Comparison of the Micromorphology and Ultrastructure of Pollen Grains of Selected Rubus idaeus L. Cultivars Grown in Commercial Plantation
Abstract
1. Introduction
1.1. Genus Rubus in the Flora of Poland
1.2. Economic Importance
1.3. Morphology of Pollen from the Subfamily Rosoideae
2. Results
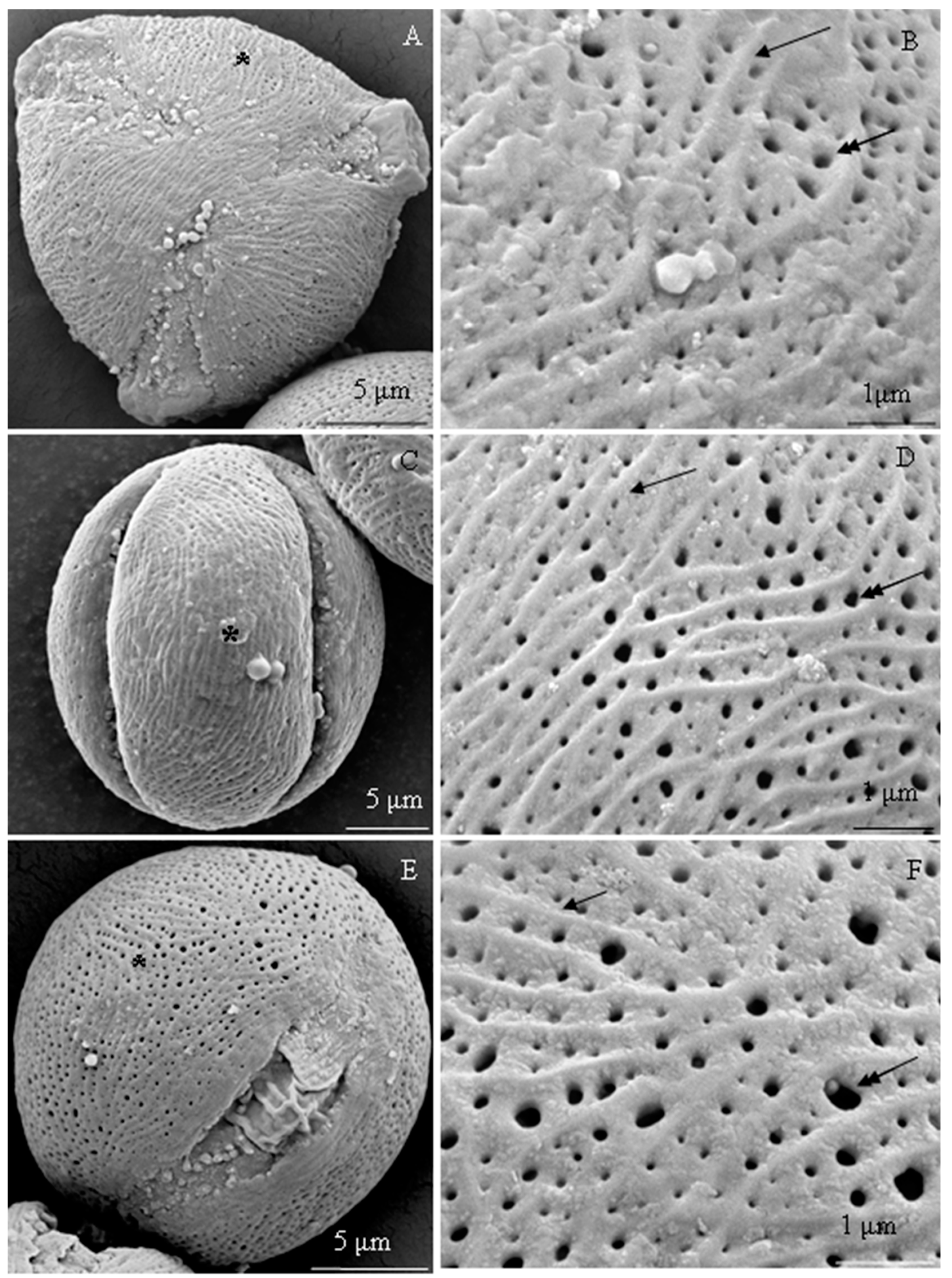
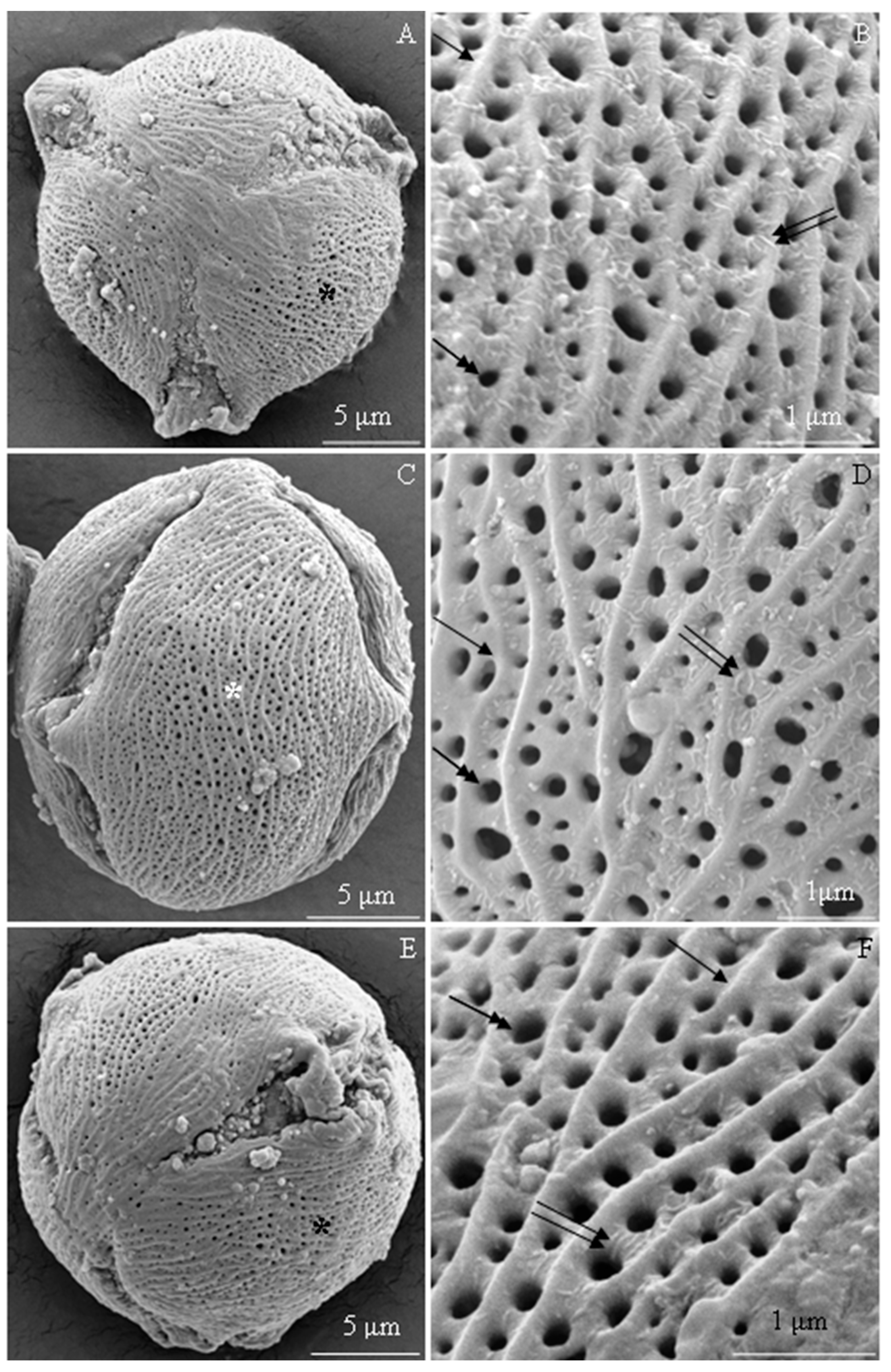
2.1. Micromorphology of Pollen Grains
2.1.1. Size and Shape of Pollen Grains
2.1.2. Exine Sculpture
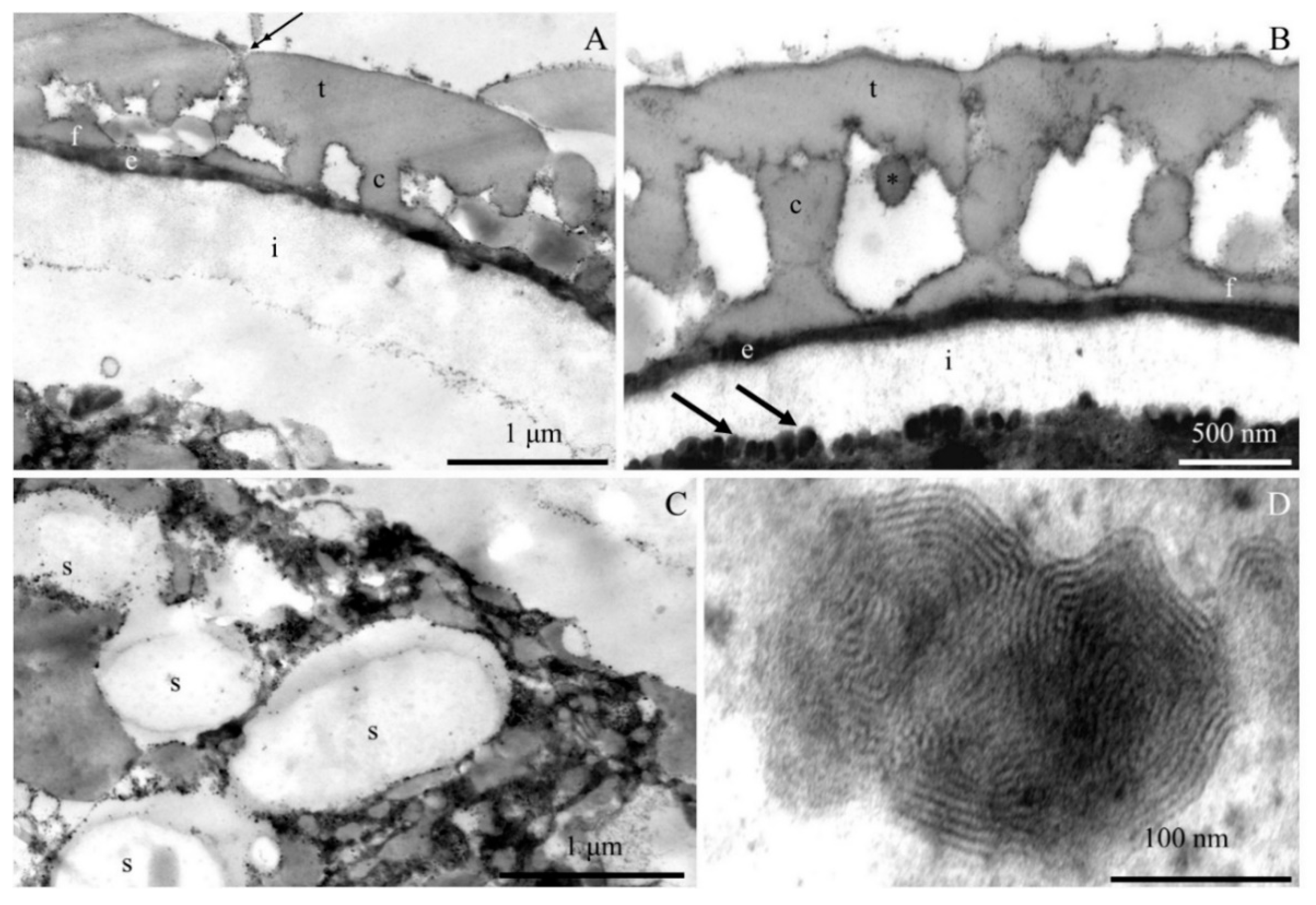
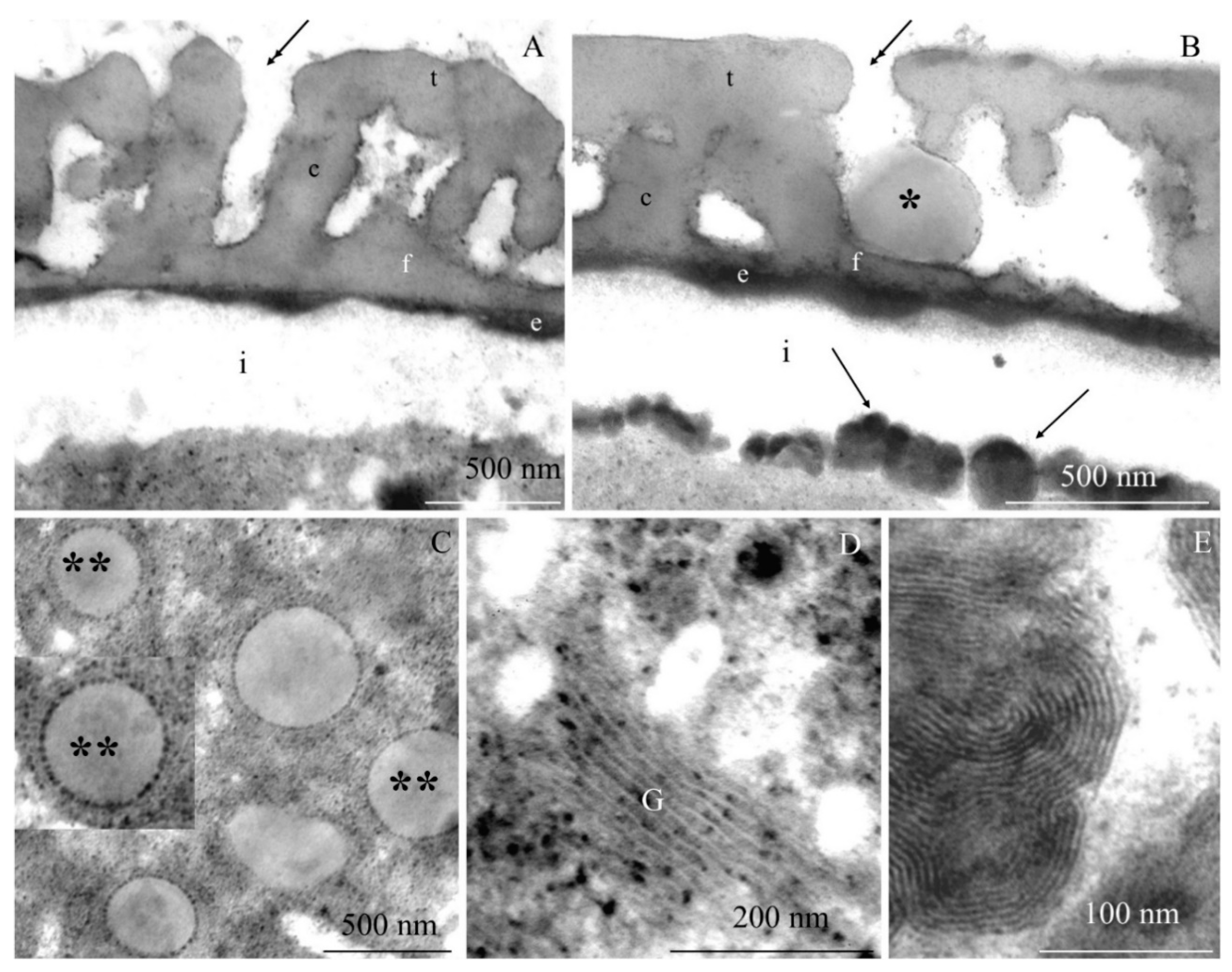
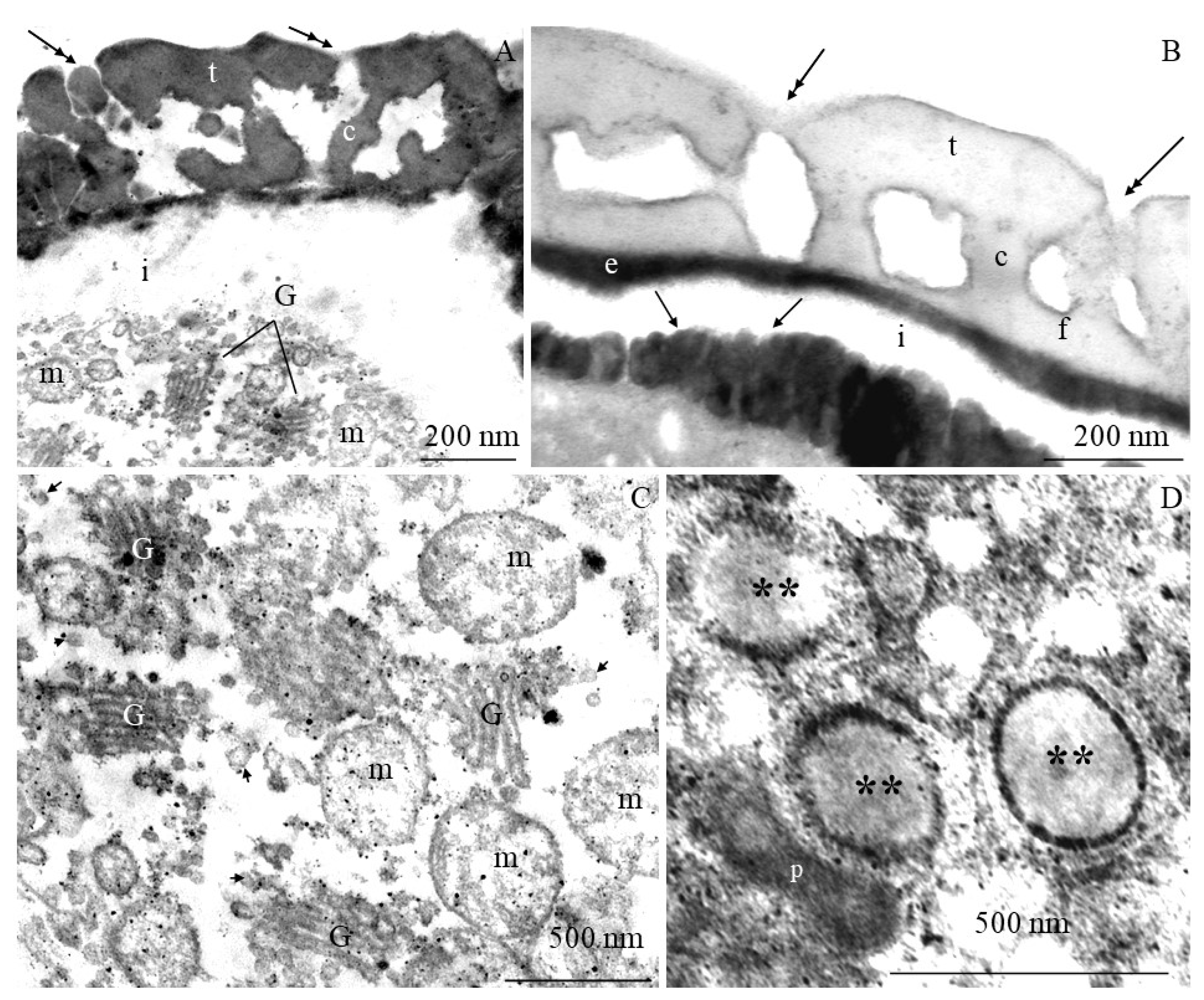
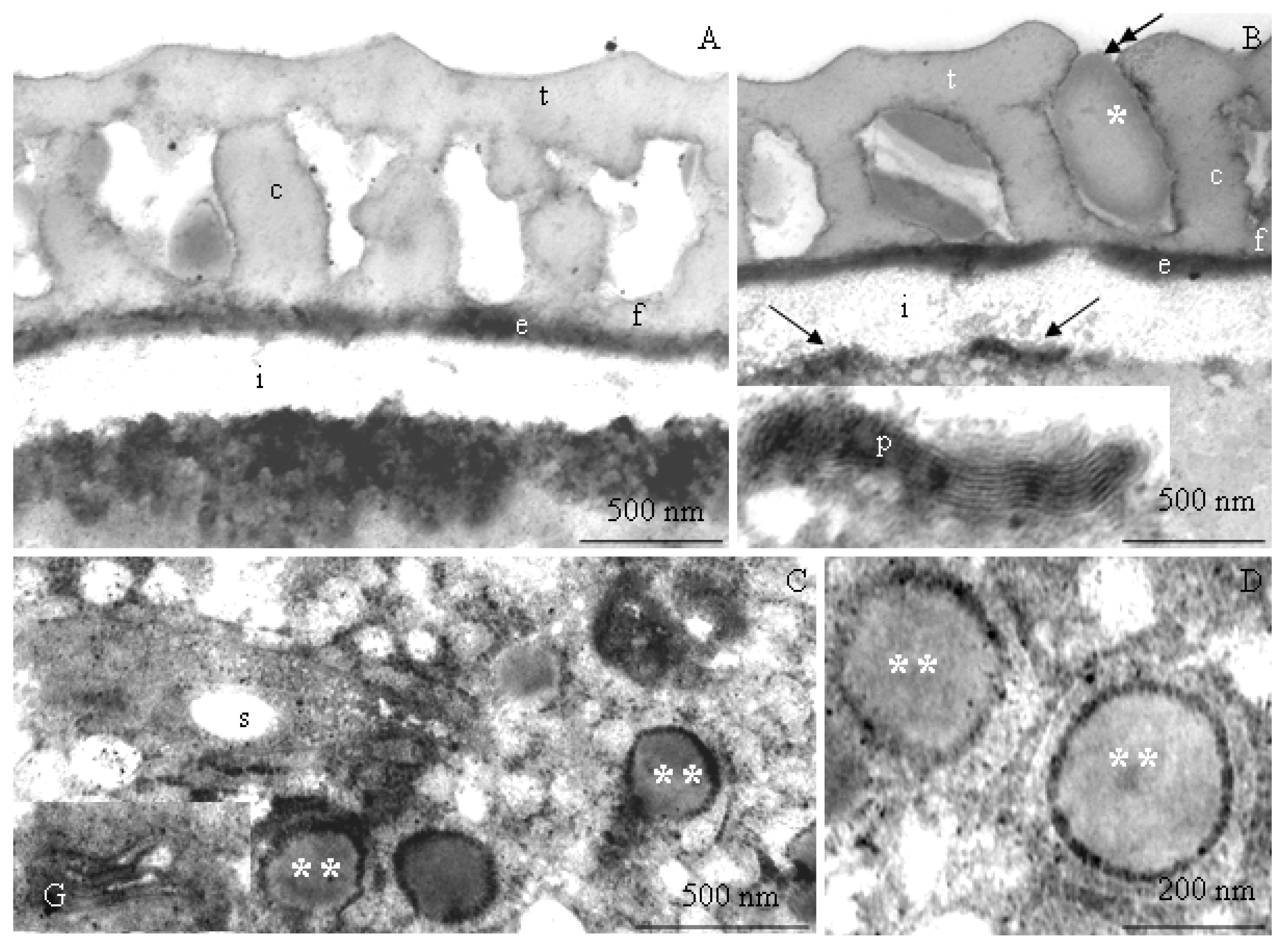
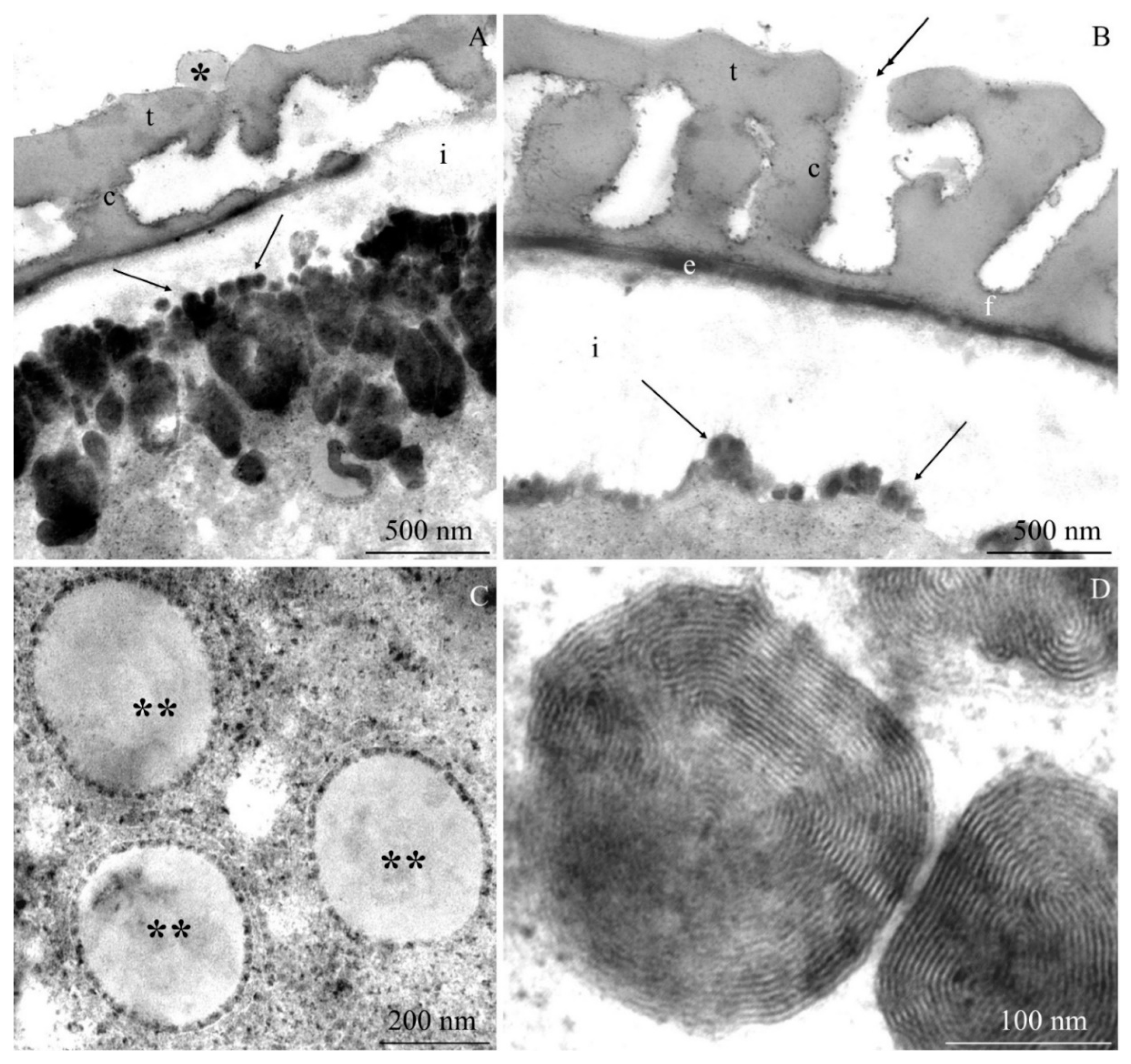
2.2. Ultrastructure of Pollen Grains
2.2.1. Pollen Grain Cell Wall
2.2.2. Protoplast
3. Discussion
3.1. Micromorphology of Pollen Grains
3.2. Ultrastructure of Pollen Grains
4. Materials and Methods
4.1. Study Area and Plant Material
4.2. Fixation of the Material
4.3. Light Microscopy (LM)
4.4. Scanning Electron Microscopy (SEM)
4.5. Transmission Electron Microscopy (TEM)
4.6. Morphometric Measurements
4.7. Statistical Analysis of Results
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ling-Ti, L. A study on the genus Rubus of China. J. Syst. Evol. 1983, 21, 13–25. [Google Scholar]
- Hummer, K.E. Rubus diversity. Hort. Sci. 1996, 31, 182–183. [Google Scholar] [CrossRef]
- Morden, C.W.; Gardner, D.E.; Weniger, D.A. Phylogeny and biogeography of Pacific Rubus subgenus Idaeobatus (Rosaceae) species: Investigating the origin of the endemic Hawaiian raspberry R. macraei. Pac. Sci. 2003, 57, 181–197. [Google Scholar] [CrossRef]
- Wang, H.; Sun, H. Rubus tetsunii, a replacement name for the Chinese, R. lobatus TT Yu & LT Lu (Rosaceae). Phytotaxa 2013, 79, 63–64. [Google Scholar]
- Wang, Y.; Chen, Q.; Fu, H.; Zhang, J.; Chen, T.; Sun, B.; Luo, Y.; Zhang, Y.; Thang, H.; Wang, X. Genome affinity in Rubus (Rosaceae) inferred from meiotic chromosome pairing of sixteen wild and cultivated bramble resources. Ind. J. Genet. Plant. Breed. 2018, 78, 496–506. [Google Scholar]
- Foster, T.M.; Bassil, N.V.; Dossett, M.; Leigh Worthington, M.; Graham, J. Genetic and genomic resources for Rubus breeding: A roadmap for the future. Hortic. Res. 2019, 6, 116. [Google Scholar] [CrossRef]
- Oklejewicz, K.; Luczaj, L. Folk taxonomy of Rubus species in Poland. In Proceedings of the Conference Dzikie Rośliny Jadalne-zapomniany Potencjal Przyrody, Przemysl-Bolestraszyce, Poland, 13 September 2007; pp. 201–218. (In Polish). [Google Scholar]
- Wang, H.C.; Wang, Y.H.; Sun, H. Nomenclatural changes in Rubus (Rosaceae) mostly from China. Phytotaxa 2013, 114, 58–60. [Google Scholar] [CrossRef]
- Zielinski, J. Rubus kuleszae (Rosaceae)—A new bramble species of section Corylifolii from Poland. Fragm. Florist. Geobot. 1996, 41, 249–254. [Google Scholar]
- Zielinski, J. The genus Rubus (Rosaceae) in Poland. Pol. Bot. Stud. 2004, 16, 1–300. [Google Scholar]
- Zielinski, J.; Travnicek, B. Rubus bohemo-polonicus (Rosaceae)—A new species of bramble from the Czech Republic and Poland. Acta Soc. Bot. Pol. 2004, 73, 311–314. [Google Scholar] [CrossRef]
- Zielinski, J.; Kosinski, P.; Tomaszewski, D. Rubus lucentifolius (Rosaceae), a new species of bramble from Poland. Pol. J. Bot. 2004, 49, 5–9. [Google Scholar]
- Zielinski, J.; Kosinski, P.; Tomaszewski, D. The genus Rubus (Rosaceae) in southeastern Lower Silesia (Poland). Pol. J. Bot. 2004, 49, 161–180. [Google Scholar]
- Wolanin, M.M.; Wolanin, M.N.; Musial, K.; Kania, I.; Oklejewicz, K. Rubus zielinskii (Rosaceae), a new species from Poland. Phytotaxa 2016, 273, 183–190. [Google Scholar] [CrossRef]
- Rumasz-Rudnicka, E.; Koszanski, Z.; Podsiadlo, C. Influence of drip irrigation and nitrogen fertilizer on the yield of raspberry cultivated on sandy soil. Inz. Rol. 2005, 9, 201–206. (In Polish) [Google Scholar]
- Celik, F.; Ercisli, S. Lipid and fatty acid composition of wild and cultivated red raspberry fruits (Rubus idaeus L.). J. Med. Plant. Res. 2009, 3, 583–585. [Google Scholar]
- Krawiec, P.; Rybczynski, R. Fertigation efficiency of pimocane-fruiting of raspberry varieties. Acta Agrophys. 2010, 16, 347–358. (In Polish) [Google Scholar]
- Nikfardjam, M.P.; Sussmann, M.; Muster, G. Influence of cultivation management, date of harvest, ripeness stage and storage time on anthocyanins in raspberries cv. ‘Tulameen’ and ‘Glen Ample’. Mitt. Klosterneubg. 2015, 65, 276–286. [Google Scholar]
- Lacis, G.; Kota-Dombrovska, I.; Strautiņa, S. Evaluation of red raspberry cultivars used for breeding and commercial growing in the Baltic region. Proc. Latv. Acad. Sci. Sec. B Nat. Exact. Appl. Sci. 2017, 71, 203–210. [Google Scholar] [CrossRef]
- Chwil, M.; Kostryco, M. Bioactive compounds and antioxidant activity of Rubus idaeus L. leaves. Acta Sci. Pol. Hortorum Cultus. 2018, 17, 135–147. [Google Scholar] [CrossRef]
- Marchi, P.M.; Carvalho, I.R.; Pereira, I.D.S.; Rosa, T.C.D.; Hohn, D.; Szareski, V.J.; Reisser, C.; Junior, A.L.E.C. Yield and quality of primocane-fruiting raspberry grown under plastic cover in southern Brazil. Sci. Agric. 2019, 76, 481–486. [Google Scholar] [CrossRef]
- Hummer, K.E.; Janick, J. Rosaceae: Taxonomy, economic importance, genomics. In Genetics and Genomics of Rosaceae. Plant Genetics and Genomics: Crops and Models, 1st ed.; Folta, K.M., Gardiner, S.E., Eds.; Springer: New York, NY, USA, 2009; Volume 6, pp. 1–17. [Google Scholar]
- Ciebien, M.; Rachon, L.; Krawiec, P. Position of Poland in the world production of raspberries in the years 2002–2012. Rocz. Nauk. Stow. Ekon. Rol. Agrob. 2015, 17, 16–19. (In Polish) [Google Scholar]
- Kljajic, N.; Subic, J.; Sredojevic, Z. Profitability of raspberry production on holdings in the territory of Arilje. Econ. Agric. 2017, 64, 57–68. [Google Scholar] [CrossRef]
- Simpson, D. The economic importance of strawberry crops. In The Genomes of Rosaceous Berries and Their Wild Relatives. Compendium of Plant Genomes, 1st ed.; Hytonen, T., Graham, J., Harrison, R., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 17–23. [Google Scholar]
- Greblikaite, J.; Ispiryan, A.; Montvydaite, D. Development of berry farms in Europe: Organisational and management issues. Mark. Menedz. Innov. 2019, 2, 141–159. [Google Scholar] [CrossRef]
- Wroblewska, W.; Pawlak, J.; Paszko, D. Economic aspects in the raspberry production on the example of farms from Poland, Serbia and Ukraine. J. Hortic. Res. 2019, 27, 71–80. [Google Scholar] [CrossRef]
- Oomah, B.D.; Ladet, S.; Godfrey, D.V.; Liang, J.; Girard, B. Characteristics of raspberry (Rubus idaeus L.) seed oil. Food Chem. 2000, 69, 187–193. [Google Scholar] [CrossRef]
- Escuredo, O.; Silva, L.R.; Valentao, P.; Seijo, M.C.; Andrade, P.B. Assessing Rubus honey value: Pollen and phenolic compounds content and antibacterial capacity. Food Chem. 2012, 130, 671–678. [Google Scholar] [CrossRef]
- Nohynek, L.; Bailey, M.; Tahtiharju, J.; Seppanen-Laakso, T.; Rischer, H.; Oksman-Caldentey, K.M.; Puupponen-Pimia, R. Cloudberry (Rubus chamaemorus) cell culture with bioactive substances: Establishment and mass propagation for industrial use. Eng. Life Sci. 2014, 14, 667–675. [Google Scholar] [CrossRef]
- Ispiryan, A.; Viskelis, J. Valorisation of raspberries by-products for food and pharmaceutical industries. Adv. Agri. Harti. Ento. 2019, 1, 102. [Google Scholar]
- Gulcin, I.; Topal, F.; Cakmakcı, R.; Bilsel, M.; Goren, A.C.; Erdogan, U. Pomological features, nutritional quality, polyphenol content analysis, and antioxidant properties of domesticated and 3 wild ecotype forms of raspberries (Rubus idaeus L.). J. Food Sci. 2011, 76, C585–C593. [Google Scholar] [CrossRef]
- Picuric-Jovanovic, K.; Milovanovic, M. Investigation on the Rubus idaeus L. seed oil (Rosaceae). J. Agric. Sci. 2016, 45, 37–42. [Google Scholar]
- Barbulova, A.; Tito, A.; Hill, J.; Carola, A.; Bimonte, M.; De Laurentis, F.; D’ambrosio, P.; Apone, F.; Colucci, G.; Monoli, I.; et al. Raspberry stem cell extract to protect skin from inflammation and oxidative stress. Cosmet. Toilet. 2010, 125, 38–47. [Google Scholar]
- Tito, A.; Bimonte, M.; Carola, A.; De Lucia, A.; Barbulova, A.; Tortora, A.; Colucci, G.; Apone, F. An oil-soluble extract of Rubus idaeus cells enhances hydration and water homeostasis in skin cells. Int. J. Cosmet. Sci. 2015, 37, 588–594. [Google Scholar] [CrossRef]
- Papaioanou, M.; Chronopoulou, E.G.; Ciobotari, G.; Efrose, R.C.; Sfichi-Duke, L.; Chatzikonstantinou, M.; Pappa, E.; Ganopoulos, I.; Madesis, P.; Nianiou-Obeidat, I.; et al. Cosmeceutical properties of two cultivars of red raspberry grown under different conditions. Cosmetics 2018, 5, 20. [Google Scholar] [CrossRef]
- Fuchigami, T.; Kimata, R.; Haneda, M.; Kakimoto, K.I. Complex three-dimensional Co3O4 nano-raspberry: Highly stable and active low-temperature CO oxidation catalyst. Nanomaterials 2018, 8, 662. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A.; Buyukbezirci, K.; Celi, C.; Kislacki, E.; Karaagac, Z.; Gokturk, E.; Kati, A.; Cimen, B.; Yilmaz, V.; Ocsoy, I. Synthesis of long-term stable gold nanoparticles benefiting from red raspberry (Rubus idaeus), strawberry (Fragaria ananassa), and blackberry (Rubus fruticosus) extracts–gold ion complexation and investigation of reaction conditions. ACS Omega 2019, 4, 18637–18644. [Google Scholar] [CrossRef]
- Demirbas, A.; Yilmaz, V.; Ildiz, N.; Baldemir, A.; Ocsoy, I. Anthocyanins-rich berry extracts directed formation of Ag NPs with the investigation of their antioxidant and antimicrobial activities. J. Mol. Liq. 2017, 248, 1044–1049. [Google Scholar] [CrossRef]
- Stevanovic, M.S.; Zvezdanovic, J.B.; Stanojevic, L.P.; Stanojevic, J.S.; Petrovic, S.M.; Cakic, M.D.; Cvetkovic, D.J. Synthesis, characterization and antioxidant activity of silver nanoparticles stabilized by aqueous extracts of wild blackberry (Rubus spp.) and raspberry (Rubus idaeus L.) leaves. Adv. Technol. 2019, 8, 47–58. [Google Scholar] [CrossRef]
- Ra, J.C.; Kang, K.S.; Lee, H.Y.; Choi, M.K.; Park, H.G. Effect of decreasing body weight with plant extracts containing Rubi fructus. Toxicol. Res. 2004, 20, 167–172. [Google Scholar]
- Kim, D.H.; Park, J.H.; Kim, J.H.; Kim, C.H.; You, J.H.; Kwon, M.C.; Lee, H.Y. Enhancement of immune activities of Ephedrae herba and Rubi fructus at low temperature extraction. Korean J. Med. Crop. Sci. 2005, 13, 81–86. [Google Scholar]
- Kim, J.S.; Jeon, W.J.; You, H.J.; Park, M.S.; Ji, G.E. Inhibitory activities of Rubi fructus on digestive enzymes. Food Sci. Biotechnol. 2010, 19, 1165–1170. [Google Scholar] [CrossRef]
- Nam, M.K.; Choi, H.R.; Cho, J.S.; Cho, S.M.; Ha, K.C.; Kim, T.H.; Ryu, H.Y.; Lee, Y.I. Inhibitory effects of Rubi Fructus extracts on hepatic steatosis development in high-fat diet-induced obese mice. Mol. Med. Rep. 2014, 10, 1821–1827. [Google Scholar] [CrossRef]
- Chwil, M.; Kostryco, M. Histochemical assays of secretory trichomes and the structure and content of mineral nutrients in Rubus idaeus L. leaves. Protoplasma 2020, 257, 119–139. [Google Scholar] [CrossRef]
- Costea, T.; Istudor, V.; Nencu, I. Researches upon indigenous herbal products for therapeutic valorification in metabolic diseases. Note I. Polyphenols’ analysis of Rubi idaei folium. Acta Med. Marisiensis 2012, 58, 281–284. [Google Scholar]
- Costea, T.; Istudor, V.; Fierascu, R.; Fierascu, I.; Botez, A. Researches upon indigenous herbal products for therapeutic valorification in metabolic diseases Note I. Betulae folium and Rubi idaei folium, sources of micro-and macroelements. Farmacia 2013, 61, 162–169. [Google Scholar]
- Costea, T.; Vlase, L.; Gostin, I.N.; Olah, N.K.; Predan, G.M.I. Botanical characterization, phytochemical analysis and antioxidant activity of indigenous red raspberry (Rubus idaeus L.) leaves. Stud. Univ. Vasile Goldis Ser. Stiint. Vietii 2016, 26, 463–472. [Google Scholar]
- Willmer, P.G.; Bataw, A.A.M.; Hughes, J.P. The superiority of bumblebees to honeybees as pollinators: Insect visits to raspberry flowers. Ecol. Entomol. 1994, 19, 271–284. [Google Scholar] [CrossRef]
- Andrikopoulos, C.J. Comparative Pollination Efficacies of Bees on Raspberry and the Management of Osmia lignaria for Late Blooming Crops. Master’s Thesis, Utah State University, Logan, UT, USA, 2018. [Google Scholar]
- Bobis, O.; Marghita, L.A.; Dezmirean, D.; Bonta, V.; Mihai, C.M. Beehive products: Source of nutrients and natural biologically active compounds. J. Agroalim. Proc. Technol. 2010, 16, 104–109. [Google Scholar]
- Ceksteryte, V.; Kurtinaitiene, B.; Balzekas, J. Pollen diversity in honey collected from Lithuania’s protected landscape areas. Proc. Estonian Acad. Sci. 2013, 62, 277–282. [Google Scholar] [CrossRef]
- Weber, H.E. Former and modern taxonomic treatment of the apomictic Rubus complex. Folia Geobot. Phytotax 1996, 31, 373–380. [Google Scholar] [CrossRef]
- Howarth, D.G.; Gardner, D.E.; Morden, C.W. Phylogeny of Rubus subgenus Idaeobatus (Rosaceae) and its implications toward colonization of the Hawaiian Islands. Syst. Bot. 1997, 22, 433–441. [Google Scholar] [CrossRef]
- Alice, L.A.; Campbell, C.S. Phylogeny of Rubus (Rosaceae) based on nuclear ribosomal DNA internal transcribed spacer region sequences. Am. J. Bot. 1999, 86, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Alice, L.A. Evolutionary relationships in Rubus (Rosaceae) based on molecular data. In: VIII International Rubus and Ribes Symposium. Acta Hortic. 2001, 585, 79–83. [Google Scholar]
- Clark, L.V.; Jasieniuk, M. Spontaneous hybrids between native and exotic Rubus in the Western United States produce offspring both by apomixis and by sexual recombination. Heredity 2012, 109, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Sarhanova, P.; Sharbel, T.F.; Sochor, M.; Vasut, R.J.; Dancak, M.; Travnicek, B. Hybridization drives evolution of apomicts in Rubus subgenus Rubus: Evidence from microsatellite markers. Ann. Bot. 2017, 120, 317–328. [Google Scholar] [CrossRef]
- Hebda, R.J.; Chinnappa, C.C. Studies on pollen morphology of Rosaceae in Canada. Rev. Paleobot. Palyno. 1990, 64, 103–108. [Google Scholar] [CrossRef]
- Monasterio-Huelin, E.; Pardo, C. Pollen morphology and wall stratification in Rubus L. (Rosaceae) in the Iberian Peninsula. Grana 1995, 34, 229–236. [Google Scholar] [CrossRef]
- Agudo, J.S.; Rico, E.; Sanchez, J.S. Palynological study of Potentilla subg. Potentilla (Rosaceae) in the western Mediterranean. Grana 1998, 37, 276–284. [Google Scholar]
- Mercado-Gomez, J.D.; Jimenez-Bulla, L.C.; Sanchez-Montano, L.R. Pollen of Magnoliopsida in El Volcan (Pamplona, Colombia) II: Families Hypericaceae, Lamiaceae, Lobeliaceae, Melastomataceae, Polygonaceae, Rhamnaceae, Rosaceae, Rubiaceae, Scrophulariaceae and Solanaceae. Caldasia 2013, 35, 409–427. [Google Scholar]
- Hebda, R.J.; Chinnappa, C.C.; Smith, B.M. Pollen morphology of the Rosaceae of Western Canada: I. Agrimonia to Crataegus. Grana 1988, 27, 95–113. [Google Scholar] [CrossRef]
- Tomlik-Wyremblewska, A. Pollen morphology of genus Rubus L. Part I. Introductory studies of the European representatives of the subgenus Rubus L. Acta Soc. Bot. Pol. 1995, 64, 187–203. [Google Scholar] [CrossRef]
- Wronska-Pilarek, D.; Jagodzinski, A.M. Pollen morphological variability of Polish native species of Rosa L. (Rosaceae). Dendrobiology 2009, 62, 71–82. [Google Scholar]
- Ghosh, A.; Saha, I. Pollen morphological study of some selected Indian taxa of Rosaceae. Indian J. Appl. Pure Biol. 2017, 32, 121–130. [Google Scholar]
- Wronska-Pilarek, D.; Boratynska, K. Pollen morphology of Rosa gallica L. (Rosaceae) from southern Poland. Acta. Soc. Bot. Pol. 2005, 74, 297–304. [Google Scholar] [CrossRef]
- Wronska-Pilarek, D.; Jagodzinski, A.M. Intra-and interindividual variability of selected quantitative features of pollen grain morphology based on the example of Rosa canina L. (Rosaceae). Dendrobiology 2012, 67, 25–39. [Google Scholar]
- Perveen, A.; Qaiser, M. Pollen flora of Pakistan–LXXI. Rosaceae. Pak. J. Bot. 2014, 46, 1027–1037. [Google Scholar]
- Chwil, M. Micromorphology of pollen grains of fruit trees of the genus Prunus. Acta Sci. Pol. Hortorum Cultus 2015, 14, 115–129. [Google Scholar]
- Padilla, F.; Soria, N.; Oleas, A.; Rueda, D.; Manjunatha, B.; Kundapur, R.R.; Maddela, N.R.; Rajeswari, B. The effects of pesticides on morphology, viability, and germination of Blackberry (Rubus glaucus Benth) and Tree tomato (Solanum betaceum Cav.) pollen grains. 3 Biotech. 2017, 7, 154. [Google Scholar]
- Motyleva, S.M.; Gruner, L.; Semenova, L. The morphology of pollen grains of some cultivars Rubus fruticosus L. Agrobiodivers. Improv. Nutr. Health Life Qual. 2018, 2, 1–6. [Google Scholar]
- Lechowicz, K.; Wronska-Pilarek, D.; Bocianowski, J.; Malinski, T. Pollen morphology of Polish species from the genus Rubus L. (Rosaceae) and its systematic importance. PLoS ONE 2020, 15, e0221607. [Google Scholar]
- Tomlik-Wyremblewska, A. Pollen morphology of genus Rubus L., Part II. Introductory studies on the Malesian species of subgenus Micranthobatus. Acta Soc. Bot. Pol. 2000, 69, 31–40. [Google Scholar]
- Tomlik-Wyremblewska, A.; Van Der Ham, R.W.; Kosinski, P. Pollen morphology of genus Rubus L. Part III. Studies on the Malesian species of subgenera Chamaebatus L. and Idaeobatus L. Acta. Soc. Bot. Pol. 2004, 73, 207–227. [Google Scholar] [CrossRef]
- Wronska-Pilarek, D.; Malinski, T.; Lira, J. Pollen morphology of Polish species of genus Rubus-Part I. Rubus gracilis. Dendrobiology 2006, 56, 69–77. [Google Scholar]
- Wei-Lin, L.I.; Shan-An, H.E.; Yin, G.U.; Pu, S.H.U.; Zu-Mao, P.U. Pollen morphology of the genus Rubus from China. J. Syst. Evol. 2001, 39, 234–247. [Google Scholar]
- Kodela, P.G. Pollen morphology of some rainforest taxa occurring in the Illawarra region of New South Wales, Australia. Telopea 2006, 11, 346–389. [Google Scholar] [CrossRef]
- Wronska-Pilarek, D.; Jagodzinski, A.; Malinski, T. Morphological studies of pollen grains of the Polish endemic species of the genus Rubus (Rosaceae). Biologia 2012, 67, 87–96. [Google Scholar] [CrossRef]
- Popek, R. Biosystematic Studies of the Ggenus Rosa L. In Poland and Neighboring Countries; Prace Monograficzne; Wydawnictwo Naukowe Wyzszej Szkoly Pedagogicznej: Krakow, Poland, 1996; pp. 1–199. (In Polish) [Google Scholar]
- Lihua, Z.; Zhongxin, W.; Zhengyi, W. Pollen morphology of Rosoideae (Rosaceae) of China. Acta Bot. Yunnanica 1999, 21, 455–460. [Google Scholar]
- Wronska-Pilarek, D. Pollen morphology of Polish native species of the Rosa genus (Rosaceae) and its relation to systematics. Acta. Soc. Bot. Pol. 2011, 80, 221–232. [Google Scholar] [CrossRef]
- Zhou, L.H.; Wei, Z.X.; Wu, Z.Y. Pollen morphology of Prunoideae of China (Rosaceae). Acta Bot. Yunnanica 1999, 22, 207–211. [Google Scholar]
- Ariizumi, T.; Toriyama, K. Genetic regulation of sporopollenin synthesis and pollen exine development. Annu. Rev. Plant. Biol. 2011, 62, 437–460. [Google Scholar] [CrossRef]
- Wiermann, R.; Gubatz, S. Pollen wall and sporopollenin. Int. Rev. Cytol. 1992, 140, 35–72. [Google Scholar]
- Dobritsa, A.A.; Shrestha, J.; Morant, M.; Pinot, F.; Matsuno, M.; Swanson, R.; Moller, B.L.; Preuss, D. CYP704B1 is a long-chain fatty acid ω-hydroxylase essential for sporopollenin synthesis in pollen of Arabidopsis. Plant. Physiol. 2009, 151, 574–589. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.Z.; Milincic, D.D.; Barac, M.B.; Shariati, A.M.; Tesic, Z.L.; Pesic, M.B. The application of pollen as a functional food and feed ingredient-the present and perspectives. Biomolecules 2020, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Suh, H.J.; Chung, I.K. Enhanced extraction of bioactive compounds from bee pollen by wet-grinding technology. J. Life Sci. 2016, 26, 651–656. [Google Scholar] [CrossRef]
- Fatemi, N.; Attar, F.; Assareh, M.N.; Hamzehee, B. Pollen morphology of the genus Rosa L. (Rosaceae) in Iran. Iran. J. Bot. 2012, 18, 284–293. [Google Scholar]
- Kuiling, W.; Qingchao, L.; Xin, H.; Qinghua, L.; Qixiang, Z. Study on palynology of Camellia japonica L. (NaiDong). Chin. Agric. Sci. Bull. 2007, 23, 267–272. [Google Scholar]
- Chung, K.S.; Elisens, W.J.; Skvarla, J.J. Pollen morphology and its phylogenetic significance in tribe Sanguisorbeae (Rosaceae). Plant Syst. Evol. 2010, 285, 139–148. [Google Scholar] [CrossRef]
- Wronska-Pilarek, D.; Jagodzinski, A.M. Systematic importance of pollen morphological features of selected species from the genus Rosa (Rosaceae). Plant Syst. Evol. 2011, 295, 55–72. [Google Scholar] [CrossRef]
- Wronska-Pilarek, D.; Daniekewicz, W.; Bocianowski, J.; Mailnski, T.; Janyszek, M. Comparative pollen morphological analysis and its systematic implications on three European oak (Quercus L., Fagaceae) species and their spontaneous hybrids. PLoS ONE 2016, 11, e0161762. [Google Scholar] [CrossRef]
- Wronska-Pilarek, D.; Wiatrowska, B.; Bocianowski, J. Pollen morphology and variability of invasive Spiraea tomentosa L. (Rosaceae) from populations in Poland. PLoS ONE 2019, 14, 1–19. [Google Scholar] [CrossRef]
- Radovic, A.; Nikolic, D.; Milatovic, D.; Djurovic, D.; Trajkovic, J. Investigation of pollen morphological characteristics in some quince (Cydonia oblonga Mill.) cultivars. Turk. J. Agric. For. 2016, 40, 441–449. [Google Scholar] [CrossRef]
- Song, J.H.; Oak, M.K.; Roh, H.S.; Hong, S.P. Morphology of pollen and orbicules in the tribe Spiraeeae (Rosaceae) and its systematic implications. Grana 2017, 56, 351–367. [Google Scholar] [CrossRef]
- Eide, F.Y. Key for northwest European Rosaceae pollen. Grana 1981, 20, 101–118. [Google Scholar] [CrossRef]
- Eide, F.Y. On the pollen morphology of Rubus chamaemorus L. (Rosaceae). Grana 1981, 20, 25–27. [Google Scholar] [CrossRef]
- Kalkman, C. The phylogeny of the Rosaceae. Bot. J. Linn. Soc. 1988, 98, 37–59. [Google Scholar] [CrossRef]
- Hebda, R.J.; Chinnappa, C.C. Studies on pollen morphology of Rosaceae. Acta Bot. Gall. 1994, 141, 183–193. [Google Scholar] [CrossRef]
- Xiong, X.H.; Zhou, X.M.; Li, M.; Xu, B.; Deng, H.N.; Yu, Q.; Gao, X.F. Pollen morphology in Rubus (Rosaceae) and its taxonomic implications. Plant. Syst. Evol. 2019, 305, 705–716. [Google Scholar] [CrossRef]
- Dar, J.A.; Wani, A.A.; Dhar, M.K. Preliminary pollen analysis of some apple cultivars in Kashmir: Towards understanding the apple pollen morphology. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 90, 431–438. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Renouard, S.; Drouet, S.; Blondeau, J.P.; Hano, C. A critical cross-species comparison of pollen from Nelumbo nucifera Gaertn. vs. Nymphaea lotus L. for authentication of Thai medicinal herbal tea. Plants 2020, 9, 921. [Google Scholar]
- Schori, M.; Furness, C.A. Pollen diversity in Aquifoliales. Bot. J. Linn. Soc. 2014, 175, 169–190. [Google Scholar] [CrossRef]
- Almeida, R.P.; Stouthamer, R. Phylogeny of the Trichogramma endosymbiont Wolbachia, an alpha-proteobacteria (Rickettsiae). Braz. J. Biol. 2018, 78, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Nagamitsu, T.; Nagamasu, H. Keys for the pollen of Ashiu, Central Japan. Contr. Biol. Lab. Kyoto Univ. 1994, 28, 261–355. [Google Scholar]
- Harder, L.D. Pollen-size comparisons among animal-pollinated angiosperms with different pollination characteristics. Bot. J. Linn. Soc. 1998, 64, 513–525. [Google Scholar] [CrossRef]
- Giovannini, A.; Macovei, A.; Caser, M.; Mansuino, A.; Ghione, G.G.; Savona, M.; Carbonera, D.; Scariot, V.; Balestrazzi, A. Pollen grain preservation and fertility in valuable commercial rose cultivars. Plants 2017, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.C.; Stephenson, A.G. Effects of soil phosphorus on pollen production, pollen size, pollen phosphorus content, and the ability to sire seeds in Cucurbita pepo (Cucurbitaceae). Sex. Plant. Reprod. 1994, 7, 215–220. [Google Scholar] [CrossRef]
- Aizen, M.A.; Raffaele, E. Flowering-shoot defoliation affects pollen grain size and postpollination pollen performance in Alstroemeria aurea. Ecology 1998, 79, 2133–2142. [Google Scholar] [CrossRef]
- Griener, K.W.; Warny, S. Nothofagus pollen grain size as a proxy for long-term climate change: An applied study on Eocene, Oligocene, and Miocene sediments from Antarctica. Rev. Paleobot. Palyno. 2015, 221, 138–143. [Google Scholar] [CrossRef]
- Ejsmond, M.J.; Wronska-Pilarek, D.; Ejsmond, A.; Dragosz-Kluska, D.; Karpinska-Kolaczek, M.; Kolaczek, P.; Kozlowski, J. Does climate affect pollen morphology? Optimal size and shape of pollen grains under various desiccation intensity. Ecosphere 2011, 2, 1–15. [Google Scholar] [CrossRef]
- Rodriguez-Damian, M.; Cernadas, E.; Formella, A.; Fernandez-Delgado, M.; De Sa-Otero, P. Automatic detection and classification of grains of pollen based on shape and texture. IEEE Trans. Syst. Man Cybern. Part C Appl. Rev. 2006, 36, 531–542. [Google Scholar] [CrossRef]
- Rodriguez-Damian, M.; Cernadas, E.; Formella, A.; Sa-Otero, R. Pollen Classification using Brightness-based and Shape-based Descriptors. In Proceedings of the 17th International Conference on Pattern Recognition ICPR, Cambridge, UK, 26 August 2004; Kittler, J., Petrou, M., Nixon, M., Eds.; IEEE Institute of Electrical and Electronics Engineers: Piscataway Township, NJ, USA, 2004; pp. 212–215. [Google Scholar]
- Naruhashi, N.; Takano, H. Size variation of pollen grains in some Rubus species. J. Phytogeograph. Taxon. 1980, 28, 27–32. [Google Scholar]
- Kosenko, V.N.; Nguen, T.K.; Iakovlev, G.P. Palynomorphological study of the representatives of the genus Rubus (Rosaceae) in the flora of Viet Nam. Bot. Zhurnal 1984, 4, 497–503. [Google Scholar]
- Fedoronchuk, M.M.; Savitsky, V.D. Comparative and morphological analysis of pollen for genera of the family Rosaceae Juss. of the Ukrainian flora. Ukrain. Bot. Zhurnal 1987, 44, 32–38. [Google Scholar]
- Ueda, Y. Pollen surface morphology in the genus Rosa and related genera. Jpn. J. Palynol. 1992, 38, 94–105. [Google Scholar]
- Ueda, Y. Systematic studies in the genus Rosa. Tech. Bull. Fac. Hort. Chiba Univ. 1994, 48, 241–328. [Google Scholar]
- Ueda, Y.; Okada, Y. Discrimination of rose cultivar groups by pollen surface structure. J. Hortic. Sci. 1994, 69, 601–607. [Google Scholar] [CrossRef]
- Wang, X.R.; Tang, H.R.; Huang, L.; He, Z.Z.; Dong, X.L.; Fu, H.Q.; Deng, Q.X. Comparative studies on pollen submicroscopic morphology of some wild species and cultivars of bramble (Rubus L.). Acta Hort. Sin. 2007, 34, 1395. [Google Scholar]
- Kasalkheh, R.; Jorjani, E.; Sabouri, H.; Habibi, M.; Sattarian, A. Pollen morphology of gnus Rubus L. subgenus Rubus (Rosaceae) in Iran. Nova Biol. Rep. 2017, 4, 9–18. [Google Scholar]
- Ulrich, S.; Hesse, M.; Weber, M.; Halbritter, H. Amorphophallus: New insights into pollen morphology and the chemical nature of the pollen wall. Grana 2017, 56, 1–36. [Google Scholar] [CrossRef]
- Doyle, J.A.; Endress, P.K. Morphological phylogenetic analysis of basal angiosperms: Comparison and combination with molecular data. Int. J. Plant. Sci. 2000, 161, 121–153. [Google Scholar] [CrossRef]
- Doyle, J. Early evolution of angiosperm pollen as inferred from molecular and morphological phylogenetic analyses. Grana 2005, 44, 227–251. [Google Scholar] [CrossRef]
- Diego-Taboada, A.; Beckett, S.T.; Atkin, S.L.; Mackenzie, G. Hollow pollen shells to enhance drug delivery. Pharmaceutics 2014, 6, 80–96. [Google Scholar] [CrossRef]
- Rowley, J.R. Why the endexine and ectexine differ in resistance to oxidation. Calluna as a model system. Grana 2001, 40, 159–162. [Google Scholar]
- Quilichini, T.D.; Grienenberger, E.; Douglas, C.J. The biosynthesis, composition and assembly of the outer pollen wall: A tough case to crack. Phytochemistry 2015, 113, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Dobritsa, A.A.; Nishikawa, S.I.; Preuss, D.; Urbanczyk-Wochniak, E.; Sumner, L.W.; Hammond, A.; Carlson, A.L.; Swanson, R.J. LAP3, a novel plant protein required for pollen development, is essential for proper exine formation. Sex. Plant. Reprod. 2009, 22, 167–177. [Google Scholar] [CrossRef]
- Jiang, J.; Yao, L.; Yu, Y.; Liang, Y.; Jiang, J.; Ye, N.; Miao, Y.; Cao, J. Pectate lyase-like 9 from Brassica campestris is associated with intine formation. Plant. Sci. 2014, 229, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Halbritter, H.; Ulrich, S.; Grimsson, F.; Weber, M.; Zetter, R.; Hesse, M.; Buchner, R.; Svojtka, M.; Frosch-Radivo, A. Pollen morphology and ultrastructure. In Illustrated Pollen Terminology, 2nd ed.; Halbritter, H., Ulrich, S., Grimsson, F., Weber, M., Zetter, R., Hesse, M., Buchner, R., Svojtka, M., Frosch-Radivo, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 37–65. [Google Scholar]
- Suarez-Cervera, M.; Arcalis, E.; Le Thomas, A.; Seoane-Camba, J.A. Pectin distribution pattern in the apertural intine of Euphorbia peplus L. (Euphorbiaceae) pollen. Sex. Plant. Reprod. 2002, 14, 291–298. [Google Scholar] [CrossRef]
- Fang, K.; Wang, Y.; Yu, T.; Zhang, L.; Baluska, F.; Samaj, J.; Lin, J. Isolation of de-exined pollen and cytological studies of the pollen intines of Pinus bungeana Zucc. Ex Endl. and Picea wilsonii Mast. Flora 2008, 203, 332–340. [Google Scholar] [CrossRef]
- Knox, R.B. The pollen grain. In Embryology of Angiosperms, 1st ed.; Johri, B.M., Ed.; Springer: Berlin, Germany, 1984; pp. 197–271. [Google Scholar]
- Knox, R.; Heslop-Harrison, J. Pollen-wall proteins: The fate of intine-held antigens on the stigma in compatible and incompatible pollinations of Phalaris tuberosa L. J. Cell Sci. 1971, 9, 239–251. [Google Scholar]
- Scott, R.J.; Spielman, M.; Dickinson, H.G. Stamen structure and function. Plant. Cell 2004, 16 (Suppl. S1), 46–60. [Google Scholar] [CrossRef]
- Pacini, E.; Guarnieri, M.; Nepi, M. Pollen carbohydrates and water content during development, presentation, and dispersal: A short review. Protoplasma 2006, 228, 73–77. [Google Scholar] [CrossRef]
- T’ai, H.R.; Buchmann, S.L. A phylogenetic reconsideration of the pollen starch–pollination correlation. Evol. Ecol. Res. 2000, 2, 627–643. [Google Scholar]
- Green, F.J. The Sigma-Aldrich Handbook of Stains, Dyes, and Indicators; Aldrich Chemical Company Inc: Milwaukee, WI, USA, 1990. [Google Scholar]
- Wood, G.D.; Gabriel, A.M.; Lawson, J.C. Palynological Techniques-processing and microscopy. In Palynology: Principles and Applications, 1st ed.; Jansonius, J., McGregor, D.C., Eds.; American Association of Stratigraphic Palynologists Foundation: Salt Lake City, UT, USA, 1996; pp. 29–52. [Google Scholar]
- Reynolds, E.S. The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Erdman, G. Pollen Morphology and Plant. Taxonomy. Angiosperms, 1st ed.; Hafner Publication Company: New York, NY, USA, 1966. [Google Scholar]
- Dybova-Jachowicz, S.; Sadowska, A. Palinology; PAN: Krakow, Poland, 2003. (In Polish) [Google Scholar]





| Cultivar | Year | Length of Axis (μm) | P/E | ||||
|---|---|---|---|---|---|---|---|
| Polar Axis (P) | Equatorial Axis (E) | ||||||
| Min.–Max. | Mean ± SD | Min.–Max. | Mean ± SD | Min.–Max. | Mean ± SD | ||
| Biennial Fruiting Cultivars | |||||||
| ‘Glen Ample’ | 2016 | 24.40–29.88 | 27.38 ± 1.31a | 24.58-29.54 | 26.92 ± 1.51a | 0.95–1.22 | 1.02 ± 0.06a |
| 2017 | 21.05–27.68 | 24.57 ± 1.38a | 20.60–25.53 | 23.01 ± 1.40b | 1.01–1.13 | 1.07 ± 0.04a | |
| 2018 | 24.57–29.71 | 26.73 ± 1.22a | 22.09–26.69 | 24.77 ± 1.08ab | 1.01–1.21 | 1.08 ± 0.06a | |
| Mean | - | 26.23 ± 1.77A | - | 24.90 ± 2.08A | - | 1.06 ± 0.06A | |
| ‘Laszka’ | 2016 | 24.68–30.02 | 27.55 ± 1.39ab | 22.06–25.33 | 23.49 ± 0.92b | 1.02–1.30 | 1.17 ± 0.08a |
| 2017 | 21.30–28.77 | 24.49 ± 1.99b | 19.82–24.84 | 22.20 ± 1.34b | 1.03–1.25 | 1.10 ± 0.05a | |
| 2018 | 25.99–28.99 | 28.29 ± 0.78a | 24.15–27.30 | 25.40 ± 0.87a | 1.06–1.20 | 1.11 ± 0.04a | |
| Mean | - | 26.78 ± 2.20A | 23.70 ± 1.69A | 1.13 ± 0.07A | |||
| ‘Radziejowa’ | 2016 | 23.00–33.07 | 26.80 ± 2.40a | 23.06–29.95 | 26.98 ± 1.69a | 0.84–1.14 | 1.01 ± 0.07a |
| 2017 | 22.73–26.43 | 25.13 ± 1.17a | 20.96–26.31 | 23.87 ± 1.61ab | 1.00–1.25 | 1.06 ± 0.06a | |
| 2018 | 21.29–25.72 | 23.83 ± 1.23a | 18.93–23.97 | 21.35 ± 1.85b | 1.02–1.25 | 1.12 ± 0.08a | |
| Mean | - | 25.31 ± 1.88A | - | 24.01 ± 2.97A | - | 1.06 ± 0.08A | |
| Cultivars With Repeated Fruiting | |||||||
| ‘Pokusa’ | 2016 | 25.20–29.04 | 27.56 ± 1.06a | 25.01–29.46 | 26.91 ± 1.19a | 0.93–1.15 | 1.03 ± 0.06a |
| 2017 | 25.21–31.29 | 27.98 ± 1.69a | 22.92–27.02 | 25.25 ± 1.46ab | 1.00–1.22 | 1.11 ± 0.07a | |
| 2018 | 24.43–28.90 | 25.86 ± 1.39a | 22.19–25.87 | 24.16 ± 1.05b | 0.98–1.17 | 1.07 ± 0.06a | |
| Mean | - | 27.13 ± 1.66A | - | 25.44 ± 1.67A | - | 1.07 ± 0.07A | |
| ‘Polana’ | 2016 | 25.07–30.63 | 27.73 ± 1.74a | 19.60–28.88 | 23.92 ± 2.25a | 0.92–1.37 | 1.17 ± 0.12a |
| 2017 | 22.00–27.00 | 25.39 ± 1.52a | 20.67–25.84 | 22.75 ± 1.68a | 1.01–1.28 | 1.12 ± 0.10a | |
| 2018 | 24.04–29.29 | 26.46 ± 1.58a | 22.20–27.97 | 25.66 ± 1.44a | 0.92–1.14 | 1.03 ± 0.07a | |
| Mean | - | 26.53 ± 1.86A | - | 24.11 ± 2.16A | - | 1.11 ± 0.11A | |
| ‘Polka’ | 2016 | 25.07–30.63 | 27.73 ± 1.74a | 19.60–28.88 | 23.92 ± 2.25b | 0.92–1.37 | 1.17 ± 0.12a |
| 2017 | 23.36–29.00 | 26.91 ± 1.42a | 25.37–28.68 | 26.68 ± 0.97ab | 0.87–1.09 | 1.01 ± 0.05a | |
| 2018 | 26.65–32.14 | 29.45 ± 1.59a | 25.46–32.20 | 28.55 ± 1.39a | 0.83–1.19 | 1.03 ± 0.08a | |
| Mean | - | 28.03 ± 1.89A | - | 26.38 ± 2.50A | - | 1.07 ± 0.11A | |
| Cultivars | Width of Muri | Distance Between Muri | Number of Muri | |||
|---|---|---|---|---|---|---|
| Per 10 µm2 | ||||||
| Min.–Max. | Mean ± SD | Min.–Max. | Mean ± SD | Min.–Max. | Mean ± SD | |
| nm | Muri/10 µm2 | |||||
| Biennial Fruiting Cultivars | ||||||
| ‘Glen Ample’ | 320–490 | 417. 50 ± 51.32a | 560–870 | 786.25 ± 91.86a | 5.48–9.04 | 8.15 ± 1.01b |
| ‘Laszka’ | 240–360 | 298.13 ± 30.16b | 490–640 | 574.38 ± 54.28bc | 8.92–12.42 | 11.22 ± 1.01a |
| ‘Radziejowa’ | 190–330 | 247.75 ± 35.87bc | 660–890 | 781.88 ± 70.26a | 7.42–13.67 | 10.77 ± 1.88ab |
| Cultivars with Repeated Fruiting | ||||||
| ‘Pokusa’ | 130–210 | 161.88 ± 23.73d | 480–780 | 685.63 ± 90.04ab | 7.67–13.56 | 11.55 ± 1.71a |
| ‘Polana’ | 180–250 | 203.13 ± 24.69c | 420–710 | 521.88 ± 69.50cd | 8.03–12.67 | 10.98 ± 1.53a |
| ‘Polka’ | 120–210 | 156.25 ± 29.18d | 360–620 | 439.83 ± 68.65d | 9.92–14.97 | 13.03 ± 1.69a |
| Cultivars | Diameter of Perforations | Number of Perforations on the Surface of | ||||||
|---|---|---|---|---|---|---|---|---|
| Min. | Max. | 2-µm Long Colpus | 10 µm2 of Exine | |||||
| Min.–Max. | Mean ± SD | Min.–Max. | Mean±SD | Min.–Max. | Mean ± SD | Min.–Max. | Mean ± SD | |
| nm | Perforations/2 µm | Perforations/10 µm2 | ||||||
| Biennial Fruiting Cultivars | ||||||||
| ‘Glen Ample’ | 90–150 | 117.50 ± 17.70e | 100–220 | 151.88 ± 28.10d | 3.70–7.02 | 5.40 ± 0.95ab | 41.14–74.29 | 59.06 ± 10.63b |
| ‘Laszka’ | 210–290 | 248.75 ± 22.77ab | 250–450 | 323.75 ± 54.39a | 2.08–5.18 | 4.24 ± 0.79b | 56.66–71.02 | 65.01 ± 4.46b |
| ‘Radziejowa’ | 200–330 | 258.13 ± 44,46a | 210–390 | 306.25 ± 59.09a | 4.53–8.03 | 6.40 ± 1.19a | 70.65–130.43 | 91.97 ± 18.87a |
| Cultivars with Repeated Fruiting | ||||||||
| ‘Pokusa’ | 120–220 | 167.50 ± 24.36cd | 160–260 | 208.75 ± 31.60bc | 2.01–3.97 | 2.68 ± 0.53c | 32.84–47.06 | 38.47 ± 4.11c |
| ‘Polana’ | 130–240 | 193.75 ± 38.62bc | 210–310 | 268.75 ± 32.84ab | 5.03–8.13 | 6.29 ± 1.01a | 60.91–89.55 | 75.35 ± 6.71ab |
| ‘Polka’ | 110–170 | 131.25 ± 21.25de | 120–220 | 173.13 ± 29.38cd | 5.05–8.79 | 6.82 ± 1.15a | 49.51–88.61 | 71.68 ± 9.64ab |
| Cultivars | Thickness of Tectum | Height of Columellae | Thickness of Columellae | Distance Between Columellae | Thickness of Foot Layer | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min.–Max. | Mean ± SD | Min.–Max. | Mean ± SD | Min.–Max. | Mean ±SD | Min.–Max. | Mean ± SD | Min.–Max. | Mean ±SD | |
| Biennial Fruiting Cultivars | ||||||||||
| ‘Glen Ample’ | 360–540 | 430.75 ± 48.51a | 260–510 | 402.50 ± 73.89a | 180–310 | 241.88 ± 40.53a | 270 ± 490 | 406.67 ± 69.73a | 110–190 | 148.63 ± 23.34a |
| ‘Laszka’ | 170–250 | 209.06 ± 23.11c | 240–390 | 299.06 ± 49.61a | 200–290 | 238.69 ± 25.35a | 230 ± 360 | 296.69 ± 37.85b | 70–90 | 77.75 ± 9.43d |
| ‘Radziejowa’ | 170–280 | 210.88 ± 36.02bc | 280–470 | 348.13 ± 61.03a | 190–260 | 228.13 ± 18.34a | 250 ± 380 | 331.88 ± 37.10b | 90–120 | 103.13 ± 11.96bc |
| Cultivars with Repeated Fruiting | ||||||||||
| ‘Pokusa’ | 200–270 | 227.50 ± 24.90bc | 270–390 | 335.00 ± 40.33a | 170–260 | 208.13 ± 28.57a | 260–370 | 321.25–37.75b | 90–130 | 109.38 ± 9.98bc |
| ‘Polana’ | 210–360 | 307.50 ± 43.13b | 300–430 | 369.38 ± 54.59a | 210–320 | 261.25 ± 35.94a | 250 ± 390 | 339.38–39.58ab | 80–130 | 110.04 ± 16.73c |
| ‘Polka’ | 190–310 | 250.00 ± 43.67bc | 290–440 | 365.01 ± 38.64a | 200–320 | 251.88 ± 34.10a | 220 ± 430 | 330.63–58.71b | 110–150 | 132.50 ± 16.13a |
| Cultivars | Ectoexine | Endoexine | Exine | Intine | Cell Wall | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min.–Max. | Mean ± SD | Min.–Max. | Mean ± SD | Min.–Max. | Mean ± SD | Min.–Max. | Mean ± SD | Min.–Max. | Mean ± SD | |
| Biennial Fruiting Cultivars | ||||||||||
| ‘Glen Ample’ | 810–1090 | 981.88 ± 82.64a | 70–110 | 90.42 ± 13.44ab | 920–1180 | 1072.26 ± 82.22a | 380–630 | 498.00 ± 74.01a | 1410–1740 | 1570.26 ± 104.13a |
| ‘Laszka’ | 500–690 | 585.87 ± 62.02c | 50–90 | 76.38 ± 15.04b | 580–780 | 662.25 ± 67.52d | 250–450 | 316.88 ± 54.46bc | 850–1177 | 979.13 ± 100.47b |
| ‘Radziejowa’ | 550–740 | 662.14 ± 54.63c | 60–90 | 82.09 ± 9.10b | 620–820 | 744.21 ± 55.30d | 320–480 | 393.75 ± 53.77b | 880–1120 | 1137.96 ± 67.42b |
| Cultivars with Repeated Fruiting | ||||||||||
| ‘Pokusa’ | 600–740 | 671.88 ± 44.46bc | 70–100 | 85.63 ± 8.92ab | 690–830 | 757.50 ± 44.35cd | 210–350 | 272.50 ± 37.15cd | 900–1130 | 1030.00 ± 64.81b |
| ‘Polana’ | 640–890 | 786.92 ± 67.50b | 70–100 | 91.88 ± 9.11ab | 740–980 | 878.75 ± 67.61bc | 410–640 | 540.63 ± 66.28a | 1170–1590 | 1419.38 ± 111.56a |
| ‘Polka’ | 680–840 | 747.51 ± 46.98b | 90–130 | 107.50 ± 13.42a | 800–940 | 855.00 ± 47.61b | 160–260 | 228.75 ± 30.30d | 990–1200 | 1083.75 ± 55.12b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostryco, M.; Chwil, M.; Matraszek-Gawron, R. Comparison of the Micromorphology and Ultrastructure of Pollen Grains of Selected Rubus idaeus L. Cultivars Grown in Commercial Plantation. Plants 2020, 9, 1194. https://doi.org/10.3390/plants9091194
Kostryco M, Chwil M, Matraszek-Gawron R. Comparison of the Micromorphology and Ultrastructure of Pollen Grains of Selected Rubus idaeus L. Cultivars Grown in Commercial Plantation. Plants. 2020; 9(9):1194. https://doi.org/10.3390/plants9091194
Chicago/Turabian StyleKostryco, Mikołaj, Mirosława Chwil, and Renata Matraszek-Gawron. 2020. "Comparison of the Micromorphology and Ultrastructure of Pollen Grains of Selected Rubus idaeus L. Cultivars Grown in Commercial Plantation" Plants 9, no. 9: 1194. https://doi.org/10.3390/plants9091194
APA StyleKostryco, M., Chwil, M., & Matraszek-Gawron, R. (2020). Comparison of the Micromorphology and Ultrastructure of Pollen Grains of Selected Rubus idaeus L. Cultivars Grown in Commercial Plantation. Plants, 9(9), 1194. https://doi.org/10.3390/plants9091194





