1. Introduction
Water scarcity represents a significant global challenge, with agriculture consuming over 70% of global freshwater usage [
1]. Inefficient irrigation methods substantially contribute to water wastage, particularly in areas experiencing drought and diminishing water resources. Optimizing irrigation efficiency is crucial for water conservation as well as improving the production of crops despite changes in climatic circumstances [
2].
Precise assessment of plant hydration levels is essential for maximizing irrigation efficiency. Conventional techniques, such as relative water content (RWC) and pressure chamber measurements, are commonly invasive, labor-intensive, and inappropriate for real-time applications [
3,
4]. Soil moisture sensors and atmospheric models, such as the Penman-Monteith equation, yield indirect estimations that are frequently affected by soil type, depth, and environmental variability, resulting in inaccurate assessments of plant water needs [
5,
6]. Advanced technologies, such as drones and hyperspectral imaging, provide extensive monitoring capabilities but are costly and complicated to deploy, particularly in small to medium-sized agricultural enterprises [
7,
8].
Although numerous strategies have been proposed to assess plant water status utilizing diverse plant tissues such as roots [
9], stems [
10], and fruits [
11], leaf-based methods persist as the most practical and efficient way for real-time measurement of plant hydration [
12]. These methods usually detect changes caused by differences in light or electrical properties or by tracking important plant activities such as transpiration rate [
13] or chlorophyll fluorescence [
14].
According to recent research, non-invasive techniques such as electrical impedance spectroscopy (EIS) and near-infrared spectroscopy (NIRS) can achieve highly accurate correlations with plant water status, facilitating early stress diagnosis compared to traditional methods [
15,
16]. In a study, hardwood tree leaves were exposed to full-range NIRS (1200–2500 nm) in a controlled laboratory environment. Using partial least squares regression (PLSR) analysis, a correlation R2 = 0.94–0.97 was obtained for estimating the water content of the leaves indoors [
17]. Similarly, Portable NIRS systems exhibit over 90% correlation with pressure chamber measurements in Shiraz grape leaves, facilitating real-time and non-destructive analysis [
18]. Additionally, the best-performing model was able to predict the leaf water content with an R
2pred of 0.85 and an RMSEP of 2.32%, according to a study on the water condition of eucalypt leaves using a handheld NIR spectrometer. [
19] On the other hand, in canola crops, EIS methods show a maximum correlation coefficient (R) of 0.99 with the RWC approach, along with a root mean square error (RMSE) of 0.30 and a coefficient of determination (R
2) of 0.98 [
20]. Likewise, a study that used an EIS with a precision LCR meter to track the dehydration of onions over three weeks in ambient storage showed good prediction performance (R2 = 0.98) [
21]. The feasibility of evaluating Labisia pumila’s water status using electrical impedance spectroscopy (EIS) in a controlled greenhouse condition was examined in [
16], where the results showed that the plant group with the lowest irrigation regime showed the highest impedance (~0.10–0.15 MΩ at 70–100 kHz), and regression analysis revealed R
2 = 0.78 (LWP) and R
2 = 0.73 (RWC).
However, each of these promising studies has been performed under highly controlled lab or greenhouse conditions with strictly regulated irrigation methods, producing results that are frequently unfeasible for actual agricultural situations characterized by fluctuating weather, soil heterogeneity, and diverse management techniques.
This work aims to investigate how direct electrical and optical measures taken on the leaf through simple, affordable, and non-invasive devices can be used to assess the general plant water status. It was shown that the combination of two methods, electric impedance spectroscopy (EIS) and infrared spectroscopy (IR), allows the extraction of information that correlates with statistical significance with the level of water stress of the plant.
Based on this objective, the study seeks to determine whether non-invasive infrared and electrical impedance measurements performed directly on the leaf can effectively distinguish plants subjected to different irrigation treatments. In addition, it explores whether the parameters derived from these sensors show meaningful correlations with standard reference measurements such as relative water content.
The measurement of a few leaf tissue parameters, indirectly related to the plant’s general water status, can thus be used to monitor the plant and inform the irrigation strategies in smart farming applications. The experiment was carried out in two phases, separated by a 10-day interruption period, over a 20-day experiment on six Hydrangea macrophylla plants in uncontrolled outdoor conditions and exposed to two different irrigation treatments. The control group consisted of three plants watered regularly, while the test group consisted of three plants watered less frequently to cause the insurgence of water stress. The effectiveness of the treatments was checked using the standard RWC method, and both sensing methods successfully distinguished between well-watered and stressed plants, showing their ability to help make quick decisions in precision irrigation systems.
3. Materials and Methods
3.1. Experimental Setup
The experiment involved six Hydrangea macrophylla plants, selected for their large leaf area, numerous leaves, and high-water needs, making them ideal for research on monitoring plant water status. The large leaf area ensured by this type of plant was considered a key feature for experimentation since it allowed consistent placement of the measurement setup without causing damage to the plant. All plant samples were grown in standard plastic pots (30 cm in diameter and 25 cm in depth) containing a uniform soil mixture of 40% peat moss, 30% perlite, 20% compost, and 10% sand, which is a commercially recommended substrate for Hydrangea cultivation. The experiment was conducted over a total of 20 days during the summer of 2024 on the terrace of the Department of Electrical and Electronic Engineering at the University of Cagliari, Italy (coordinates: 39°13′47.3″ N, 9°06′31.3″ E), under uncontrolled outdoor conditions. The plants were placed on a partially shaded area of the terrace, where they received indirect sunlight during most of the day, minimizing exposure to direct solar radiation and preventing stress unrelated to the irrigation treatments such as rain or other harsh conditions. Weather conditions in the exact location of experiment were not collected by direct measures. Historical recordings for the area show that the temperature conditions were quite regular, with an average temperature of 27.4° (average minimum 18°, average maximum 39°), an average humidity of 66% (min 50%, max 79%) and three summer storm events occurring in the period.
The plants were divided into two distinct groups, each subjected to different irrigation treatments to induce varying hydration levels. The control group consisted of three well-irrigated plants (Awell, Bwell, and Cwell), which received daily watering to maintain optimal hydration, while the test group (Abad, Bbad, and Cbad) was irrigated only once every three days to induce water stress. The three-day irrigation interval for the stressed group was chosen based on preliminary observations, showing it effectively induces water stress in Hydrangea macrophylla without leading to leaf loss or permanent damage. Before starting the measurements, all plants underwent a one-week pre-irrigation period to equalize their hydration levels.
The first phase of the experiment lasted for 12 consecutive days. This was followed by a 10-day interruption, during which all plants were irrigated in the same way and data collection was paused. To restore the initial conditions, a second one-week pre-irrigation period was applied, ensuring uniform hydration across all plants once again. The experiment then resumed for another 8 days of consecutive measurements, following the same initial treatment conditions.
Throughout the study, a representative healthy leaf was tagged on each plant to ensure consistency in measurements. Daily recordings were carried out in the late evening to minimize variability caused by sunlight and temperature fluctuations, using the two sensing methods described in the following sections. Additionally, RWC measurements were performed just every three days to minimize plant damage.
3.2. Relative Water Content Method
This study used the RWC method as a reference for assessing each plant’s hydration status. RWC estimates the current water content of the sampled leaf tissue relative to its fully hydrated state [
22]. RWC levels typically vary from 98% in completely turgid leaves to 30–40% in severely dehydrated and withering leaves, depending on the plant type [
23].
For each RWC assessment, at midday, a healthy, mature leaf was selected and cut from each plant and then placed directly in aluminum bags to prevent loss of turgidity. Eight discs were carefully cut from each leaf using a cork borer with diameter of approximately 16 mm and immediately weighed using a high-precision laboratory balance with a sensitivity of 0.1 mg [
24] to obtain fresh weight (
FW).
After that, the discs are then immersed in distilled water for 4 h at room temperature to reach full turgidity and will be directly weighed, after gently removing the excess surface water, to obtain the turgid weight (
TW). Lastly, we dried the samples at 70 °C for 10 min and weighed them to determine their dry weight (
DW).
RWC was calculated using the standard formula shown in Equation (1):
3.3. Infrared Spectroscopy
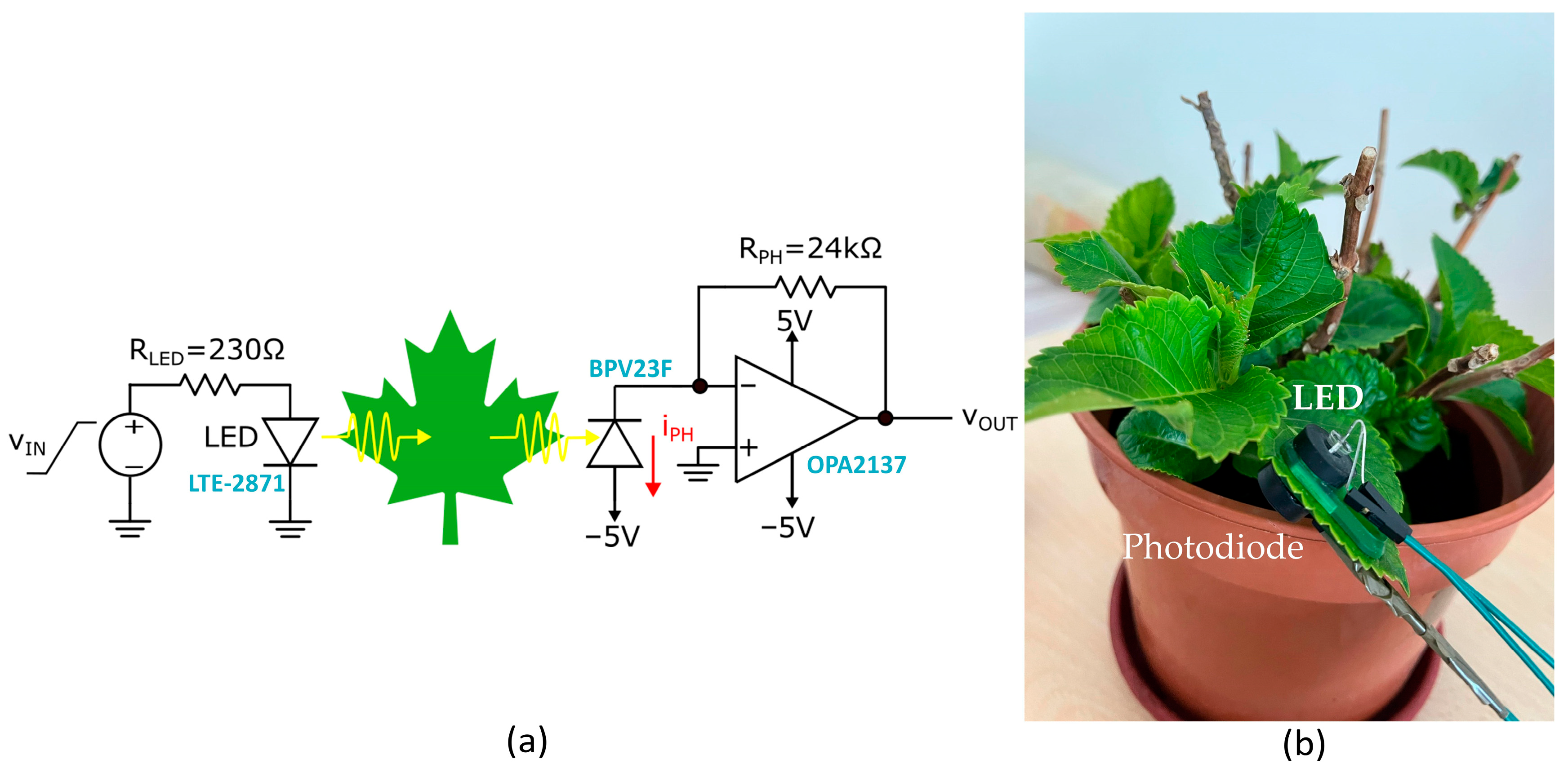
The infrared spectroscopy method was implemented by a simple experimental setup including three main elements: an infrared LED “LTE-2871 (Lite-on Technology, Taipei, Taiwan)” with a peak wavelength at 940 nm [
25] which serves as the light source, a photodiode “BPV23F (Vishay Intertechnology, Malvern, PA, USA)” with a spectral range between 400 and 1100 nm [
26] acts as a transducer and converts light into a current, and a transimpedance amplifier to be used as current-to-voltage converter. The infrared LED was driven by a ramp-up voltage, from 0 to 5 V, generated by the Analog Discovery 2 (AD2) [
27], and the reported final voltage was computed as the average of the photodiode responses across the entire LED active range where generator voltages V
gen ∈ [∼1.25 V, V
max] as shown in the
Supplementary Material (Figure S1), this minimizes sensitivity to noise or local fluctuations and improves signal resolution. The incident infrared (IR) light penetrates the leaf tissue, where part of it is absorbed by the water content in the leaf, while the transmitted portion is detected by the photodiode. Then, the output photocurrent was amplified and converted into a voltage signal (V
out) using a transimpedance amplifier (TIA) realized with a “OPA2137 (Texas Instruments, Dallas, TX, USA)” [
28] in a voltage-to-current configuration with a feedback resistor of 24 kΩ. The two input channels of AD2 were used to display both the input and output waveforms. Both the LED and the Photodiode were mounted on two faces of a custom 3D-printed clip equipped with magnets to ensure stable placement between the leaf, as shown in
Figure 1. Since ambient light could alter the reading of IR information, to minimize its impact all measurements were consistently conducted during shaded periods in the late evening, when direct sunlight was reduced.
Measurements were taken daily over the 20-day experiment, subdivided into 2 time periods of 12 and 8 days with a 10-day interruption in between, and the collected absorption data were calculated using the formula of Equation (2). A baseline reading (
Vbaseline) was recorded, without a leaf, indicating 100% light transmittance, which is emitted from the LED directly to the Photodiode. This approach was used to calibrate all the daily measurements.
3.4. Electrical Impedance Spectroscopy
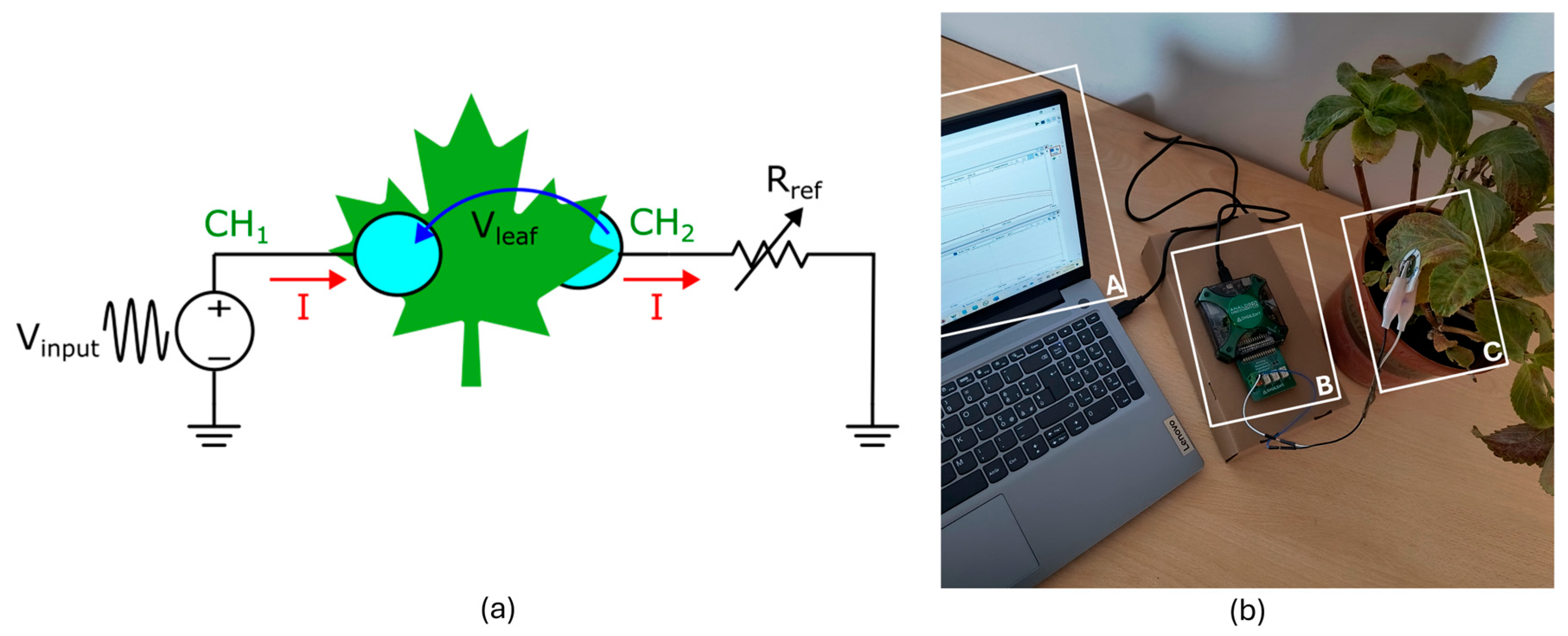
The impedance spectroscopy method was implemented using a Digilent (AD2) device equipped with its impedance analyzer adapter and two custom ECG electrodes (
Figure 2). The adapter’s printed circuit board (PCB) includes multiple precision reference resistors, automatically selected via relays based on the leaf’s impedance range. The AD2 waveform generator produces a known sinusoidal AC signal at a specified frequency through the adapter’s terminals, where the leaf is connected. Channel 1 of the oscilloscope (CH
1) measures the applied waveform voltage (V
input), and channel 2 (CH
2) measures the voltage across the leaf (V
leaf). The known reference resistor (R
ref) in series with the leaf is used to determine the current flowing through the leaf (I). Ohm’s law calculates the impedance of the leaf (Z
leaf) by dividing the applied voltage (Vl
eaf) by the measured current (I). The software directly calculates the phase difference between the displayed signals (V
input and V
leaf). The impedance measurements were calculated over a frequency sweep from 10 Hz to 1 MHz, using 151 logarithmically spaced frequency points. This number of points provides sufficient data density for reliable fitting of the impedance spectra.
To improve consistency and minimize electrode–leaf variability, we followed a standardized procedure to place the electrodes. The electrodes were placed in direct contact with the upper and lower surfaces at the same marked spot on each leaf. To ensure good contact between the electrodes and the leaf tissue, light pressure was applied so that the gel layer adhered properly to both sides of the leaf, ensuring alignment with the leaf surface structure. All electrode applications were performed by the same operator to maintain consistency. New ECG electrodes were used for each measurement to avoid tissue damage and ensure the measurement’s repeatability crosses the same leaf. The impedance data (magnitude and phase) were recorded and collected daily with the same protocol used for the infrared spectroscopy.
Data measurements were exported and processed in Python version 3.10 using the impedance.py library [
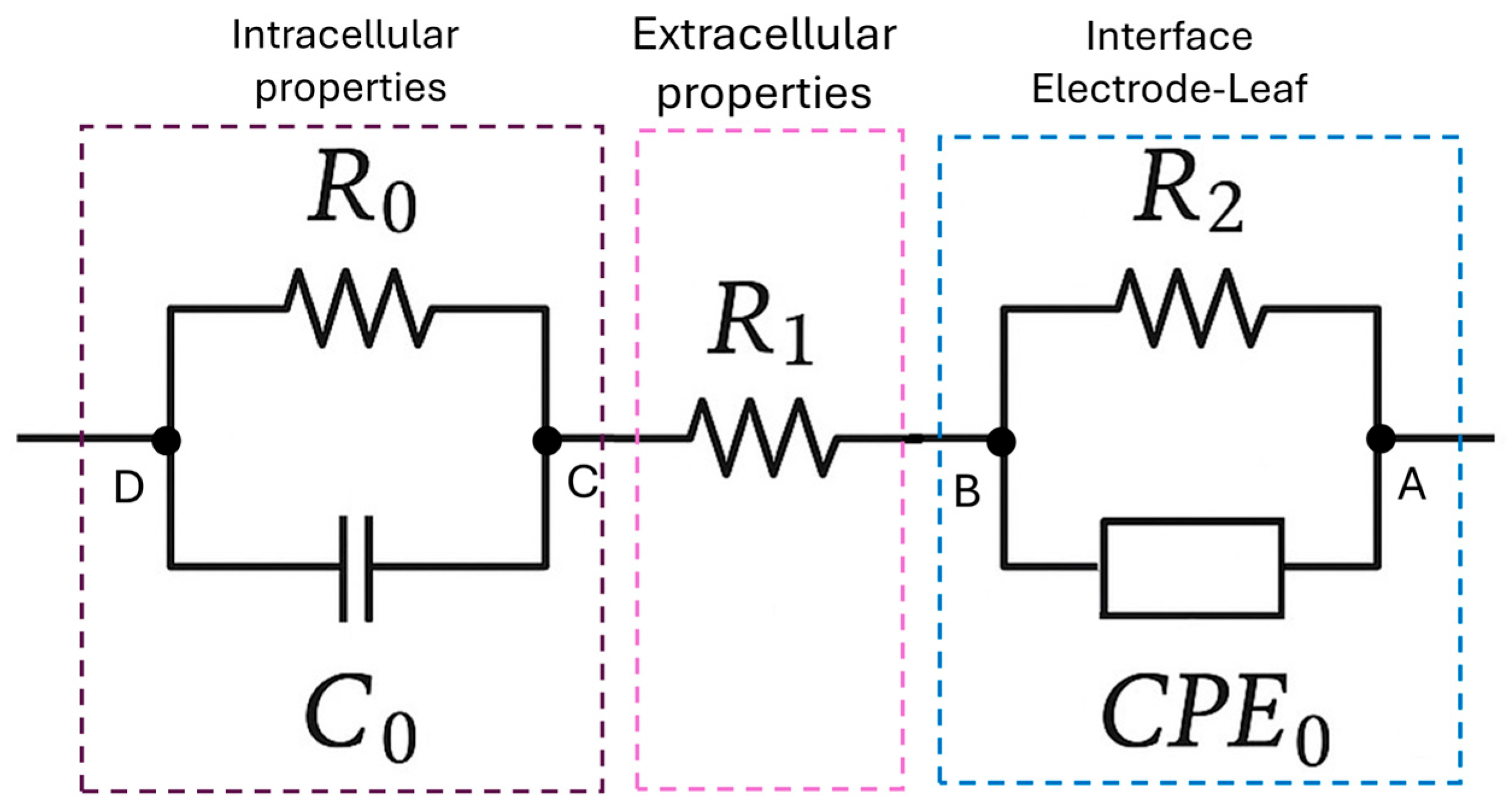
29]. Impedance values were transformed into complex formats (real and imaginary components) and fitted into a defined equivalent electric circuit (
Figure 3) utilizing the
CustomCircuit() function from the same library.
The suggested model consisted of a resistance in series between two parallel branches. Each component was selected to reflect known electrophysiological properties of leaf tissues. The series resistance R1 is associated with extracellular ionic pathways, capturing conductivity through apoplastic spaces, while the parallel branch (R0, C0) captures intracellular properties, with R0 representing the intracellular resistance and C0 modeling the cell membrane capacitance. The other branch (R2‖
CPE0) was designed to represent the contact interface between the electrode and the leaf, where R2 reflects interfacial resistance and
CPE0 accounts for non-ideal capacitive behavior (Equation (3)) due to surface irregularities, with the phase factor
n indicating the degree of non-ideality [
30].
where Z
CPE = impedance of the CPE;
CPE0 = pseudo-capacitance; ω = angular frequency;
j = imaginary unit and
n = phase factor (0 ≤
n ≤ 1).
The fitting was performed using a hybrid optimization approach to ensure accurate and efficient estimation of circuit parameters. First, a global optimization algorithm was employed using the SciPy optimize library [
31] to determine optimal initial guesses for the model parameters, improving convergence speed and avoiding local minima. These initial values were then used in a nonlinear least squares (NLLS) fitting process, which uses the Levenberg–Marquardt (LM) algorithm [
32] to minimize the disparity between the measured and predicted impedance data over different frequencies.
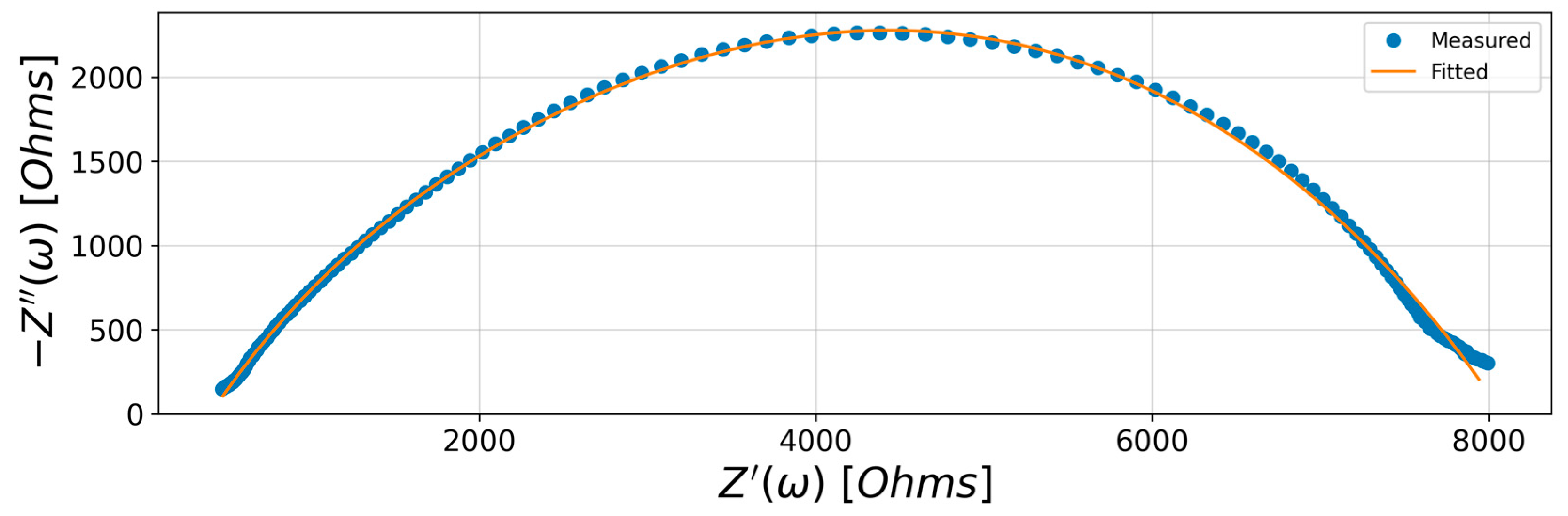
Performance of the fit is confirmed with the coefficient of determination R2, and a Nyquist plot is displayed to exhibit the impedance response and check the validity of the fitted model.
5. Discussion
This study assessed two non-invasive sensing methods, infrared spectroscopy and electrical impedance spectroscopy (EIS), to directly monitor the water status of six Hydrangea macrophylla plants from their leaf tissues. The experiment spanned 20 days and was divided into two phases: the first phase lasted 12 days, followed by a 10-day interruption and one-week pre-irrigation period, after which measurements resumed for an additional 8 days. Both sensing methods successfully distinguished between well-watered and water-stressed plants throughout the experiment. The effectiveness of the sensors was supported by statistical analyses and strong model fitting results, as indicated by high coefficients of determination (R2) between the normalized sensor parameters and the reference RWC values, confirming their potential for non-destructive water status monitoring.
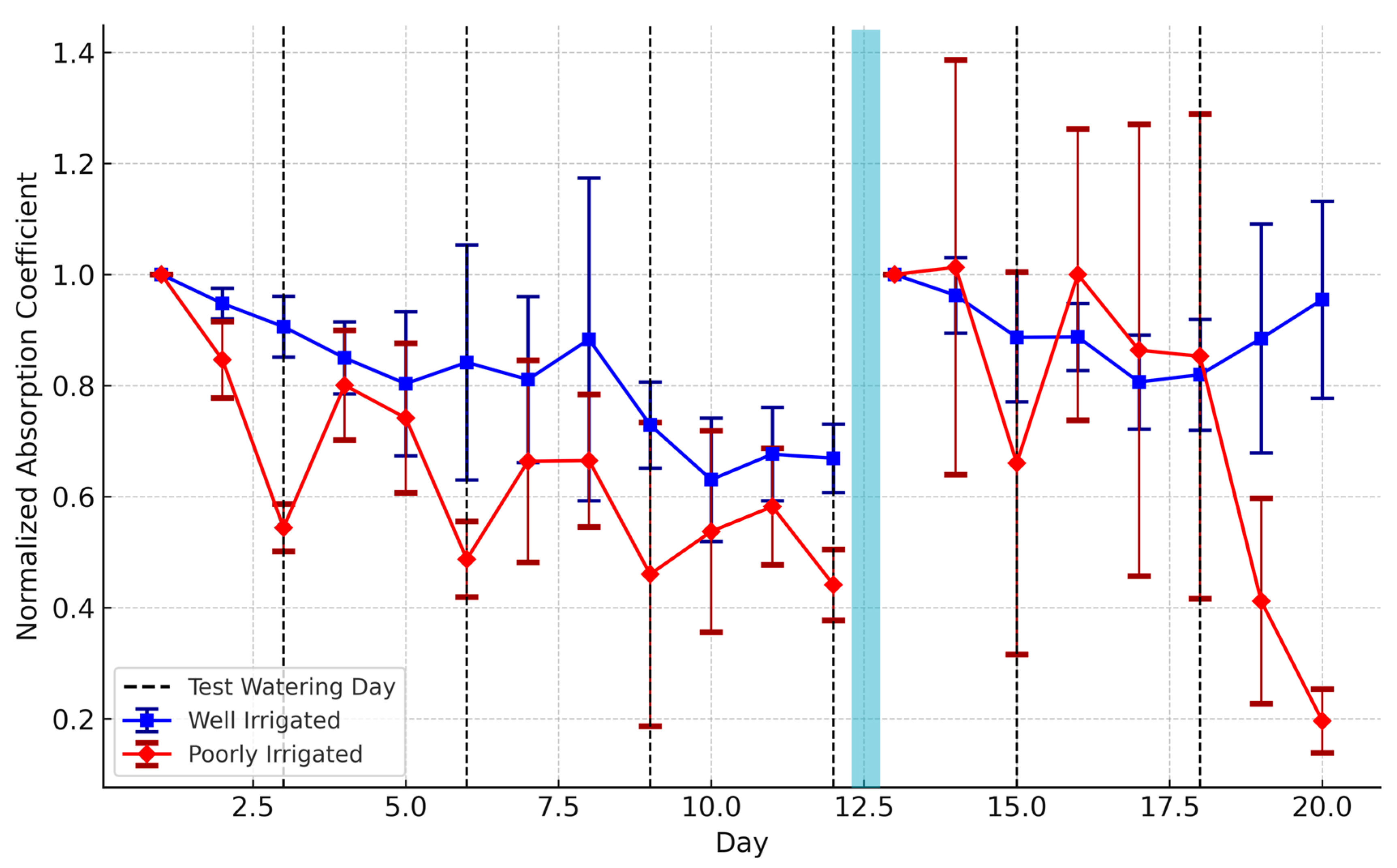
The observed decline in normalized absorption coefficients in water-stressed plants in both phases (
Figure 5) can be explained by structural changes in leaf tissues during dehydration. A decrease in the water content reduces leaf thickness and turgor pressure, which in turn leads to a decrease in optical path length and a change in absorption behavior. Researchers often quantify such results with an equivalent water thickness (EWT) in mm: the thicker the “water layer” in the leaf, the deeper the absorption features [
30]. These findings align with previous studies that highlight how leaf internal structure and moisture content affect optical properties in the near-infrared regions [
34,
35]. Furthermore, in [
17], it was reported that well-hydrated plants exhibit higher absorbance values in the NIR spectrum compared to water-stressed plants. This trend was also observed in our control group during the first phase of the experiment, where the normalized absorption values remained relatively stable and consistently higher than those of the test group. In the second phase, the response of the normalized absorption coefficients for both groups was unexpected. Specifically, between days 16 and 18, the poorly irrigated group exhibited high normalized absorption values followed by a sharp decline in the final two days and for the well-irrigated group it showed lower normalized absorption values than the test group followed by slight recovery in last 2 days. This phenomenon could be explained by the physiological adaptation response of the plants [
36,
37]. The combination of two stress periods, first phase and 10 days of interruption, may have triggered an adaptive mechanism in the poorly irrigated group, enhancing their tolerance to harsh conditions. the subsequent one-week irrigation period. may have enabled the poorly irrigated group to temporarily recover and build some resistance to water stress. As a result, in the early days of the second phase, these plants showed a transient improvement in optical responses. However, once water stress was reintroduced, their response may have become desensitized due to prior stress memory leading to a sharp decrease in the last two days reduced sensitivity to further irrigation events. Conversely, the well-irrigated plants were not previously exposed to water deficits before experiencing unexpected stress during the interruption period that affected their ability to recover fully even after the one-week irrigation period. This could explain their low infrared response during the early days up to day 18 following by a slight recovery in the last 2 days.
Another noteworthy observation in this study was that increases in the normalized absorption coefficients of the water-stressed plants often occurred the day after irrigation rather than on the same day. Although measurements were taken approximately 10 h after watering, no immediate change was detected. This slow reaction could suggest that plant physiological recovery mechanisms do not happen instantly but rather develop over time. Research indicated in [
38] demonstrates that xylem embolism significantly reduces stem-specific conductivity in maize plants during daylight hours; however, they can recover overnight if sufficient soil moisture is available.
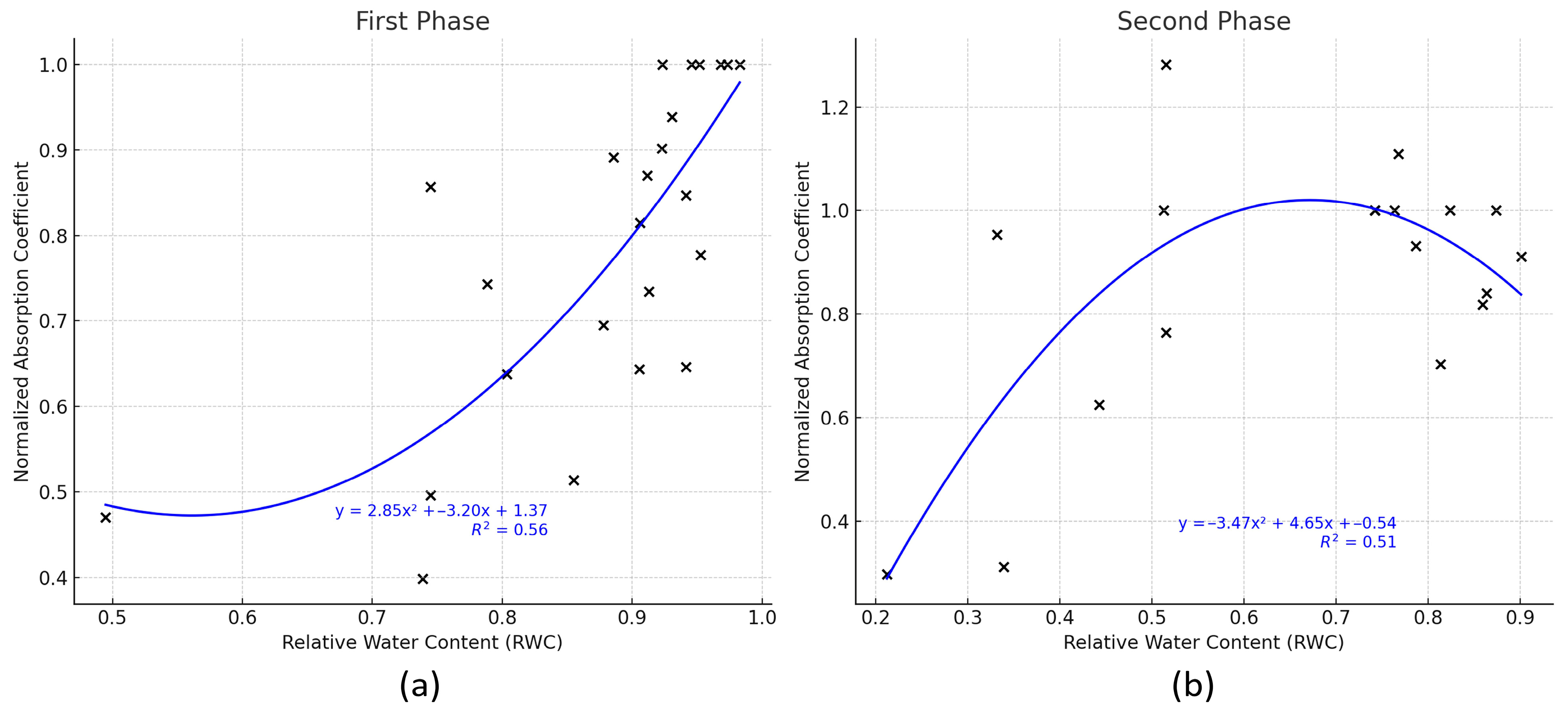
The infrared spectroscopy method showed a high correlation with the RWC method by using a quadratic regression approach where the determination coefficients R
2 = 0.56 and R
2 = 0.51, respectively, to the first and second phases of the experiment, confirming the potential of this non-destructive technique to monitor plant hydration levels. These results may appear weaker compared to some other studies that used similar optical sensing techniques as the study mentioned in [
39] where they achieved high determination coefficients up to 0.9899 in the correlation between leaf water content and diffuse reflectance spectra in Miscanthus. However, it is worth noting that our results were achieved in uncontrolled outdoor conditions with plants exposed to any real weather event, rather than in highly controlled environments as in the above-mentioned studies.
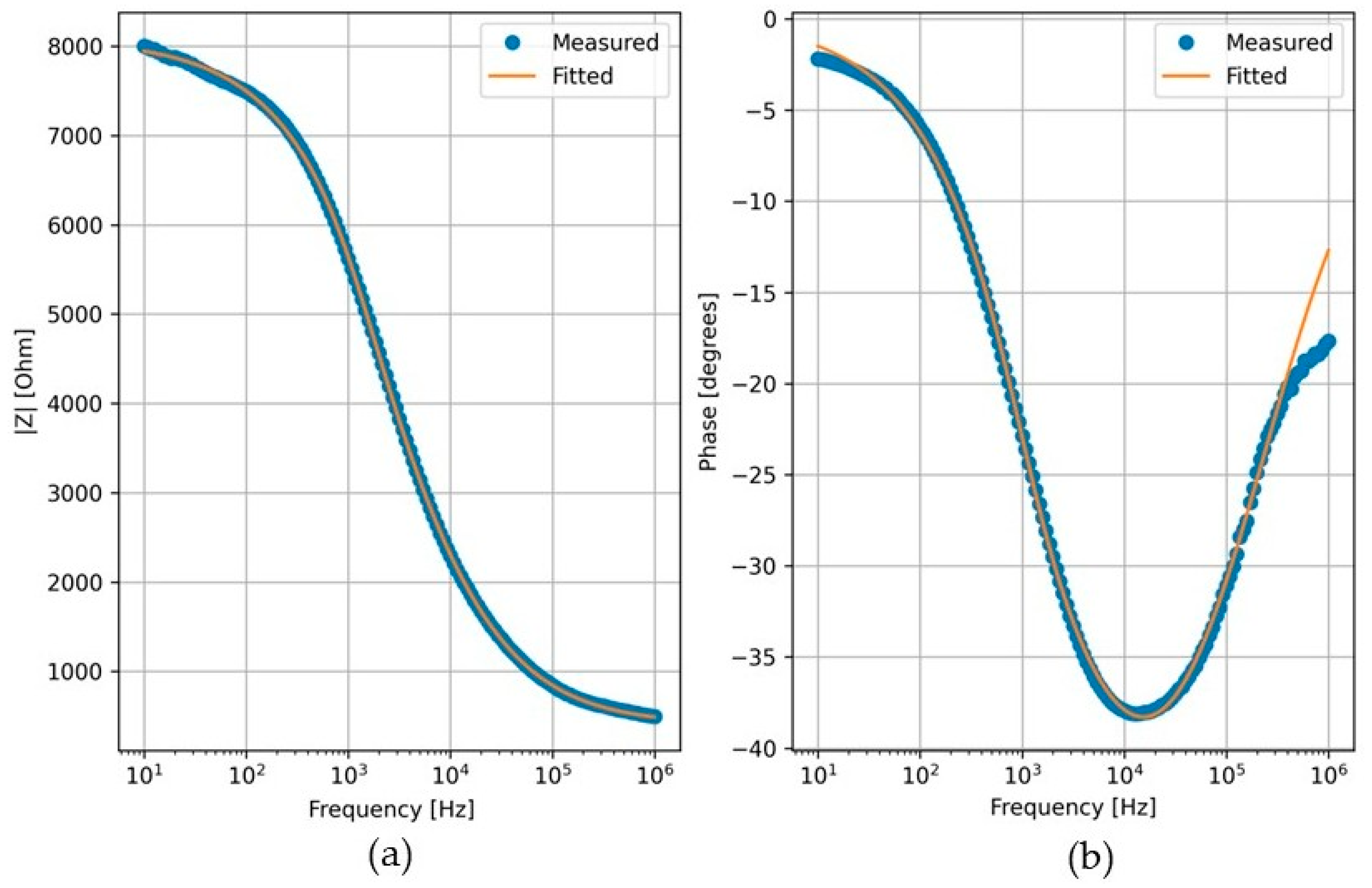
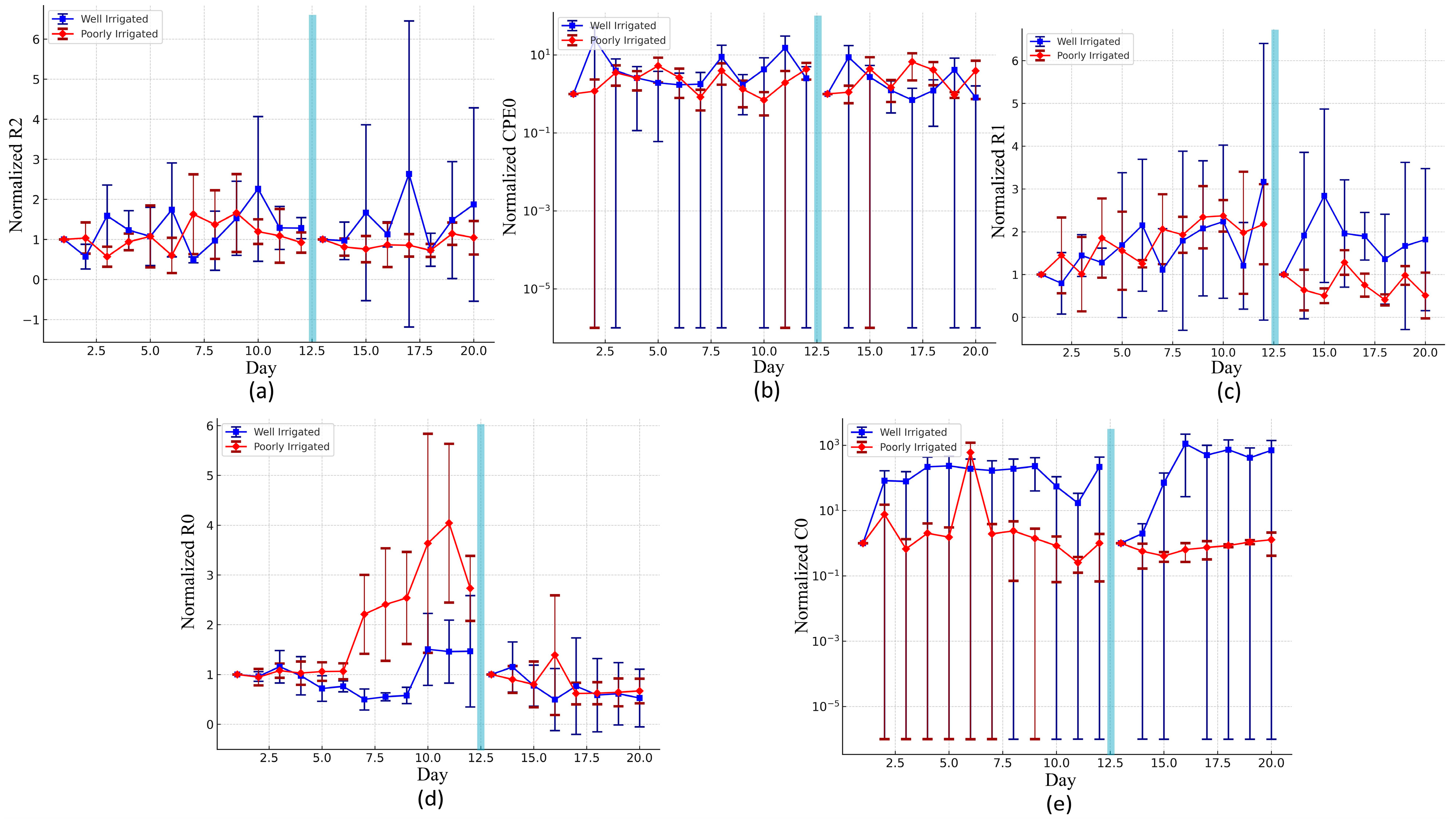
The impedance spectroscopy analysis started from suggesting an equivalent electrical circuit model (
Figure 3) that fits well the impedance spectra as shown in Nyquist and Bode plots (
Figure 7 and
Figure 8). This model is selected based on established bioimpedance literature, including both plant and biomedical applications, where electrical components are commonly associated with biological structures [
40,
41,
42,
43,
44]. Each component represents the electrophysiological properties of leaf tissues. These extracted parameters were plotted during both phases of the experiment for well and poorly irrigated groups (
Figure 9). The results showed that some of these parameters could distinguish between both treatments whether immediately at a very early stage as normalized C0 or after some days as normalized R0. Statistical analysis confirmed that only R0 and C0 exhibited significant differences (
p < 0.001) between treatment groups, indicating that they are effective indicators of plant water status.
Figure 9d shows how the normalized R0 increases over time for the poorly irrigated plants after 5 days, maintaining a steady level for the well-hydrated plants. This increase in resistance values for the stressed plants is probably associated with reduced ionic mobility in the intracellular spaces due to the water deficit [
45]. Water acts as the principal medium for ion transport; thus, tissue dryness increases resistance and makes the current harder to flow [
46]. However, in
Figure 9e, it emerges that the normalized C0 starts increasing over time for the well-watered group immediately after one day in both phases of the experiment, while it remains low with little fluctuations for the stressed plants. These features may be related to turgor pressure in the cells, where any loss can damage the membranes and reduce their ability to store charge. This is confirmed by a study where capacitance sensors on chili pepper and tomato leaves show that higher leaf moisture causes changes in the dielectric constant, which in turn yields higher capacitance readings [
47].
While the IR measurements showed a clear and quantifiable relationship with RWC allowing the use of regression models with acceptable R2 values, the same was not true for the impedance-derived parameters. Although we attempted to model the relationship between the normalized parameters like R0, C0, and RWC using various regression types, the resulting R2 values were too low to be considered statistically meaningful. This suggests that the impedance parameters may not follow a simple or direct functional relationship with RWC under the current measurement conditions.
These encouraging results could be further improved by addressing a few issues that emerged during the experiments, particularly the impedance spectroscopy. The use of two-point electrodes to measure the impedance is prone to a reduced sensitivity due to leaf–electrode contact impedance. To address this, adopting four-terminal electrode configurations, such as those implemented in [
48], could improve the reliability of the impedance sensor by minimizing contact-related artifacts. Additionally, it was observed that prolonged application of the custom electrodes on the leaf surface may have caused tissue damage, likely due to the obstruction of stomatal pores responsible for transpiration. As a result, new electrodes had to be reapplied for each daily measurement. This daily reapplication introduced variability, particularly affecting the stability of fitted parameters such as CPE0 and C0, and contributed to the large standard deviations observed in these normalized values. These parameters are especially sensitive to factors such as electrode–leaf contact quality, alignment precision, and natural structural differences in the leaf surface. Developing bioelectrodes that are more compatible with the leaf’s surface structure could offer a promising solution, as suggested in [
44,
49]. As concerns infrared spectroscopy, integrating methods to eliminate background light interference is critical to improving the precision of optical sensor readings, particularly under daylight conditions where ambient light can affect measurement accuracy.
Future improvements should focus on increasing the sample size for each treatment group and incorporating multiple leaf measurements per plant to reduce variability, as well as taking measurements at different spots on the same leaf to assess intra-leaf variability and also, should consider mixed-effects modeling to better account for temporal dynamics and plant-specific variability, which may be masked by pooled statistical approaches like Welch’s t-test. Additionally, continuous monitoring of the environmental parameters such as temperature, humidity, and light intensity should be incorporated to improve data interpretation and the impact of these parameters on such measurements.
The study will also need to be extended to various crop types, such as tomatoes and grapevines, to better understand the model’s generalizability and the practical use of the proposed sensors in the field. Furthermore, integrating impedance parameters with IR data through a formal sensor fusion could provide a more complete picture of plant water status.
Due to the low sample size, the results, although promising, should be considered a testbench for the proposed approaches. The number of plants and leaves involved in the experimentation must be increased to improve the robustness of statistical considerations. The system employed should be miniaturized and integrated into a custom printed circuit board (PCB), including a front end for measurement and a microcontroller for data processing and communication. The size of the device should be appropriately scaled to be used in an operative field, like, for example, a vineyard or an orchard [
48]. In this context, microcontrollers such as Arduino or ESP32 make them suitable for scalable deployment in precision agriculture thanks to their low power consumption, integrated wireless communication capabilities (e.g., Wi-Fi, Bluetooth, or LoRa) and cost-effective hardware.