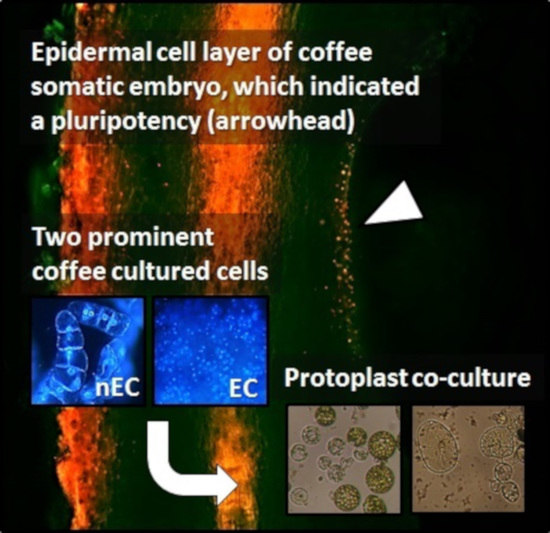
Establishment of Pluripotent Cell Cultures to Explore Allelopathic Activity of Coffee Cells by Protoplast Co-Culture Bioassay Method
Abstract
:1. Introduction
2. Results
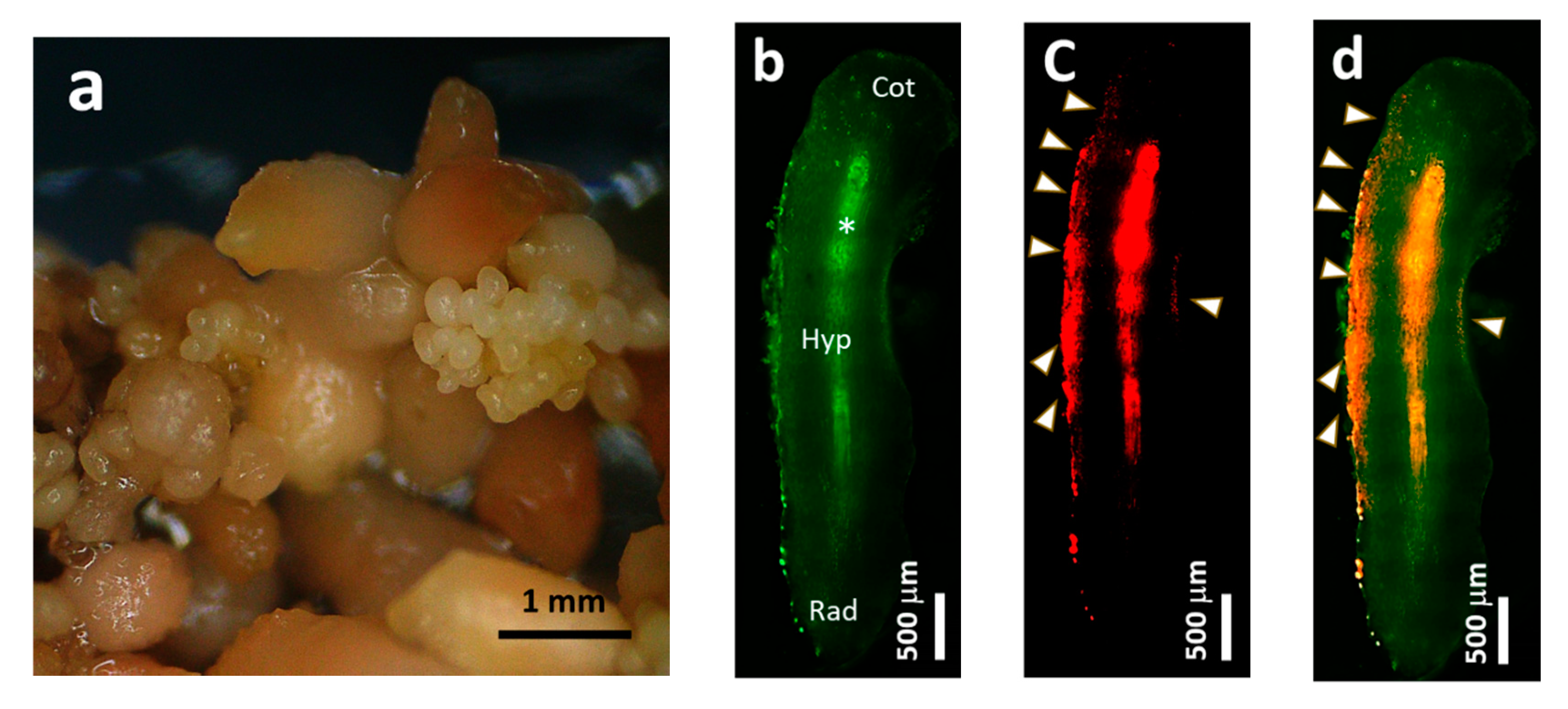
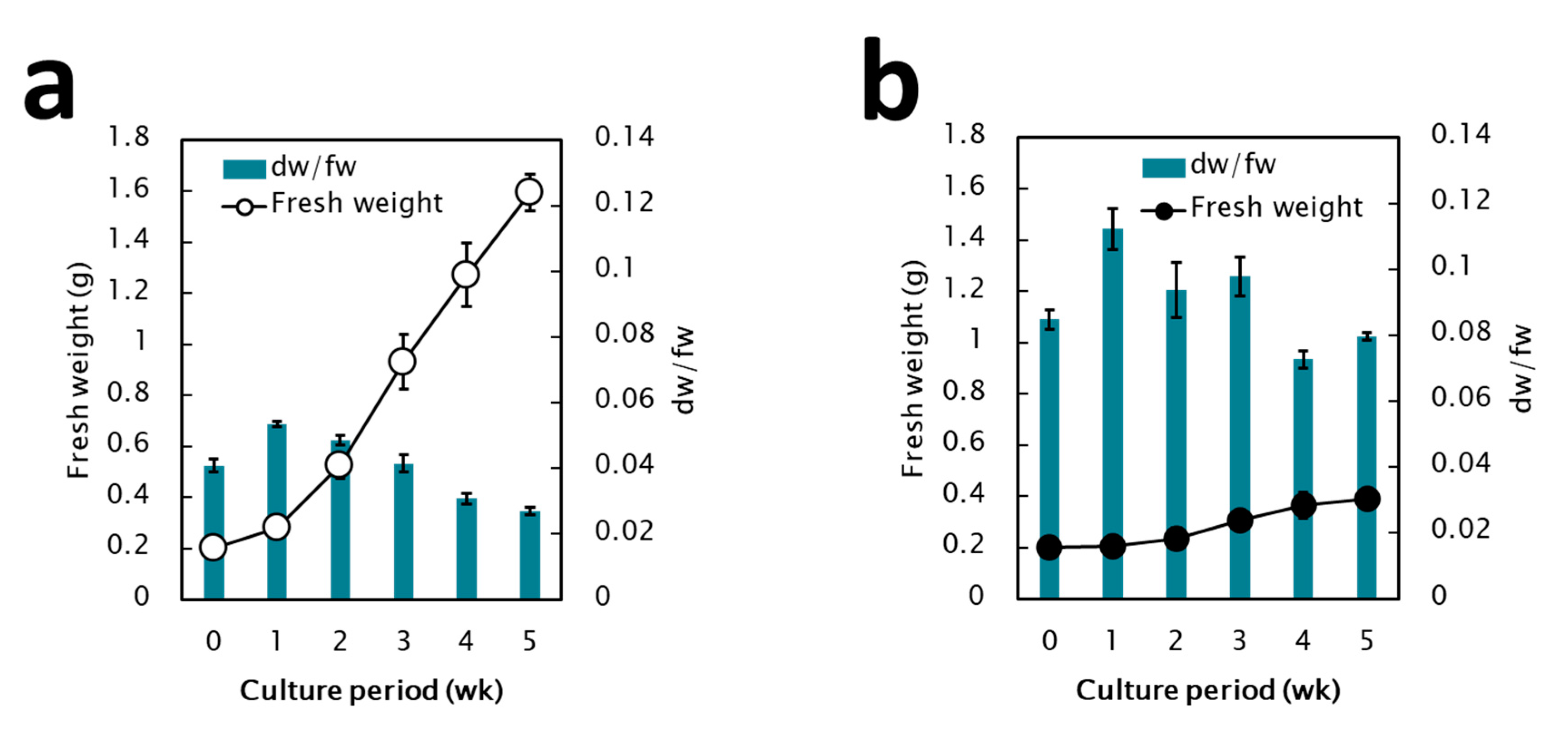
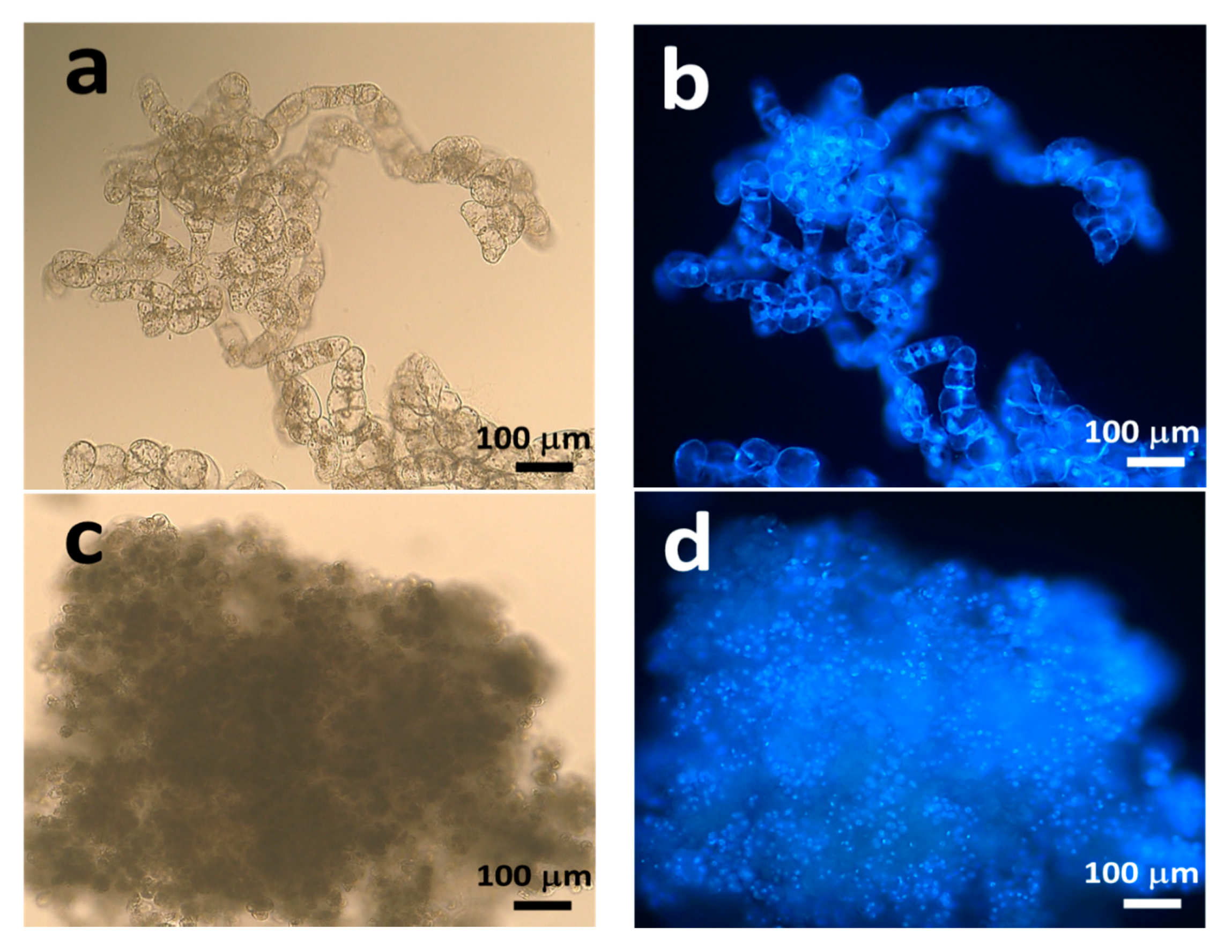
2.1. Establishment of Pluripotent Coffee Cell Cultures
2.2. Protoplast Isolation
2.3. Protoplasts Co-Culture
2.4. Effect of Coffee Protoplasts on Non-Spherical Enlargement and Cell Wall Formation Stage of Lettuce Protoplasts
2.5. Effect of Coffee Protoplasts on the Colony Formation Stage of Lettuce Protoplasts
2.6. Effect of Coffee Protoplasts on Yellow Accumulation
3. Discussion
3.1. Control of the Pluripotent Cell Culture System through Coffee Somatic Embryogenesis
3.2. Allelopathic Activity of Coffee Cells by the Protoplast Co-Culture Bioassay Method with Digital Image Analysis
4. Materials and Methods
4.1. Establishment of Pluripotent Coffee Cell Cultures
4.2. Fluorescence Microscopy
4.3. Protoplast Isolation
4.4. Protoplast Co-Culture of Coffee with Lettuce
4.5. HPLC Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Greb, T.; Lohmann, J.U. Plant stem cells. Cur. Biol. 2016, 26, R816–R821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaillochet, C.; Lohmann, J.U. The never-ending story: From pluripotency to plant developmental plasticity. Development 2015, 142, 2237–2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragni, L.; Greb, T. Secondary growth as a determinant of plant shape and form. Semi. Cell Dev. Biol. 2018, 79, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Ogita, S. Plant cell, tissue and organ culture: The most flexible foundations for plant metabolic engineering applications. Nat. Prod. Commun. 2015, 6, 815–820. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, M. Historical review of research on plant cell dedifferentiation. J. Plant Res. 2015, 128, 349–359. [Google Scholar] [CrossRef]
- Rose, R.J.; Mantiri, F.R.; Kurdyukov, S.; Chen, S.K.; Wang, X.D.; Nolan, K.E.; Sheahan, M.B. Chapter 1; Developmental biology of somatic embryogenesis. In Plant Developmental Biology–Biotechnological Perspectives; Pua, E.C., Davey, M.R., Eds.; Springer: Berlin, Germany, 2010; Volume 2, pp. 3–26. [Google Scholar] [CrossRef]
- Verdeil, J.L.; Alemanno, L.; Niemenak, N.; Tranbarger, T.J. Pluripotent versus totipotent plant stem cells: Dependence versus autonomy? Trends Plant Sci. 2007, 12, 245–252. [Google Scholar] [CrossRef]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Ogita, S.; Nomura, T.; Kato, Y.; Uehara-Yamaguchi, Y.; Inoue, K.; Yoshida, T.; Sakurai, T.; Shinozaki, K.; Mochida, K. Transcriptional alterations during proliferation and lignification in Phyllostachys nigra cells. Sci. Rep. 2018, 8, 11347. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Liu, W.; Liu, J.; Qin, P.; Xu, L. Auxin control of root organogenesis from callus in tissue culture. Front. Plant Sci. 2017, 8, 1385. [Google Scholar] [CrossRef] [Green Version]
- Altamura, M.M.; Della, R.F.; Fattorini, L.; D’Angeli, S.; Falasca, G. Recent advances on genetic and physiological bases of in vitro somatic embryo formation. In In Vitro Embryogenesis in Higher Plants; Germana, M., Lambardi, M., Eds.; Humana Press: New York, NY, USA, 2016; pp. 47–85. [Google Scholar] [CrossRef]
- Annual Review: Addressing the Coffee Price Crisis; International Coffee Organization: London, UK, 2019; p. 32. Available online: http://www.ico.org/documents/cy2019-20/annual-review-2018-19-e.pdf (accessed on 13 July 2020).
- Ashihara, H.; Crozier, A. Caffeine: A well known but little mentioned compound in plant science. Trends Plant Sci. 2001, 6, 407–413. [Google Scholar] [CrossRef]
- Ogita, S.; Uefuji, H.; Morimoto, M.; Sano, H. Application of RNAi to confirm theobromine as the major intermediate for caffeine biosynthesis in coffee plants with potential for construction of decaffeinated varieties. Plant Mol. Biol. 2004, 54, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Sasamoto, H.; Murashige-Baba, T.; Inoue, A.; Sato, T.; Hayashi, S.; Hasegawa, A. Development of a new method for bioassay of allelopathy using protoplasts of a leguminous plant, Mucuna pruriens with a high content of the allelochemical L-DOPA. J. Plant Stud. 2013, 2, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Steward, F.C.; Mapes, M.O.; Mears, K. Growth and organized development of cultured cells. II. Organization in cultures grown from freely suspended cells. Am. J. Bot. 1958, 45, 705–708. [Google Scholar] [CrossRef]
- Kim, S.W.; Oh, S.C.; Liu, J.R. Control of direct and indirect somatic embryogenesis by exogenous growth regulators in immature zygotic embryo cultures of rose. Plant Cell Tiss. Org. Cult. 2003, 74, 61–66. [Google Scholar] [CrossRef]
- Paul, S.; Dam, A.; Bhattacharyya, A.; Bandyopadhyay, T.K. An efficient regeneration system via direct and indirect somatic embryogenesis for the medicinal tree Murraya koenigii. Plant Cell Tiss. Org. Cult. 2011, 105, 271–283. [Google Scholar] [CrossRef]
- Glover, B.J. Differentiation in plant epidermal cells. J. Exp. Bot. 2000, 51, 497–505. [Google Scholar] [CrossRef]
- Sasamoto, H.; Fujii, Y.; Ashihara, H. Effect of purine alkaloids on the proliferation of lettuce cells derived from protoplasts. Nat. Prod. Commun. 2015, 5, 751–754. [Google Scholar] [CrossRef] [Green Version]
- Hess, D. Alkaloids. In Plant Physiology: Molecular, Biochemical, and Physiological Fundamentals of Metabolism and Development; Springer: Berlin, Germany, 1975; pp. 144–159. [Google Scholar] [CrossRef]
- Fuentes-Cerda, C.F.J.; Monforte-González, M.; Méndez-Zeel, M.; Rojas-Herrera, R.; Loyola-Vargas, V.M. Modification of the embryogenic response of Coffea arabica by the nitrogen source. Biotechnol. Lett. 2001, 23, 1341–1343. [Google Scholar] [CrossRef]
- Ogita, S.; Sasamoto, H.; Yeung, E.C.; Thorpe, T.A. The effects of glutamine on the maintenance of embryogenic cultures of Cryptomeria japonica. Vitro Cell. Dev. Biol. Plant 2001, 37, 268–273. [Google Scholar] [CrossRef]
- Inoue, A.; Ogita, S.; Tsuchiya, S.; Minagawa, R.; Sasamoto, H. A protocol for axenic liquid cell cultures of a woody leguminous mangrove, Caesalpinia crista, and their amino acids profiling. Nat. Prod. Commun. 2015, 5, 755–760. [Google Scholar] [CrossRef] [Green Version]
- Mori, D.; Ogita, S.; Fujise, K.; Inoue, A.; Sasamoto, H. Protoplast co-culture bioassay for allelopathy in leguminous plants, Leucaena leucocephala and Mucuna gigantea, containing allelochemical amino acids, mimosine and L-DOPA. J. Plant Stud. 2015, 4, 1–11. [Google Scholar] [CrossRef]
- Asrori, M.I.; Sasamoto, H.; Ogita, S. In vitro bioassay of allelopathy in robusta coffee callus using sandwich method. Adv. Eng. Res. 2020, 194, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Chou, C.H.; Waller, G.R. Possible allelopathic constituents of Coffea arabica. J. Chem. Ecol. 1980, 6, 643–654. [Google Scholar] [CrossRef]
- Asrori, M.I. Studies on Pluripotent Cell Cultures for Exploring the Allelopathic Capacity of Robusta Coffee (Coffea canephora). Master’s Thesis, Prefectural University of Hiroshima, Hiroshima, Japan, 2020. [Google Scholar]
- Kumar, R.; Bohra, A.; Pandey, A.K.; Pandey, M.K.; Kumar, A. Metabolomics for plant improvement: Status and prospects. Front. Plant Sci. 2017, 8, 1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambla, J.L.; López-Gresa, M.P.; Bellés, J.M.; Granell, A. Metabolomic profiling of plant tissues. In Plant Functional Genomics; Alonso, J., Stepanova, A., Eds.; Humana Press: New York, NY, USA, 2015; pp. 221–235. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogita, S.; Asrori, M.I.; Sasamoto, H. Establishment of Pluripotent Cell Cultures to Explore Allelopathic Activity of Coffee Cells by Protoplast Co-Culture Bioassay Method. Plants 2020, 9, 1170. https://doi.org/10.3390/plants9091170
Ogita S, Asrori MI, Sasamoto H. Establishment of Pluripotent Cell Cultures to Explore Allelopathic Activity of Coffee Cells by Protoplast Co-Culture Bioassay Method. Plants. 2020; 9(9):1170. https://doi.org/10.3390/plants9091170
Chicago/Turabian StyleOgita, Shinjiro, Muchamad Imam Asrori, and Hamako Sasamoto. 2020. "Establishment of Pluripotent Cell Cultures to Explore Allelopathic Activity of Coffee Cells by Protoplast Co-Culture Bioassay Method" Plants 9, no. 9: 1170. https://doi.org/10.3390/plants9091170
APA StyleOgita, S., Asrori, M. I., & Sasamoto, H. (2020). Establishment of Pluripotent Cell Cultures to Explore Allelopathic Activity of Coffee Cells by Protoplast Co-Culture Bioassay Method. Plants, 9(9), 1170. https://doi.org/10.3390/plants9091170