation speed to 600 rpm, it dips to 262.5 MPa, marking a reduction of about 20%. Concurrently, the maximum stress decreased by 33%, going from 4.8 MPa at 2400 rpm to 3.2 MPa at 600 rpm. The elongation at break experiences a slight decrease from 2.7% to 1.8% as the rotation speed is dialed back. Conclusively, these findings accentuate the profound influence of the solution viscosity on the scaffolds’ mechanical properties. The scaffolds prepared from the more viscous solution of 1460 mPa·s register a higher Young’s modulus. On the contrary, those from the less viscous solution of 460 mPa·s showcase a markedly lower Young’s modulus and maximum stress but with significant variations in the elongation at break. These data underscore the nuanced interplay of the manufacturing variables in determining the mechanical behavior of scaffolds.

Table 2.
Mechanical Properties of Electrospun P(NIPAM-co-NtBA) Scaffolds from the Copolymer 2 Solutions as Influenced by the Solution Viscosity and the Collector’s Rotation Speed.
3.4. Optimizing the Scaffold Morphology via Forced Airflow Activation (FAA)
Electroforming experiments utilizing forced airflow activation (FAA) highlight the significance of this method related to the scaffold’s structural characteristics, as evidenced by Figure 5. Scaffolds formed with FAA exhibited a porosity of 95.2 ± 1.6% compared to 79.6 ± 3.1% for scaffolds without FAA. The increased porosity can be attributed to more intensive fiber stretching when forced airflow is activated. The thickness of the scaffold with FAA was 220 µm, which is 4.7 times greater than that of the scaffolds without FAA (47 µm). This change in thickness suggests that FAA facilitates the formation of thicker scaffolds due to more even distribution of the copolymer solution.
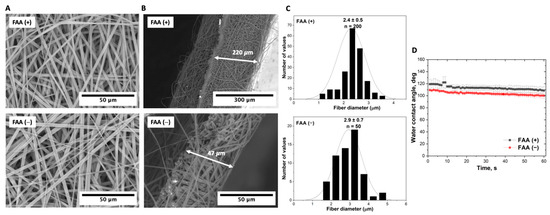
Figure 5.
Comparative analysis of electrospun P(NIPAM-co-NtBA) scaffolds with and without FAA: (A) SEM images of the surface morphology; (B) cross-sectional SEM views of scaffolds, following their detachment from the foil, illustrating their inherent thickness; (C) variation in the fiber thickness distribution; (D) contact angle measurements. These scaffolds were produced using the dynamic collector rotating at 600 RPM from 1 mL of the 15% Copolymer 2 solution at a nozzle-to-collector distance of 15 cm.
While fiber diameters were comparable for both methods, being 2.4 ± 0.5 µm versus 2.9 ± 0.7 µm, this finding indicates that the influence of FAA has a more profound effect on the overall structure of the scaffold than on the individual characteristics of fibers. The contact angle observations showed a slight difference in the surface hydrophobicity. For the scaffold with activated FAA, the contact angle started at 120°, decreasing to 110° by the 60 s mark, whereas for the scaffold without FAA, the angle started at 110°, dropping to 100° by the 60 s mark.
3.5. Analysis of the Scaffold Thickness and Surface Hydrophilicity: Effects of the Distance between the Nozzle and Collector
The distance between the nozzle and collector undeniably influences the morphology and properties of the resultant scaffold in the electrospinning process. As demonstrated in Figure 6, at a distance of 15 cm, the resulting scaffold had a thickness of 80 µm; the scaffold’s porosity was 94.9 ± 1.4%. The flat fibers here, with a diameter of 2.0 ± 0.6 µm, presented a rather uniform distribution. This could be attributed to a shorter trajectory the polymer solution travels, resulting in a shorter time for the solvent evaporation and, thereby, yielding thicker fibers. Contrarily, increasing the distance to 23 cm resulted in a scaffold thickness of 110 µm. The porosity here was slightly augmented to 95.1 ± 1.2%, and intriguingly, fibers were narrower at 1.5 ± 0.4 µm in width. The extended trajectory allows for more efficient solvent evaporation, leading to the formation of thinner fibers. The longer pathway possibly promotes greater fiber stretching and alignment before deposition, influencing the fiber morphology.
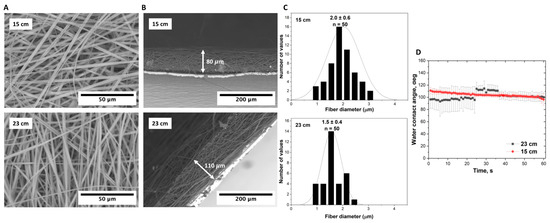
Figure 6.
Electroformed P(NIPAM-co-NtBA) scaffolds at different nozzle-to-collector distances (15 and 23 cm): (A) Surface SEM images; (B) cross-sectional SEM views depicting the scaffold thickness on foil; (C) distribution of the fiber thickness; (D) measurements of contact angle. All scaffolds were fabricated using a dynamic collector rotating at 600 RPM and 1 mL of the Copolymer 1 solution with FAA.
The observed shifts in the initial contact angle, from 110 degrees at 15 cm to 95 degrees at 23 cm, represent another notable result, reflecting a less hydrophobic surface for the scaffolds produced at the greater distance. The observed decrease in the contact angle when the distance is increased might be due to an altered surface energy. As the distance increases and fibers become narrower, there might be an increase in the surface area exposed to ambient conditions, which could induce certain alterations in the surface chemistry, thus influencing hydrophobicity.
3.6. The Solution Volume in Electrospinning: Implications for the Scaffold Thickness, Wetting Dynamics, and Mechanical Properties
Altering the solution volume from 1 mL to 3 mL led to an increase in the scaffold thickness, as detailed in Figure 7. This change profoundly affected not only the fiber morphology but also the mechanical properties of the scaffolds. Specifically, the scaffold prepared from 3 mL of the solution had fibers with a width of 2.1 µm, in contrast to the 1.5 µm-wide fibers of the scaffold produced from 1 mL of the solution. The Young’s modulus varied significantly, as well. The modulus for the scaffold prepared from 1 mL of the solution was recorded at 6.8 ± 3.0 MPa, considerably higher than 0.15 ± 0.03 MPa for the scaffold from 3 mL of the solution.
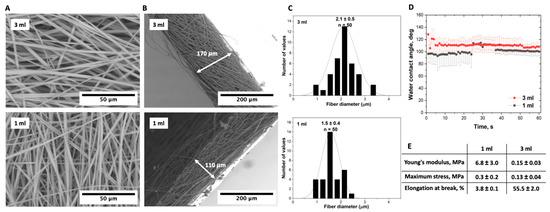
Figure 7.
Comparative analysis of electrospun P(NIPAM-co-NtBA) scaffold characteristics prepared from different volumes (1 mL and 3 mL) of a Copolymer 1 solution. The tests were conducted at a maximum nozzle-to-collector distance of 23 cm with FAA enabled using a dynamic collector rotating at 600 RPM: (A) SEM images of the surface morphology; (B) SEM images of the scaffold thickness on foil; (C) distribution of the scaffold fiber thickness; (D) contact angle values for scaffolds prepared from different solution volumes; (E) mechanical data.
The maximum stress of the scaffold prepared from 1 mL was measured at 0.3 ± 0.2 MPa, while the scaffold from 3 mL showed a maximum stress of 0.13 ± 0.04 MPa. Furthermore, the elongation at break of the 3 mL-based scaffold was recorded at 55.5 ± 2.0%, higher than the 3.8 ± 0.1% observed for the 1 mL-based scaffold. These distinctions likely originate from varying solution volumes influencing the fiber packing, orientation, and overall mechanical behavior of the resulting scaffolds. The scaffold produced from 3 mL of the solution demonstrated a porosity of 92.6 ± 2.6%, closely matching the porosity of 95.1 ± 1.2% of the scaffold prepared from 1 mL of the solution. This finding implies that meticulous selection of the polymer solution volume is crucial for optimizing both the structure and mechanical properties of scaffolds for specific biomedical applications.
3.7. Cell Viability Analysis
The cell experiments were conducted with Copolymer 2 due to its lower dispersity. The analysis of the P(NIPAM-co-NtBA) copolymers’ cytotoxicity showed that none of the tested copolymer concentrations influenced the metabolic and proliferative activity of cells compared to the control culture without the addition of the polymer (Figure 8A,B).
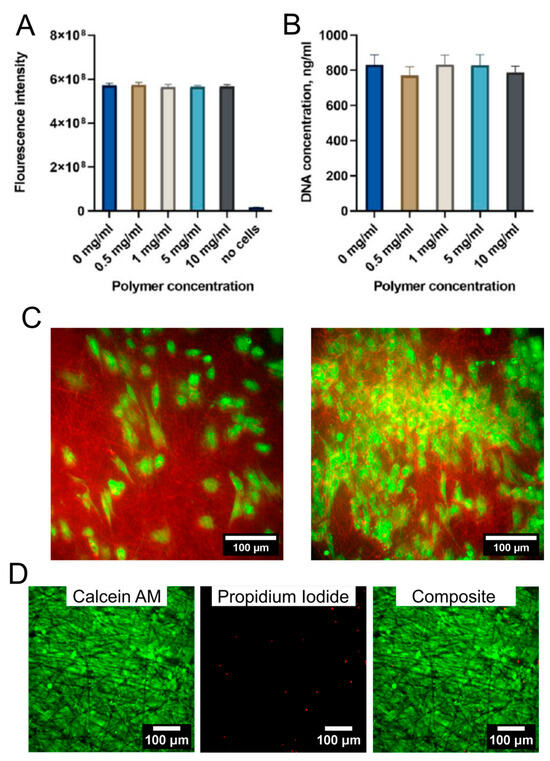
Figure 8.
Cytotoxicity of the pNIPAm-co-NtBA copolymer (A,B) and the cell behavior on the electrospun scaffolds (C). (A) Analysis of cell metabolic activity; Alamar Blue test. (B) Analysis of the proliferative activity of cells in the presence of the polymer; PicoGreen assay. (C) The REF52 cells’ morphology on the electrospun scaffolds, with low and high density, confocal microscopy of cells stained with Calcein AM and electrospun scaffolds fibers stained with Rhodamin C. (D) Analysis of the cell viability on the electrospun scaffolds using the Live/Dead assay. Calcein AM and propidium iodide staining of ARPE-19 cells grown on scaffolds for 3 weeks.
The scaffolds obtained from 1 mL of Copolymer 2 at a collector rotation speed of 600 rpm were selected to test cell spreading and migration inside the scaffold due to their large porosity. REF52 cells seeded on the electrospun scaffolds were viable after 1 week and had a spread morphology typical for fibroblasts (Figure 8C). However, the cells did not migrate inside the scaffold and were located only on its surface, probably due to the high fiber density and small interfibrillar distance. It can be assumed that the formation of the cell sheet will occur mostly on the surface of the matrix, and there is no significant migration of cells into the scaffold.
For analysis of long-term cell viability, APRE-19 human retinal pigment epithelial cells were seeded on the same electrospun scaffolds for 3 weeks. At the end of experiment, the cells formed a dense cell sheet (Figure 8D). The number of viable cells was high (96 ± 3%), which confirms a good cytocompatibility of scaffolds.
Thus, fibrous thermosensitive matrices made from P(NIPAM-co-NtBA) are nontoxic and promote cell growth on their surface, and the functional activity of cells is preserved. At the same time, such a system makes it possible to achieve the detachment of cellular structures much faster than in more common monolayer systems.
3.8. Selection of the Optimal Parameters for Electrospinning of the P(NIPAM-co-NtBA) Scaffolds
Optimization of electrospinning parameters to achieve the desired scaffold parameters is an important step in scaffold production [42,43,44]. In the process of investigating electrospinning techniques for scaffold fabrication, several pivotal dependencies were discerned. The viscosity of the solution emerges as a critical parameter influencing the fiber diameter; more viscous solutions tended to produce finer fibers. The collector’s rotation speed also significantly impacted the resultant scaffold morphology. While an increase in the speed can facilitate the formation of more aligned structures, ideal for directional tissue growth, a decrease in the speed led to a more random arrangement. This random orientation, coupled with larger pores, potentially offers room for deep cellular penetration and integration, which is particularly beneficial for various biomedical applications. In our specific case, the scaffold obtained from a solution of Copolymer 2 with a viscosity of 460 mPa·s at a collector rotation speed of 600 rpm, using 1 mL of the solution, showed a scaffold structure with larger pores. This larger porosity was seen as advantageous, as it potentially allows for deeper cellular penetration and integration into the scaffold, which can be beneficial for certain biomedical applications. Yet, in this configuration, we observed cell growth only on the surface of the scaffold, suggesting that further refinement and optimization of the technique might still be needed. Increasing the distance between the nozzle and the collector resulted in thinner fibers due to the extended solvent evaporation time. Enlarging the solution volume led to an increase in the scaffold thickness and alteration of mechanical properties. The application of FAA enhanced fiber stretching, thereby influencing the overall scaffold morphology. In general, the appropriate combination of all these parameters allows for the tailored control of the scaffold morphology and mechanical properties, positioning electrospinning as a potent tool for crafting materials that meet the demands of diverse biomedical applications.
4. Conclusions
Our study offers an important insight into the development of scaffolds using thermoresponsive copolymers, specifically copolymers of N-isopropylacrylamide and N-tert-butylacrylamide (P(NIPAM-co-NtBA)). Our findings confirm that these copolymers have the potential to significantly enhance the capabilities of scaffolds in supporting efficient cell attachment, growth, and the non-invasive detachment of 3D tissue constructs. We demonstrated that the process parameters used in electrospinning, such as the distance from nozzle to collector and the volume of the solution, can significantly affect the properties of the resulting scaffolds, including fiber diameter, thickness, wettability, and mechanical properties. Such parameters as the solution viscosity, collector’s rotation speed, and polymer composition critically dictate the scaffold morphology. For instance, higher solution viscosities tend to generate finer fibers, while the implementation of forced airflow activation (FAA) enhances fiber stretching. Specifically, in our research, the optimal scaffold was achieved using a solution of Copolymer 2 with a viscosity of 460 mPa·s at a collector rotation speed of 600 rpm. This configuration resulted in a scaffold with larger pores, thereby facilitating deeper cellular penetration. However, further optimization is needed, as cell growth was predominantly observed on the scaffold’s surface. The absence of cytotoxicity of the copolymers and effective cell spreading on the obtained scaffolds were demonstrated. These insights underscore the robust potential of electrospinning in producing materials tailored to diverse biomedical requirements. We believe that this study provides valuable insights into scaffold technologies for tissue engineering. By exploring the role of thermoresponsive polymers in scaffold development, we aim to contribute to the creation of more efficient tissue substitutes. Such advancements are important steps toward the broader goals of regenerative medicine.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/technologies11050145/s1, Figure S1: 1H NMR spectrum of poly(N-isopropylacrylamide-co-N-tert-butylacrylamide) P(NIPAM-co-NtBA) copolymer (NIPAM:NtBA=86/14); Figure S2: Size-exclusion chromatography (SEC) traces of poly(N-isopropylacrylamide-co-N-tert-butylacrylamide)s used in this study.
Author Contributions
Conceptualization, P.S.T., Y.A.R., S.V.K. and S.L.K.; methodology, G.K.K., V.S.P., A.A.F., S.V.K. and Y.M.E.; investigation, G.K.K., V.S.P., A.A.F., Y.M.E. and S.V.K.; resources, P.S.T., Y.A.R. and S.V.K.; writing—original draft preparation, G.K.K. and Y.M.E.; writing—review and editing, S.V.K., P.S.T., Y.A.R. and S.L.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation (grant No. 21-15-00349, https://rscf.ru/en/project/21-15-00349/, electrospinning, cell experiments), by the State Program for Scientific Research of Belarus “Chemical processes, reagents and technologies, bioregulators and bioorganic chemistry” (project 2.2.02.04, synthesis and characterization of copolymers), and by the Ministry of Science and Higher Education of the Russian Federation within the framework of state support for the creation and development of World-Class Research Centers “Digital biodesign and personalized healthcare” N◦075-15-2022-304 (confocal microscopy).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
The authors extend their gratitude to Irina Zurina for her exemplary coordination of the cellular work and to Georgy Vladimirov, Polina Sirotkina, and Altynay Baygunusova for their invaluable assistance during the experiments. The study was carried out using the unique scientific facility Transgenebank.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials Design for Bone-Tissue Engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Mousaei Ghasroldasht, M.; Seok, J.; Park, H.-S.; Liakath Ali, F.B.; Al-Hendy, A. Stem Cell Therapy: From Idea to Clinical Practice. Int. J. Mol. Sci. 2022, 23, 2850. [Google Scholar] [CrossRef]
- Laurencin, C.T.; McClinton, A. Regenerative Cell-Based Therapies: Cutting Edge, Bleeding Edge, and Off the Edge. Regen. Eng. Transl. Med. 2020, 6, 78–89. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution Properties of Poly(N-Isopropylacrylamide). J. Macromol. Sci. Part A Chem. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Aseyev, V.; Tenhu, H.; Winnik, F.M. Non-Ionic Thermoresponsive Polymers in Water. In Self Organized Nanostructures of Amphiphilic Block Copolymers II; Müller, A.H.E., Borisov, O., Eds.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2011; pp. 29–89. ISBN 978-3-642-22297-9. [Google Scholar]
- Pasparakis, G.; Tsitsilianis, C. LCST Polymers: Thermoresponsive Nanostructured Assemblies towards Bioapplications. Polymer 2020, 211, 123146. [Google Scholar] [CrossRef]
- Zubanova, E.M.; Kostjuk, S.V.; Timashev, P.S.; Rochev, Y.A.; Kokorin, A.I.; Melnikov, M.Y.; Golubeva, E.N. Inhomogeneities in PNIPAM Aqueous Solutions: The Inside View by Spin Probe EPR Spectroscopy. Polymers 2021, 13, 3829. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Cao, M.; Wang, Y.; Hu, X.; Gong, H.; Li, R.; Cox, H.; Zhang, J.; Waigh, T.A.; Xu, H.; Lu, J.R. Reversible Thermoresponsive Peptide–PNIPAM Hydrogels for Controlled Drug Delivery. Biomacromolecules 2019, 20, 3601–3610. [Google Scholar] [CrossRef]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T. Thermoresponsive Polymers and Their Biomedical Application in Tissue Engineering—A Review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef]
- Hogan, K.J.; Mikos, A.G. Biodegradable Thermoresponsive Polymers: Applications in Drug Delivery and Tissue Engineering. Polymer 2020, 211, 123063. [Google Scholar] [CrossRef]
- Lu, H.; Leng, J.; Du, S. A Phenomenological Approach for the Chemo-Responsive Shape Memory Effect in Amorphous Polymers. Soft Matter. 2013, 9, 3851–3858. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive Polymers with Lower Critical Solution Temperature: From Fundamental Aspects and Measuring Techniques to Recommended Turbidimetry Conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Li, J.; Fan, X.; Yang, L.; Wang, F.; Zhang, J.; Wang, Z. A Review on Thermoresponsive Cell Culture Systems Based on Poly(N-Isopropylacrylamide) and Derivatives. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 371–382. [Google Scholar] [CrossRef]
- Tang, Z.; Akiyama, Y.; Okano, T. Recent Development of Temperature-Responsive Cell Culture Surface Using Poly(N-Isopropylacrylamide). J. Polym. Sci. Part B Polym. Phys. 2014, 52, 917–926. [Google Scholar] [CrossRef]
- Pardeshi, S.; Damiri, F.; Zehravi, M.; Joshi, R.; Kapare, H.; Prajapati, M.K.; Munot, N.; Berrada, M.; Giram, P.S.; Rojekar, S.; et al. Functional Thermoresponsive Hydrogel Molecule to Material Design for Biomedical Applications. Polymers 2022, 14, 3126. [Google Scholar] [CrossRef]
- Rana, M.M.; De La Hoz Siegler, H. Tuning the Properties of PNIPAm-Based Hydrogel Scaffolds for Cartilage Tissue Engineering. Polymers 2021, 13, 3154. [Google Scholar] [CrossRef]
- Nagase, K. Thermoresponsive Interfaces Obtained Using Poly(N-Isopropylacrylamide)-Based Copolymer for Bioseparation and Tissue Engineering Applications. Adv. Colloid Interface Sci. 2021, 295, 102487. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S. Poly(N-Isopropylacrylamide)-Based Thermo-Responsive Surfaces with Controllable Cell Adhesion. Sci. China Chem. 2014, 57, 552–557. [Google Scholar] [CrossRef]
- Nash, M.E.; Healy, D.; Carroll, W.M.; Elvira, C.; Rochev, Y.A. Cell and Cell Sheet Recovery from pNIPAm Coatings; Motivation and History to Present Day Approaches. J. Mater. Chem. 2012, 22, 19376–19389. [Google Scholar] [CrossRef]
- Yamato, M.; Akiyama, Y.; Kobayashi, J.; Yang, J.; Kikuchi, A.; Okano, T. Temperature-Responsive Cell Culture Surfaces for Regenerative Medicine with Cell Sheet Engineering. Prog. Polym. Sci. 2007, 32, 1123–1133. [Google Scholar] [CrossRef]
- Matsuda, N.; Shimizu, T.; Yamato, M.; Okano, T. Tissue Engineering Based on Cell Sheet Technology. Adv. Mater. 2007, 19, 3089–3099. [Google Scholar] [CrossRef]
- Cooperstein, M.A.; Canavan, H.E. Biological Cell Detachment from Poly(N-Isopropyl Acrylamide) and Its Applications. Langmuir 2010, 26, 7695–7707. [Google Scholar] [CrossRef]
- Kotova, S.; Kostjuk, S.; Rochev, Y.; Efremov, Y.; Frolova, A.; Timashev, P. Phase Transition and Potential Biomedical Applications of Thermoresponsive Compositions Based on Polysaccharides, Proteins and DNA: A Review. Int. J. Biol. Macromol. 2023, 249, 126054. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly(N-Isopropylacrylamide)-Based Thermoresponsive Composite Hydrogels for Biomedical Applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef]
- Ansari, M.J.; Rajendran, R.R.; Mohanto, S.; Agarwal, U.; Panda, K.; Dhotre, K.; Manne, R.; Deepak, A.; Zafar, A.; Yasir, M.; et al. Poly(N-Isopropylacrylamide)-Based Hydrogels for Biomedical Applications: A Review of the State-of-the-Art. Gels 2022, 8, 454. [Google Scholar] [CrossRef]
- Yifang, S.; Huang, L.; Wang, X.; Li, Y.; Shen, R. Intelligent Drug Delivery System Based on Silk Fibroin/Wool Keratin. Math. Probl. Eng. 2022, 2022, e6748645. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, P.; Chen, L.; Wang, X.; Lu, S. Genipin-Cross-Linked Thermosensitive Silk Sericin/Poly(N-Isopropylacrylamide) Hydrogels for Cell Proliferation and Rapid Detachment. J. Biomed. Mater. Res. Part A 2014, 102, 76–83. [Google Scholar] [CrossRef]
- Zhang, Q.; Xue, R.; Li, X.; Wang, Z.; Zhou, Z.; Chen, L. Silk Sericin/Poly (NIPAM/LMSH) Nanocomposite Hydrogels: Rapid Thermo-Responsibility and Good Carrier for Cell Proliferation. Pure Appl. Chem. 2014, 86, 721–731. [Google Scholar] [CrossRef]
- Li, J.; Wang, B.-X.; Cheng, D.-H.; Liu, Z.-M.; Lv, L.-H.; Guo, J.; Lu, Y.-H. Electrospun Sericin/PNIPAM-Based Nano-Modified Cotton Fabric with Multi-Function Responsiveness. Coatings 2021, 11, 632. [Google Scholar] [CrossRef]
- Brunelle, A.R.; Horner, C.B.; Low, K.; Ico, G.; Nam, J. Electrospun Thermosensitive Hydrogel Scaffold for Enhanced Chondrogenesis of Human Mesenchymal Stem Cells. Acta Biomater. 2018, 66, 166–176. [Google Scholar] [CrossRef]
- Kim, I.L.; Khetan, S.; Baker, B.M.; Chen, C.S.; Burdick, J.A. Fibrous Hyaluronic Acid Hydrogels That Direct MSC Chondrogenesis through Mechanical and Adhesive Cues. Biomaterials 2013, 34, 5571–5580. [Google Scholar] [CrossRef]
- Bauri, K.; Roy, S.G.; Arora, S.; Dey, R.K.; Goswami, A.; Madras, G.; De, P. Thermal Degradation Kinetics of Thermoresponsive Poly(N-Isopropylacrylamide-Co-N,N-Dimethylacrylamide) Copolymers Prepared via RAFT Polymerization. J. Therm. Anal. Calorim. 2013, 111, 753–761. [Google Scholar] [CrossRef]
- Moran, M.T.; Carroll, W.M.; Selezneva, I.; Gorelov, A.; Rochev, Y. Cell Growth and Detachment from Protein-Coated PNIPAAm-Based Copolymers. J. Biomed. Mater. Res. Part A 2007, 81, 870–876. [Google Scholar] [CrossRef]
- De Pieri, A.; Korntner, S.H.; Capella-Monsonis, H.; Tsiapalis, D.; Kostjuk, S.V.; Churbanov, S.; Timashev, P.; Gorelov, A.; Rochev, Y.; Zeugolis, D.I. Macromolecular Crowding Transforms Regenerative Medicine by Enabling the Accelerated Development of Functional and Truly Three-Dimensional Cell Assembled Micro Tissues. Biomaterials 2022, 287, 121674. [Google Scholar] [CrossRef] [PubMed]
- Rochev, Y.; O’Halloran, D.; Gorelova, T.; Gilcreest, V.; Selezneva, I.; Gavrilyuk, B.; Gorelov, A. Rationalising the Design of Polymeric Thermoresponsive Biomaterials. J. Mater. Sci. Mater. Med. 2004, 15, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, S.J.; Sampson, W.W. Statistical Geometry of Pores and Statistics of Porous Nanofibrous Assemblies. J. R. Soc. Interface 2005, 2, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Haider, S.; Kang, I.-K. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun Poly(ε-Caprolactone) Microfiber and Multilayer Nanofiber/Microfiber Scaffolds: Characterization of Scaffolds and Measurement of Cellular Infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; Wise, S.G.; Li, Z.; Maitz, P.K.M.; Young, C.J.; Wang, Y.; Weiss, A.S. Tailoring the Porosity and Pore Size of Electrospun Synthetic Human Elastin Scaffolds for Dermal Tissue Engineering. Biomaterials 2011, 32, 6729–6736. [Google Scholar] [CrossRef]
- Dehghan, S.F.; Golbabaei, F.; Maddah, B.; Latifi, M.; Pezeshk, H.; Hasanzadeh, M.; Akbar-Khanzadeh, F. Optimization of Electrospinning Parameters for Polyacrylonitrile-MgO Nanofibers Applied in Air Filtration. J. Air Waste Manag. Assoc. 2016, 66, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Kanu, N.J.; Gupta, E.; Vates, U.K.; Singh, G.K. Electrospinning Process Parameters Optimization for Biofunctional Curcumin/Gelatin Nanofibers. Mater. Res. Express 2020, 7, 035022. [Google Scholar] [CrossRef]
- Singh, Y.P.; Dasgupta, S.; Nayar, S.; Bhaskar, R. Optimization of Electrospinning Process & Parameters for Producing Defect-Free Chitosan/Polyethylene Oxide Nanofibers for Bone Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2020, 31, 781–803. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).