Ecological Niche Models Reveal Climate Change Effect on Biogeographical Regions: The Iberian Peninsula as a Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area: Iberian Peninsula
2.2. Species Data Sources
2.3. Environmental Data
2.4. Ecological Niche Models
2.5. Identification of Biogeographical Regions
3. Results
4. Discussion and Conclusions
- -
- Atlantic amphibians included species with northern distribution (chorotype B; Figure 2) and cold-adapted species with their range restricted to Pyrenean-Cantabrian mountains (chorotype A; Figure 2). This pattern agrees with the mentioned previous studies, with some exceptions: for instance, Alytes obstetricans was placed in an Atlantic chorotype (chorotype B; Figure 2), when it was previously considered as mainly Mediterranean [10,90]. However, this species is distributed in central Europe, although it has a high percentage of occurrence in both Iberian regions [10].
- -
- -
- Atlantic reptiles included species with northern distribution (chorotype B; Figure 3), species with their range restricted to the Pyrenean-Cantabrian mountains (chorotype A; Figure 3) and species with their range limited to the Cantabrian region (chorotype E; Figure 3). Some species fluctuated between different chorotypes depending on the temporal period. For instance, Lacerta schreiberi was placed in a Mediterranean chorotype in the three scenarios of the past (LIG, LGM and Mid Holocene; chorotype D; Figure 3), but in an Atlantic chorotype in the present and future scenarios (chorotype B; Figure 3). This species was previously associated with Atlantic climates [10], but it does occupy riverine habitats; thus, it can persist in Mediterranean habitats [65].
- -
- Mediterranean reptiles included one chorotype with all the widespread species in most periods (chorotype D; Figure 3), except for the Mid Holocene that included two chorotypes, with a species composition very similar to the one obtained by Sillero et al. [10]: the chorotype C (Figure 3) includes species that commonly avoid the eastern and northern part of the peninsula and also species occurring in North Africa (such as Hemorrhois hippocrepis); the chorotype D (Figure 3) includes species with Palearctic distributions, species present in both Africa and in southern France.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pettorelli, N.; Laurance, W.F.; O’Brien, T.G.; Wegmann, M.; Nagendra, H.; Turner, W. Satellite remote sensing for applied ecologists: Opportunities and challenges. J. Appl. Ecol. 2014, 51, 839–848. [Google Scholar] [CrossRef]
- He, K.S.; Bradley, B.A.; Cord, A.F.; Rocchini, D.; Tuanmu, M.N.; Schmidtlein, S.; Turner, W.; Wegmann, M.; Pettorelli, N. Will remote sensing shape the next generation of species distribution models? Remote Sens. Ecol. Conserv. 2015, 1, 4–18. [Google Scholar] [CrossRef]
- Leitão, P.J.; Santos, M.J. Improving models of species ecological niches: A remote sensing overview. Front. Ecol. Evol. 2018, 7, 9. [Google Scholar] [CrossRef]
- Pasetto, D.; Arenas-Castro, S.; Bustamante, J.; Casagrandi, R.; Chrysoulakis, N.; Cord, A.F.; Dittrich, A.; Domingo-Marimon, C.; Serafy, E.; Karnieli, A. Integration of satellite remote sensing data in ecosystem modelling at local scales: Practices and trends. Methods Ecol. Evol. 2018, 9, 1810–1821. [Google Scholar] [CrossRef]
- Sillero, N.; Vale, C.G.; Beukema, W. GIS for spatial biology: The geographical component of life. Front. Inf. Syst. 2018, 1, 149–183. [Google Scholar] [CrossRef]
- Vargas, J.M.; Real, R.; Guerrero, J.C. Biogeographical regions of the Iberian Peninsula based on freshwater fish and amphibian distributions. Ecography 1998, 21, 371–382. [Google Scholar] [CrossRef]
- Lenormand, M.; Papuga, G.; Argagnon, O. Biogeographical network analysis of plant species distribution in the Mediterranean region. Ecol. Evol. 2018, 9, 237–250. [Google Scholar] [CrossRef]
- Baroni-Urbani, C.; Ruffo, S.; Taglianti, V. Materiali per uma biogeografia italiana fondata se alcuni generi di coleotteri Cicindelidi, Carabidi, e Crisomelidi. Mem. Soc. Entomol. Ital. 1978, 56, 35–92. [Google Scholar]
- Passalacqua, N.G. On the definition of element, chorotype and component in biogeography. J. Biogeogr. 2015, 42, 611–618. [Google Scholar] [CrossRef]
- Sillero, N.; Brito, J.C.; Skidmore, A.K.; Toxopeus, A.G. Biogeographical patterns derived from remote sensing variables: The amphibians and reptiles of the Iberian Peninsula. Amphib. Reptil. 2009, 30, 185–206. [Google Scholar] [CrossRef]
- Humboldt, A. Essai sur la Geographie des Plantes; Accompagne d'un Tableau Physique des Régions Equinoxiales; Levrault, Schoell et Compagnie: Paris, France, 1805. [Google Scholar]
- Sclater, P. On the geographical distribution of the members of the class Aves. J. Linn. Soc. Zool. 1858, 2, 130–145. [Google Scholar] [CrossRef]
- Wallace, A.R. The Geographical Distribution of Animals; Macmillan: London, UK, 1876. [Google Scholar]
- Vilhena, D.A.; Antonelli, A. A network approach for identifying and delimiting biogeographical regions. Nat. Commun. 2015, 6, 6848. [Google Scholar] [CrossRef] [PubMed]
- Holt, B.G.; Lessard, J.-P.; Borregaard, M.K.; Fritz, S.A.; Araújo, M.B.; Dimitrov, D.; Fabre, P.H.; Graham, C.H.; Graves, G.R.; Jønsoon, K.A. An update of Wallace’s zoogeographic regions of the world. Science 2013, 339, 74–78. [Google Scholar] [CrossRef]
- Kreft, H.; Jetz, W. A framework for delineating biogeographical regions based on species distributions. J. Biogeogr. 2010, 37, 2029–2053. [Google Scholar] [CrossRef]
- Chapin, J.P. Ecological aspects of bird distribution in tropical Africa. Am. Nat. 1923, 57, 106–125. [Google Scholar] [CrossRef]
- Simpson, G.G. Too many lines: The limits of the Oriental and Australian zoogeographic regions. Proc. Am. Philos. Soc. 1977, 121, 107–120. [Google Scholar]
- Crowe, T.M.; Crowe, A.A. Patterns of distribution, diversity and endemism in Afrotropical birds. J. Zool. 1982, 198, 417–442. [Google Scholar] [CrossRef]
- Smith, S.A.; Bermingham, E. The biogeography of lower Mesoamerican freshwater fishes. J. Biogeogr. 2005, 32, 1835–1854. [Google Scholar] [CrossRef]
- Sillero, N.; Campos, J.C.; Bonardi, A.; Corti, C.; Creemers, R.; Crochet, P.A.; Crnobrnja-Isailovic, J.; Denoel, M.; Ficetola, G.F.; Gonçalves, J. Updated distribution and biogeography of amphibians and reptiles of Europe. Amphib. Reptil. 2014, 35, 1–31. [Google Scholar] [CrossRef]
- Moreno Saiz, J.C.; Donato, M.; Katinas, L.; Crisci, J.V.; Posadas, P. New insights into the biogeography of south-western Europe: Spatial patterns from vascular plants using cluster analysis and parsimony. J. Biogeogr. 2013, 40, 90–104. [Google Scholar] [CrossRef]
- Freitas, R.; Romeiras, M.; Silva, L.; Cordeiro, R.; Madeira, P.; González, J.A.; Wirtz, P.; Falcón, J.M.; Brito, A.; Floeter, S.R. Restructuring of the ‘Macaronesia’ biogeographic unit: A marine multi-taxon biogeographical approach. Sci. Rep. 2019, 9, 15792. [Google Scholar] [CrossRef] [PubMed]
- López, H.L.; Menni, R.C.; Donato, M.; Miquelarena, A.M. Biogeographical revision of Argentina (Andean and Neotropical Regions): An analysis using freshwater fishes. J. Biogeogr. 2008, 35, 1564–1579. [Google Scholar] [CrossRef]
- Peterson, A.T. Ecological niche conservatism: A time-structured review of evidence. J. Biogeogr. 2011, 38, 817–827. [Google Scholar] [CrossRef]
- Jarnevich, C.S.; Stohlgren, T.J.; Kumar, S. Caveats for correlative species distribution modelling. Ecol. Inf. 2015, 29, 6–15. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Feng, X.; Park, D.S.; Walker, C.; Peterson, A.T.; Merow, C.; Papes, M. A checklist for maximizing reproducibility of ecological niche models. Nat. Ecol. Evol. 2019, 3, 1382–1395. [Google Scholar] [CrossRef]
- Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Sillero, N.; Carretero, M.A. Modelling the past and future distribution of contracting species. The Iberian lizard Podarcis carbonelli (Squamata: Lacertidae) as a case study. Zool. Anz. 2013, 252, 289–298. [Google Scholar] [CrossRef]
- Peterson, A.T.; Pape, M.; Eaton, M. Transferability and model evaluation in ecological niche modeling: A comparison of GARP and Maxent. Ecography 2007, 30, 550–560. [Google Scholar] [CrossRef]
- Nogués-Bravo, D. Predicting the past distribution of species climatic niches. Glob. Ecol. Biogeogr. 2009, 18, 521–531. [Google Scholar] [CrossRef]
- Franklin, J. Moving beyond static species distribution models in support of conservation biogeography. Divers. Distrib. 2010, 16, 321–330. [Google Scholar] [CrossRef]
- Seabra, R.; Wethey, D.; Santos, A.M.; Lima, F.P. Understanding complex biogeographic responses to climate change. Sci. Rep. 2015, 5, 12930. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, F. Climate changes hot-spots. Geophys. Res. Lett. 2006, 33, L08707. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Lopez-Moreno, J.-I.; Beguería, S.; Lorenzo-Lacruz, J.; Sanchez-Lorenzo, A.; García-Ruiz, J.M.; Azorin-Molina, C.; Morán-Tejeda, E.; Revuelto, J.; Trigo, R. Evidence of increasing drought severity caused by temperature rise in southern Europe. Environ. Res. Lett. 2014, 9, 044001. [Google Scholar] [CrossRef]
- Thiébault, S.; Moatti, J.-P. (Eds.) The Mediterranean Region under Climate Change: A Scientific Update; IRD: Marseille, France, 2016; p. 133. [Google Scholar]
- Knapp, A.K.; Smith, M.D. Variation among biomes in temporal dynamics of aboveground primary production. Science 2001, 291, 481–484. [Google Scholar] [CrossRef]
- Salazar, L.F.; Nobre, C.A.; Oyama, M.D. Climate change consequences on the biome distribution in tropical South America. Geophys. Res. Lett. 2007, 34, L09708. [Google Scholar] [CrossRef]
- Carvalho, S.B.; Brito, J.C.; Crespo, E.J.; Possingham, H.P. From climate change predictions to actions—Conserving vulnerable animal groups in hotspots at a regional scale. Glob. Chang. Biol. 2010, 16, 3257–3270. [Google Scholar] [CrossRef]
- Rebelo, H.; Tarroso, P.; Jones, G. Predicted impact of climate change on European bats in relation to their biogeographic patterns. Glob. Chang. Biol. 2010, 16, 561–576. [Google Scholar] [CrossRef]
- Serra-Diaz, J.M.; Franklin, J. What’s hot in conservation biogeography in a changing climate? Going beyond species range dynamics. Divers. Distrib. 2019, 25, 492–498. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Moreno-Rueda, G.; Pleguezuelos, J.M.; Pizarro, M.; Montori, A. Northward shifts of the distributions of Spanish reptiles in association with climate change. Conserv. Biol. 2011, 26, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Auer, S.K.; King, D.I. Ecological and life-history traits explain recent boundary shifts in elevation and latitude of western North American songbirds. Glob. Ecol. Biogeogr. 2014, 23, 867–875. [Google Scholar] [CrossRef]
- Williams, J.E.; Blois, J.L. Range shift in response to past and future climate change: Can climate velocities and species’ dispersal capabilities explain variation in mammalian range shifts? J. Biogeogr. 2018, 45, 2175–2189. [Google Scholar] [CrossRef]
- Borzée, A.; Andersen, D.; Groffen, J.; Kim, H.T.; Bae, Y.; Jang, Y. Climate change-based models predict range shifts in the distribution of the only Asian plethodontid salamander: Karsenia koreana. Sci. Rep. 2019, 9, 11838. [Google Scholar] [CrossRef] [PubMed]
- Rosalino, L.M.; Guedes, D.; Cabecinha, D.; Serronha, A.; Grilo, C.; Santos-Reis, M.; Monterroso, P.; Carvalho, J.; Fonseca, C.; Pardavila, X. Climate and landscape changes as driving forces for future range shift in southern populations of the European badger. Sci. Rep. 2019, 9, 3155. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 6772, 853–858. [Google Scholar] [CrossRef]
- Pascual, L.L.; Luigi, M.; Alessandra, F.; Emilio, B.; Luigi, B. Hotsposts of species richness, threat and endemism for terrestrial vertebrates in SW Europe. Acta Oecol. 2011, 37, 399–412. [Google Scholar] [CrossRef]
- Sillero, N. What does ecological modelling model? A proposed classification of ecological niche models based on their underlying methods. Ecol. Model. 2011, 222, 1343–1346. [Google Scholar] [CrossRef]
- Álvarez-López, E.A. Los caracteres geográficos de la herpetofauna ibérica (Contribución al estudio de la Zoogeografía peninsular). Bol. Real Soc. Hist. Nat. (Secc. Biol.) 1934, 34, 327–374. [Google Scholar]
- Williams, P.H.; Humphries, C.; Araújo, M.B.; Lampinen, R.; Hagemeijer, W.; Gasc, J.P.; Mitchell-Jones, T. Endemism and important areas for representing European biodiversity: A preliminary exploration of atlas data for plants and terrestrial vertebrates. Belg. J. Entomol. 2000, 2, 21–46. [Google Scholar]
- Hewitt, G.M. Some genetic consequences of ice ages, and their role in divergence and speciation. Biol. J. Linn. Soc. 1996, 58, 247–276. [Google Scholar] [CrossRef]
- Hewitt, G.M. The genetic legacy of the Quaternary ice ages. Nature 2000, 22, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Martínez, S. Mapa de Series, Geoseries y Geopermaseries de Vegetación de España, Parte 1. Memoria del Mapa de Vegetación Potencial de España; Universidad Complutense: Madrid, Spain, 2005; pp. 1–28. [Google Scholar]
- European Environment Agency. Biogeographical Regions; European Environment Agency: Copenhagen, Denmark, 2016. [Google Scholar]
- Blondel, J.; Aronson, J. Biology and Wildlife of the Mediterranean Region; Oxford University Press: Oxford, UK, 1999; p. 352. [Google Scholar]
- Gibbons, J.W.; Scott, D.E.; Ryan, T.J.; Buhlmann, K.A.; Tuberville, T.D.; Metts, B.S.; Greene, J.L.; Mills, T.; Leiden, Y.; Poppy, S.; et al. The global decline of reptiles, déjà vu amphibians. BioScience 2000, 50, 653–661. [Google Scholar] [CrossRef]
- Houlahan, J.E.; Findlay, C.S.; Schmidt, B.R.; Meyer, A.H.; Kuzmin, S.L. Quantitative evidence for global amphibian population declines. Nature 2000, 404, 752–755. [Google Scholar] [CrossRef]
- Aragón, P.; Rodríguez, M.A.; Olalla-Tárraga, M.A.; Lobo, J.M. Predicted impact of climate change on threatened terrestrial vertebrates in central Spain highlights differences between endotherms and ectotherms. Anim. Conserv. 2010, 13, 363–373. [Google Scholar] [CrossRef]
- Stuart, S.N.; Chanson, J.S.; Cox, N.; Young, B.E.; Rodrigues, A.S.L.; Fischman, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef]
- Böhm, M.; Collen, B.; Baillie, J.E.M.; Bowles, P.; Chanson, J.; Cox, N.; Hammerson, G.; Hoffmann, M.; Livingstone, S.R.; Ram, M. The conservation status of the world’s reptiles. Biol. Conserv. 2013, 157, 372–385. [Google Scholar] [CrossRef]
- Pleguezuelos, J.M.; Márquez, R.; Lizana, M. Atlas de distribución y Libro Rojo de los Anfibios y Reptiles de España; Dirección General de Conservación de la Naturaleza-Asociación Herpetológica Española: Madrid, Spain, 2002. [Google Scholar]
- Loureiro, A.; Ferrand de Almeida, N.; Carretero, M.A.; Paulo, O.S. (Eds.) Atlas dos Anfíbios e Répteis de Portugal, 1st ed.; Instituto de Conservação da Natureza e da Biodiversidade: Lisbon, Portugal, 2010; p. 257. [Google Scholar]
- Comisión de Taxonomía de la AHE. Lista Patrón de los Anfibios y Reptiles de España: Conclusiones de Nomenclatura y Taxonomía para las Especies de Anfibios y Reptiles de España; Asociación Herpetológica Española: Barcelona, Spain, 2018. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Bedia, J.; Herrera, S.; Gutiérrez, J.M. Dangers of using global bioclimatic datasets for ecological niche modeling. Limitations for future climate projections. Glob. Planet. Chang. 2013, 107, 1–12. [Google Scholar] [CrossRef]
- Varela, S.; Lima-Ribeiro, M.S.; Terribile, L.C. A short guide to the climatic variables of the Last Glacial Maximum for biogeographers. PLoS ONE 2015, 10, e0129037. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Reisinger, A., Eds.; IPCC: Geneva, Switzerland, 2007; p. 104. [Google Scholar]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Otto-Bliesner, B.L.; Marshall, S.J.; Overpeck, J.T. Simulating Arctic climate warmth and icefield retreat in the last interglaciation. Science 2006, 311, 1751–1753. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, J.D. A Short History of Planet Earth: Mountains, Mammals, Fire and Ice; John Wiley & Sons, Inc.: New York, NY, USA, 1996; p. 274. [Google Scholar]
- Clark, P.U.; Dyke, A.S.; Shakun, J.D.; Carlson, A.E.; Clark, J.; Wohlfarth, B.; Mitrovica, J.X.; Hostetler, S.W.; McCabe, M. The last glacial maximum. Science 2009, 325, 710–714. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, C.F.; Jones, R.G.; Lee, R.W.; Rowell, D.P. Selecting CMIP5 GCMs for downscaling over multiple regions. Clim. Dyn. 2015, 44, 3237–3260. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modelling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudik, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J. Maxent is not a presence-absence method: A comment on Thibaud et al. Methods Ecol. Evol. 2014, 5, 1192–1197. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Elith, J.; Graham, C.H.; Lehmann, A.; Leathwick, J.; Ferrier, S. Sample selection bias and presence-only distribution models: Implications for background and pseudo-absence data. Ecol. Appl. 2009, 19, 181–197. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting thresholds of occurrence in the prediction of species distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Raes, N.; ter Steege, H. A null-model for significance testing of presence-only species distribution models. Ecography 2007, 30, 727–736. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling. R Package Version 1.1-4. 2017. Available online: http://rspatial.org/sdm/ (accessed on 12 March 2020).
- Mac Nally, R.; Fleishman, E.; Bulluck, L.P.; Betrus, C.J. Comparative influence of spatial scale on beta diversity within regional assemblages of birds and butterflies. J. Biogeogr. 2004, 31, 917–929. [Google Scholar] [CrossRef]
- Smith, K.G. Patterns of nonindigenous herpetofaunal richness and biotic homogenization among Florida counties. Biol. Conserv. 2006, 127, 327–335. [Google Scholar] [CrossRef]
- Garcia, R.A.; Cabeza, M.; Rahbek, C.; Araújo, M.B. Multiple dimensions of climate change and their implications for biodiversity. Science 2014, 344, 486. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, M.; Foden, W.; Visconti, P.; Watson, J.E.M.; Butchart, S.H.M.; Kovacs, K.M.; Scheffers, B.R.; Hole, D.G.; Martin, T.G.; Akçakaya, R. Assessing species vulnerability to climate change. Nat. Clim. Chang. 2015, 5, 215–224. [Google Scholar] [CrossRef]
- Enriquez-Urzelai, U.; Bernardo, N.; Moreno-Rueda, G.; Montori, A.; Llorente, G. Are amphibians tracking their climatic niches in response to climate warming? A test with Iberian amphibians. Clim. Chang. 2019, 154, 289–301. [Google Scholar] [CrossRef]
- Vargas, J.M.; Real, R. Biogeografía de los anfibios y reptiles de la Península Ibérica. In Distribución y Biogeografía de los Anfibios y Reptiles en España y Portugal, Monografías de Herpetología; Pleguezuelos, J.M., Ed.; Asociación Herpetológica Española y Universidad de Granada: Granada, Spain, 1997; pp. 310–320. [Google Scholar]
- Couto, M.A.; Sánchez, G.; Tavares, C.D.; Barceló, A.M.; Nunes, L.F.; Herráez, C.F.; Pires, V.; Marques, J.; Mendes, L.; Chazarra, A. Atlas Climático Ibérico; Instituto de Meteorologia de Portugal and Agencia Estatal de Meteorología, Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2011; p. 80. [Google Scholar]
- Hewitt, G.M. Ice ages: Their impact on species distributions and evolution. In Evolution on Planet Earth; Rothschild, L.J., Lister, A.M., Eds.; Academic Press: New York, NY, USA, 2003; pp. 339–361. [Google Scholar]
- Araújo, M.B.; Whittaker, R.J.; Ladle, R.J.; Erhard, M. Reducing uncertainty in projections of extinction risk from climate change. Glob. Ecol. Biogeogr. 2005, 14, 529–538. [Google Scholar] [CrossRef]
- Araújo, M.B.; Nogués-Bravo, D.; Diniz Filho, J.A.; Haywood, A.M.; Valdes, P.J.; Rahbek, C. Quaternary climate changes explain diversity among reptiles and amphibians. Ecography 2008, 31, 8–15. [Google Scholar] [CrossRef]
- Dormann, C.F. Promising the future? Global change projections of species distributions. Basic Appl. Ecol. 2007, 8, 387–397. [Google Scholar] [CrossRef]
- Ortega, Z.; Mencía, A.; Pérez-Mellado, V. Behavioral buffering of global warming in a cold-adapted lizard. Ecol. Evol. 2016, 6, 4582–4590. [Google Scholar] [CrossRef]
- Paniagua, L.L.; García-Martín, A.; Moral, F.J.; Rebollo, F.J. Aridity in the Iberian Peninsula (1960–2017): Distribution, tendencies, and changes. Theor. Appl. Climatol. 2019, 138, 811. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Araújo, M.B.; Jepson, P.; Ladle, R.; Watson, J.E.M.; Willis, K.J. Conservation Biogeography: Assessment and prospect. Divers. Distrib. 2005, 11, 3–23. [Google Scholar] [CrossRef]
- Gao, X.; Giorgi, F. Increased aridity in the Mediterranean region under greenhouse gas forcing estimated from high resolution simulations with a regional climate model. Glob. Planet. Chang. 2008, 62, 195–209. [Google Scholar] [CrossRef]
- Sánchez, E.; Domínguez, M.; Romera, R.; López de la Franca, N.; Gaertner, M.A.; Gallardo, C.; Castro, M. Regional modeling of dry spells over the Iberian Peninsula for present climate and climate change conditions. Clim. Chang. 2011, 107, 625–634. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Hughes, L.; McIntyre, S.; Lindenmayer, D.B.; Parmesan, C.; Possingham, H.P.; Thomas, C.D. Assisted colonization and rapid climate change. Science 2008, 321, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Maurer, B.A. Relating human population growth to the loss of biodiversity. Biodivers. Lett. 1996, 1, 1–15. [Google Scholar] [CrossRef]


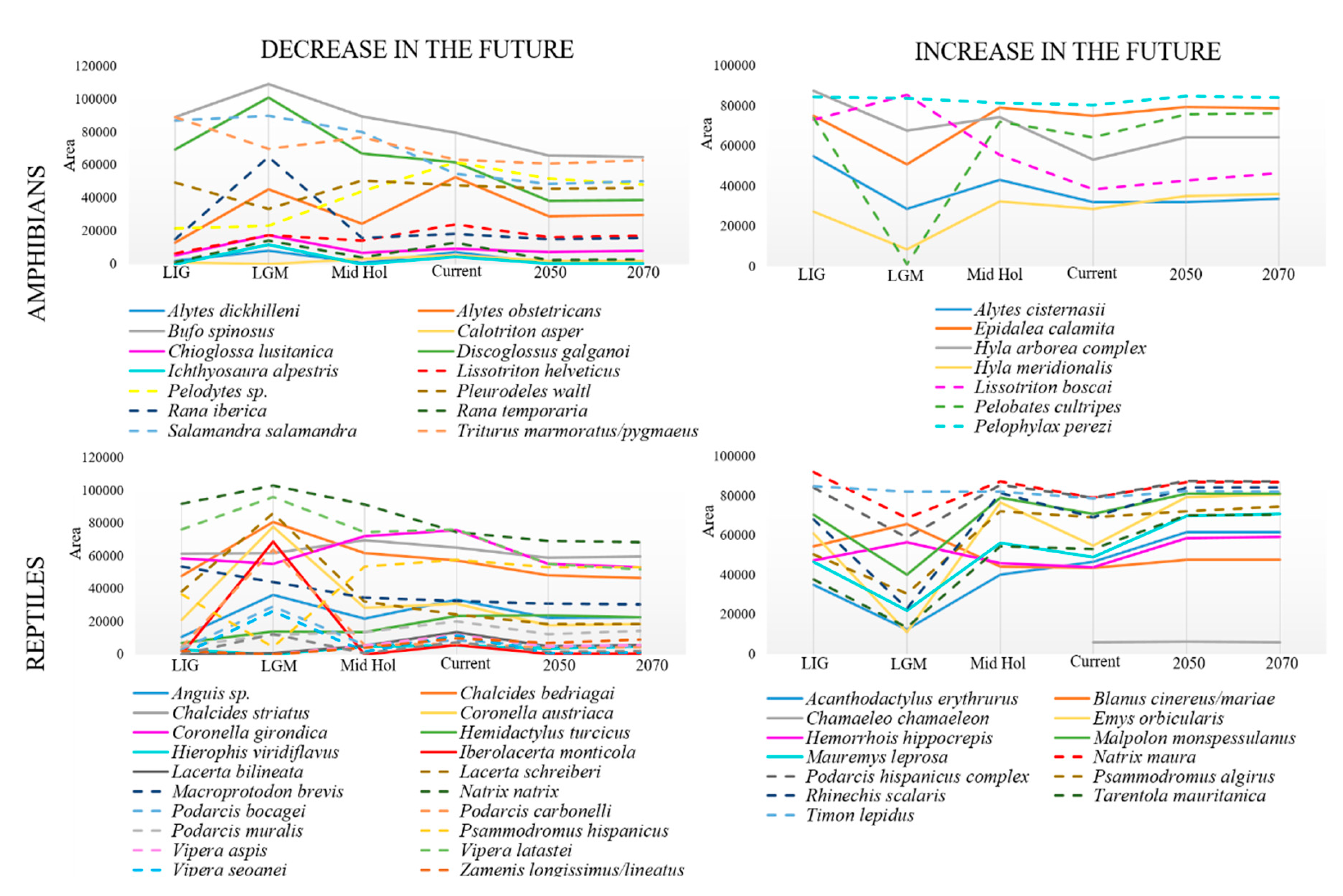
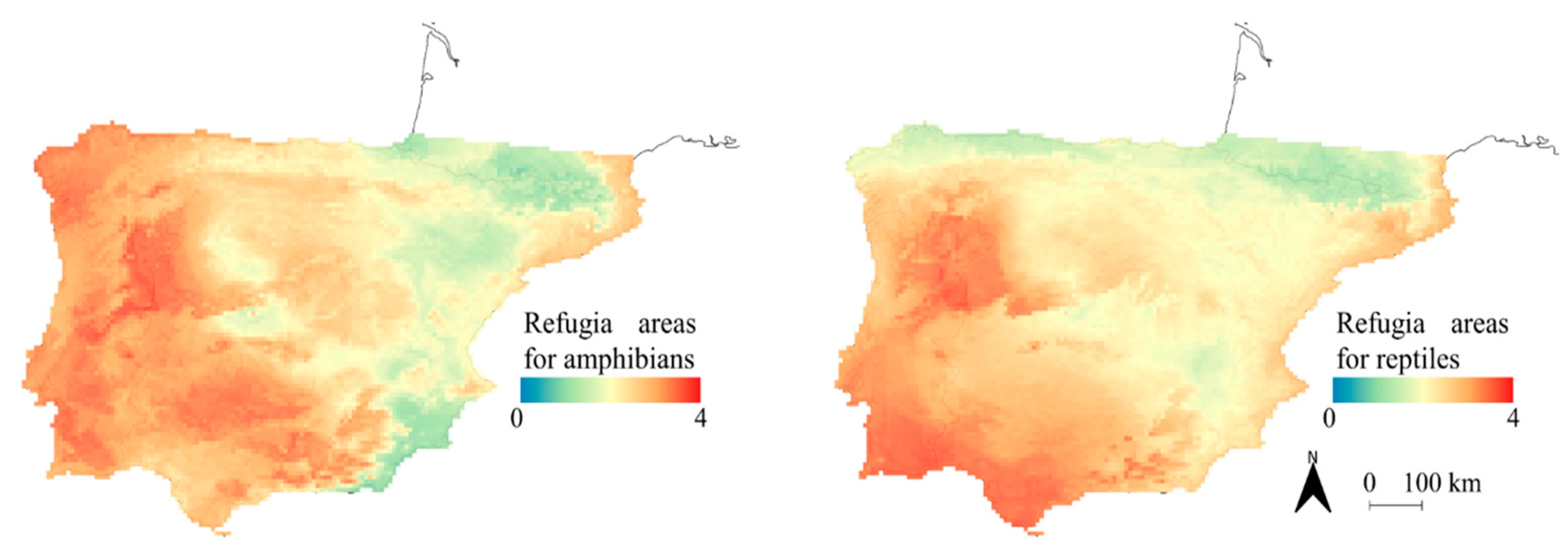
| Species | Training Records | Training AUC | Variable Contribution (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Bio4 | Bio6 | Bio8 | Bio9 | Bio12 | Bio17 | ||||
| Amphibians | Alytes cisternasii | 752 | 0.842 | 2.6 | 2.4 | 11.9 | 14.1 | 5.0 | 63.9 |
| Alytes dickhilleni | 119 | 0.968 | 41.8 | 11.8 | 7.1 | 21.6 | 4.7 | 13.0 | |
| Alytes obstetricans | 1504 | 0.732 | 8.3 | 1.2 | 1.6 | 1.5 | 0.5 | 86.9 | |
| Bufo spinosus | 2908 | 0.568 | 8.9 | 10.3 | 9.4 | 25.8 | 28.7 | 16.8 | |
| Calotriton asper | 138 | 0.974 | 6.1 | 2.3 | 1.8 | 9.7 | 2.0 | 78.1 | |
| Chioglossa lusitanica | 262 | 0.958 | 7.1 | 2.5 | 0.2 | 5.2 | 69.3 | 15.6 | |
| Discoglossus galganoi | 1214 | 0.698 | 9.4 | 8.8 | 32.3 | 19.0 | 5.7 | 24.9 | |
| Epidalea calamita | 2574 | 0.603 | 14.9 | 0.9 | 2.4 | 4.6 | 13.1 | 64.0 | |
| Hyla arborea complex | 971 | 0.758 | 13.3 | 13.1 | 26.8 | 29.2 | 0.9 | 16.9 | |
| Hyla meridionalis | 847 | 0.845 | 4.5 | 23.7 | 2.3 | 4.6 | 16.9 | 48.0 | |
| Ichthyosaura alpestris | 79 | 0.980 | 15.3 | 0.1 | 2.7 | 7.5 | 5.0 | 69.5 | |
| Lissotriton boscai | 1013 | 0.817 | 3.6 | 10.8 | 20.7 | 21.1 | 23.2 | 20.6 | |
| Lissotriton helveticus | 472 | 0.887 | 11.7 | 1.0 | 1.0 | 7.1 | 1.5 | 77.7 | |
| Pelobates cultripes | 1421 | 0.693 | 5.9 | 8.6 | 2.0 | 4.8 | 29.8 | 48.9 | |
| Pelodytes sp. | 1187 | 0.716 | 6.8 | 2.2 | 33.8 | 8.9 | 40.7 | 7.7 | |
| Pelophylax perezi | 3615 | 0.563 | 2.9 | 3.7 | 3.8 | 4.2 | 3.3 | 82.2 | |
| Pleurodeles waltl | 1194 | 0.769 | 1.1 | 0.7 | 2.0 | 14.8 | 10.5 | 70.8 | |
| Rana iberica | 575 | 0.910 | 3.4 | 0.4 | 10.5 | 8.3 | 52.0 | 25.4 | |
| Rana temporaria | 346 | 0.936 | 11.1 | 0.6 | 1.6 | 0.5 | 12.5 | 73.5 | |
| Salamandra salamandra | 1510 | 0.735 | 5.0 | 9.3 | 6.1 | 4.4 | 71.0 | 4.2 | |
| Triturus marmoratus/pygmaeus | 1548 | 0.689 | 8.9 | 1.7 | 6.4 | 36.0 | 35.2 | 11.8 | |
| Reptiles | Acanthodactylus erythrurus | 626 | 0.812 | 2.5 | 3.9 | 10.5 | 2.9 | 17.9 | 62.3 |
| Anguis sp. | 870 | 0.829 | 10.8 | 0.6 | 2.3 | 1.8 | 22.3 | 62.2 | |
| Blanus cinereus/mariae | 1216 | 0.780 | 2.2 | 0.2 | 2.4 | 8.7 | 1.1 | 85.4 | |
| Chalcides bedriagai | 603 | 0.757 | 7.5 | 4.3 | 5.2 | 15.6 | 2.1 | 65.3 | |
| Chalcides striatus | 1041 | 0.702 | 6.1 | 6.6 | 0.5 | 24.8 | 46.2 | 15.7 | |
| Chamaeleo chamaeleon | 108 | 0.974 | 9.5 | 5.7 | 2.0 | 1.3 | 0.6 | 80.9 | |
| Coronella austriaca | 428 | 0.862 | 3.0 | 1.3 | 14.6 | 35.4 | 5.0 | 40.7 | |
| Coronella girondica | 1267 | 0.644 | 15.1 | 8.3 | 25.6 | 20.3 | 14.2 | 16.6 | |
| Emys orbicularis | 402 | 0.762 | 18.9 | 7.5 | 13.7 | 14.0 | 14.5 | 31.4 | |
| Hemidactylus turcicus | 474 | 0.886 | 4.9 | 18.1 | 17.8 | 2.7 | 4.5 | 52.0 | |
| Hemorrhois hippocrepis | 1006 | 0.795 | 4.0 | 9.9 | 6.4 | 4.3 | 2.1 | 73.4 | |
| Hierophis viridiflavus | 75 | 0.977 | 2.0 | 0.9 | 0.9 | 11.8 | 2.2 | 82.2 | |
| Iberolacerta monticola | 97 | 0.975 | 10.7 | 0.9 | 8.8 | 9.8 | 36.7 | 33.2 | |
| Lacerta bilineata | 312 | 0.934 | 3.4 | 1.5 | 1.4 | 4.0 | 3.0 | 86.7 | |
| Lacerta schreiberi | 649 | 0.877 | 3.5 | 0.8 | 6.5 | 27.1 | 50.8 | 11.3 | |
| Macroprotodon brevis | 458 | 0.854 | 0.9 | 0.9 | 2.9 | 6.7 | 1.9 | 86.7 | |
| Malpolon monspessulanus | 2409 | 0.629 | 5.7 | 5.5 | 10.6 | 7.3 | 4.2 | 66.5 | |
| Mauremys leprosa | 1428 | 0.753 | 4.4 | 18.2 | 2.5 | 6.0 | 3.6 | 65.2 | |
| Natrix maura | 2655 | 0.585 | 12.0 | 2.1 | 10.0 | 4.9 | 15.1 | 55.9 | |
| Natrix natrix | 1340 | 0.642 | 11.3 | 1.1 | 13.4 | 19.0 | 38.8 | 16.4 | |
| Podarcis bocagei | 303 | 0.953 | 3.9 | 0.6 | 3.4 | 6.7 | 50.5 | 35.0 | |
| Podarcis carbonelli | 76 | 0.970 | 36.1 | 3.0 | 6.2 | 3.3 | 19.3 | 32.0 | |
| Podarcis hispanicus complex | 2753 | 0.581 | 8.5 | 6.8 | 15.7 | 14.1 | 9.5 | 45.5 | |
| Podarcis muralis | 448 | 0.910 | 1.9 | 2.4 | 1.0 | 3.2 | 2.4 | 89.0 | |
| Psammodromus algirus | 2684 | 0.626 | 5.8 | 4.6 | 2.9 | 10.5 | 6.1 | 70.1 | |
| Psammodromus hispanicus | 1115 | 0.706 | 4.7 | 1.4 | 5.5 | 4.0 | 26.5 | 57.9 | |
| Rhinechis scalaris | 2114 | 0.637 | 11.8 | 5.3 | 11.5 | 8.3 | 5.5 | 57.5 | |
| Tarentola mauritanica | 1725 | 0.731 | 5.4 | 10.3 | 14.3 | 12.4 | 10.1 | 47.5 | |
| Timon lepidus | 2973 | 0.578 | 5.8 | 3.5 | 5.6 | 6.3 | 3.6 | 75.1 | |
| Vipera aspis | 234 | 0.949 | 15.1 | 0.6 | 1.8 | 1.5 | 0.7 | 80.3 | |
| Vipera latastei | 707 | 0.740 | 6.6 | 34.8 | 6.3 | 5.2 | 12.0 | 35.3 | |
| Vipera seoanei | 287 | 0.942 | 14.5 | 1.2 | 3.1 | 13.1 | 23.0 | 45.1 | |
| Zamenis longissimus/lineatus | 96 | 0.967 | 2.8 | 4.3 | 8.6 | 1.3 | 1.6 | 81.4 | |
| Zooteca vivipara | 143 | 0.967 | 11.5 | 0.8 | 7.8 | 1.5 | 2.4 | 75.9 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa-Guedes, D.; Arenas-Castro, S.; Sillero, N. Ecological Niche Models Reveal Climate Change Effect on Biogeographical Regions: The Iberian Peninsula as a Case Study. Climate 2020, 8, 42. https://doi.org/10.3390/cli8030042
Sousa-Guedes D, Arenas-Castro S, Sillero N. Ecological Niche Models Reveal Climate Change Effect on Biogeographical Regions: The Iberian Peninsula as a Case Study. Climate. 2020; 8(3):42. https://doi.org/10.3390/cli8030042
Chicago/Turabian StyleSousa-Guedes, Diana, Salvador Arenas-Castro, and Neftalí Sillero. 2020. "Ecological Niche Models Reveal Climate Change Effect on Biogeographical Regions: The Iberian Peninsula as a Case Study" Climate 8, no. 3: 42. https://doi.org/10.3390/cli8030042
APA StyleSousa-Guedes, D., Arenas-Castro, S., & Sillero, N. (2020). Ecological Niche Models Reveal Climate Change Effect on Biogeographical Regions: The Iberian Peninsula as a Case Study. Climate, 8(3), 42. https://doi.org/10.3390/cli8030042







