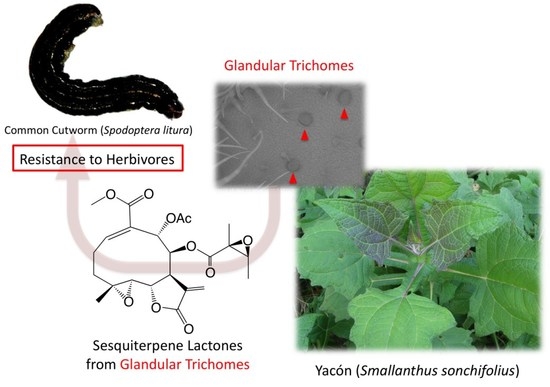
Chemical Defense of Yacón (Smallanthus sonchifolius) Leaves against Phytophagous Insects: Insect Antifeedants from Yacón Leaf Trichomes
Abstract
1. Introduction
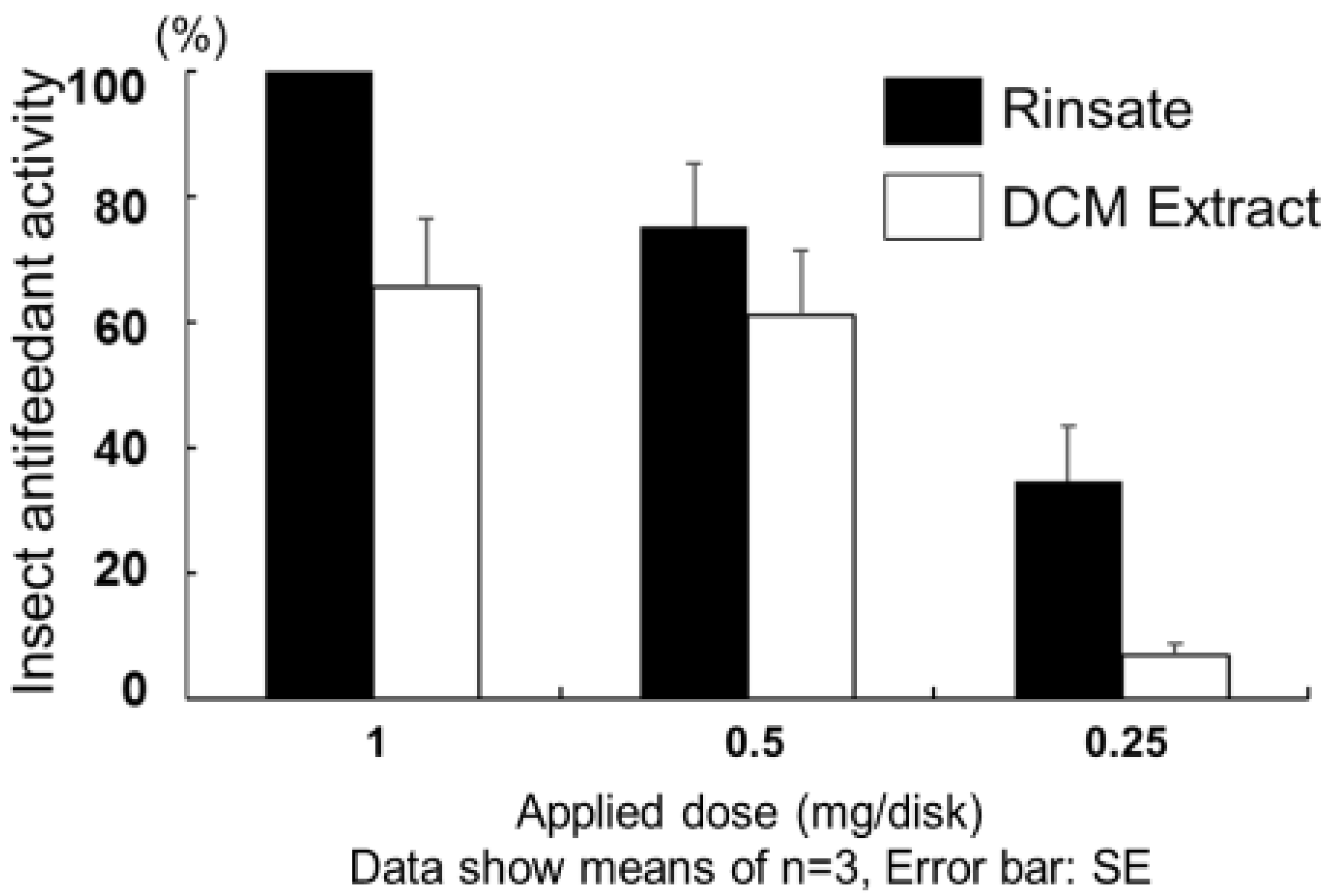
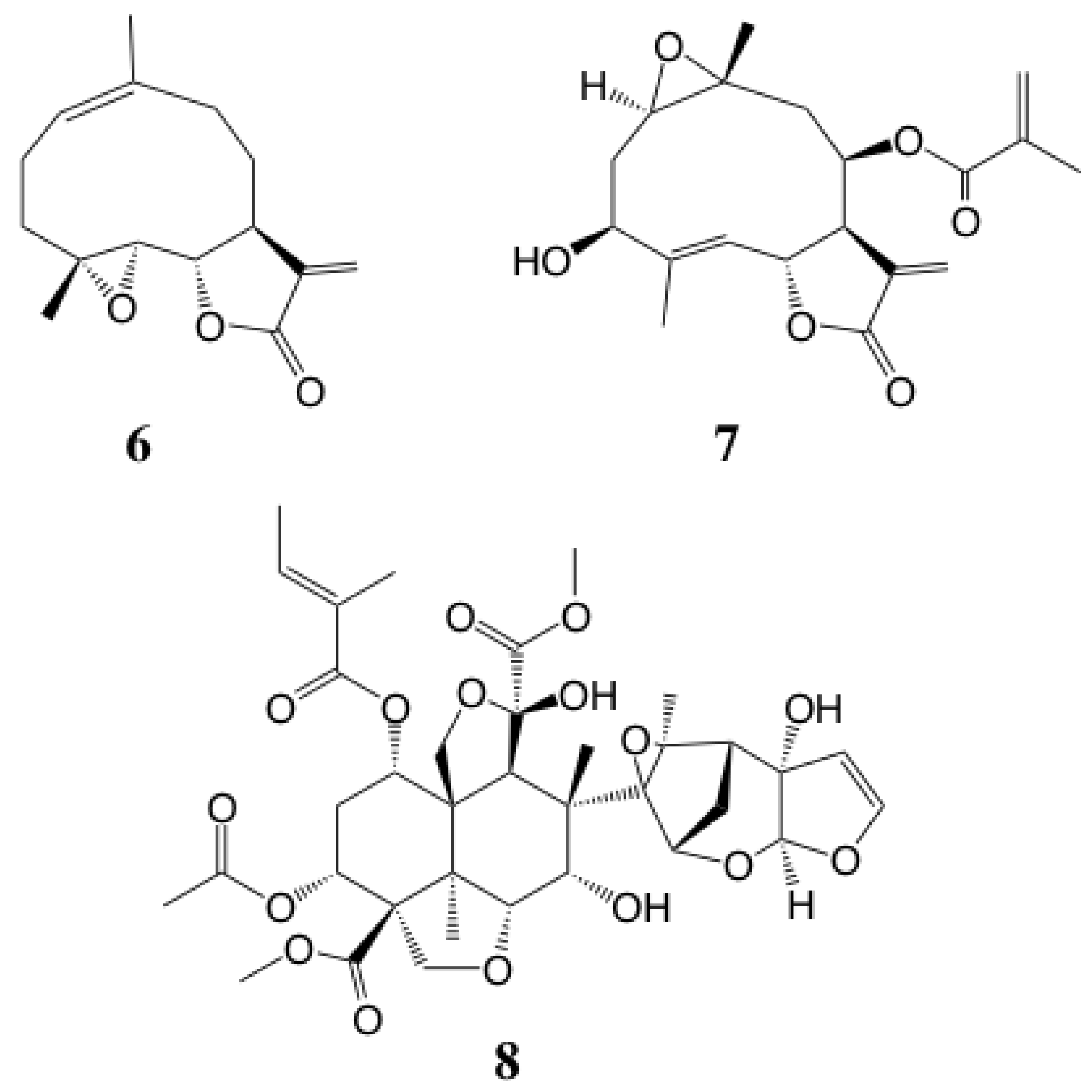
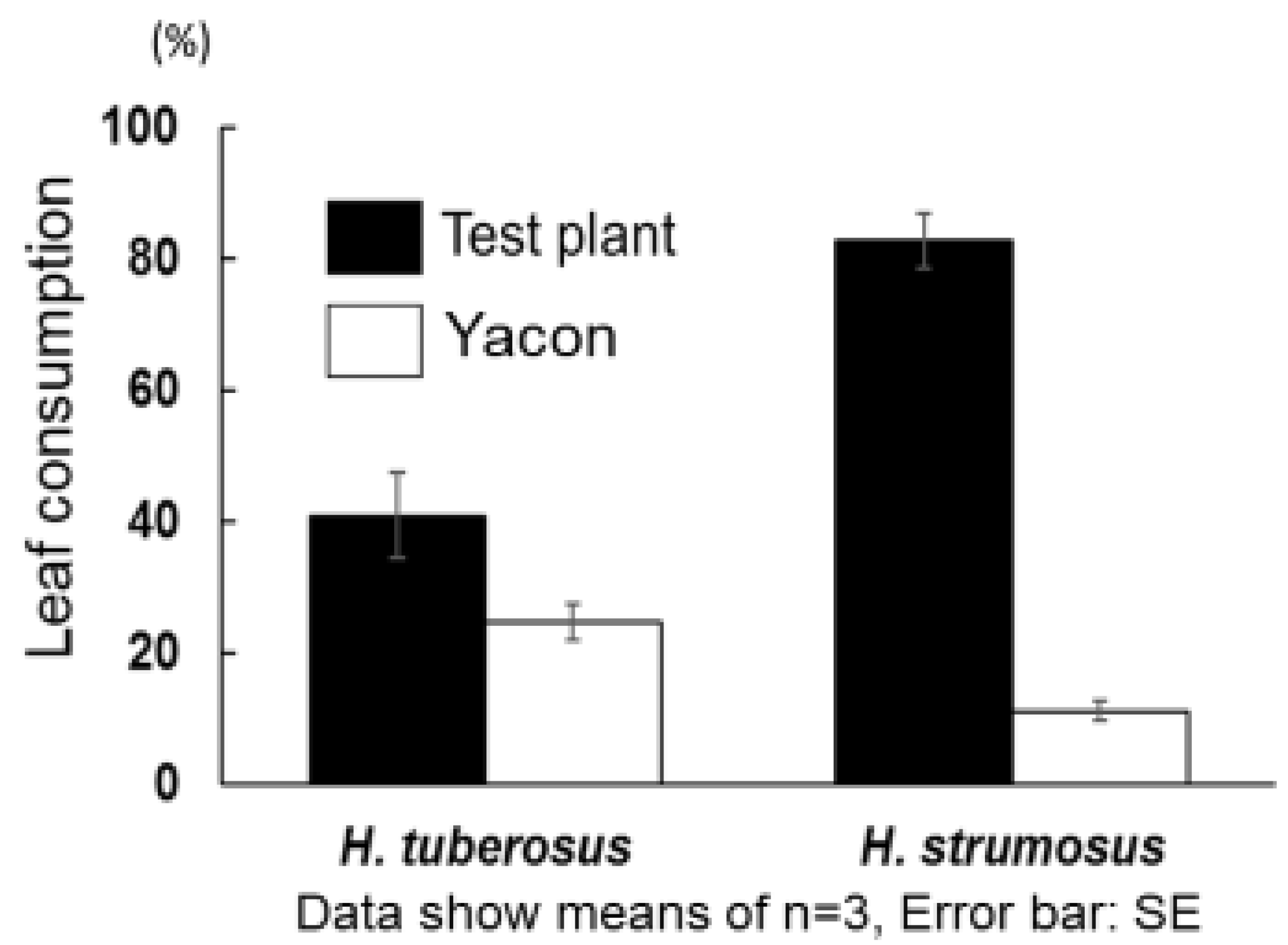
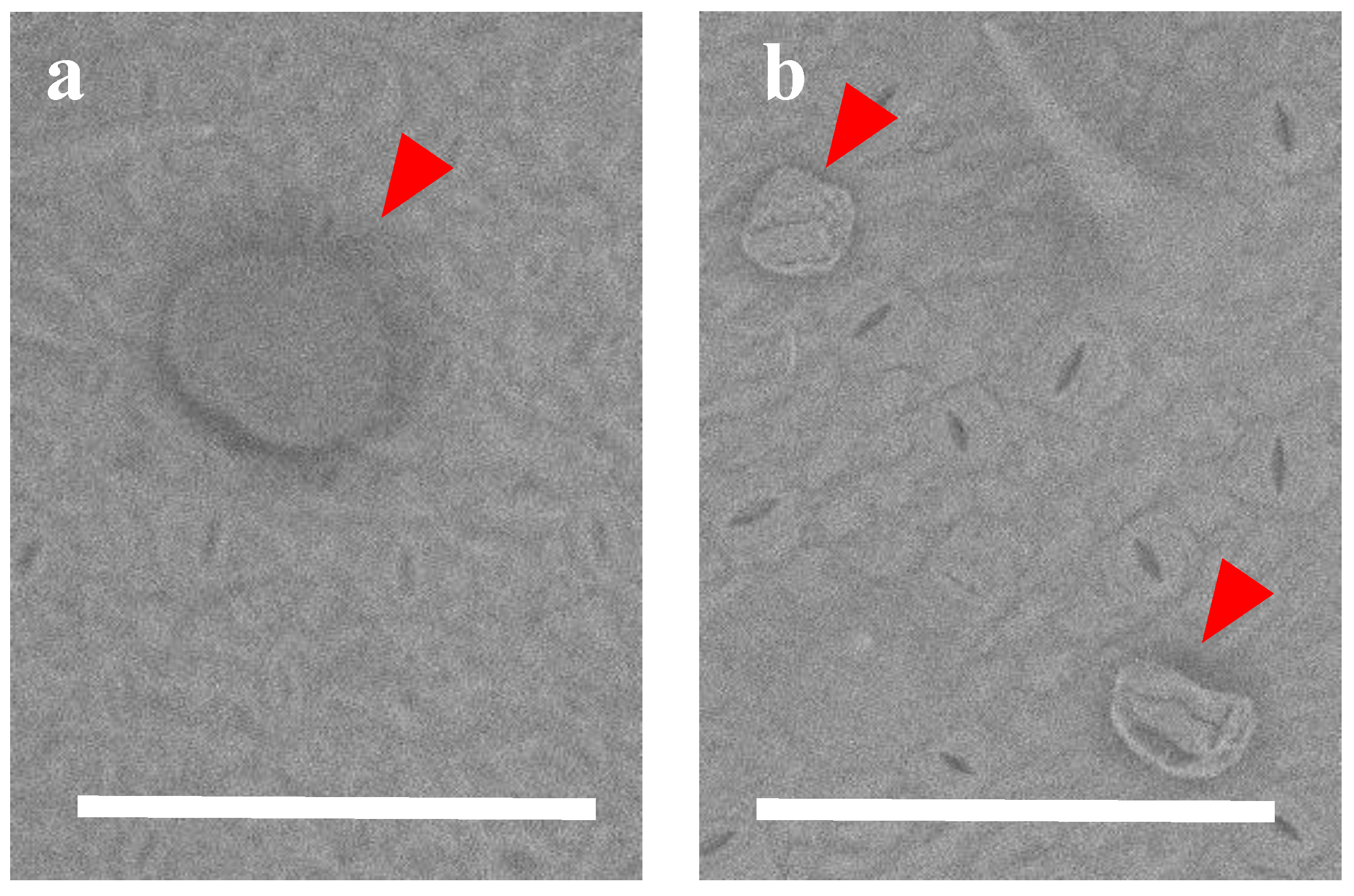
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Chemicals and Insects
3.3. Plant Materials
3.4. LVSEM Observation
3.5. Extraction and Isolation
3.6. Insect Antifeedant Biologic Assay (Dual-choice Type Test)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Doskotch, R.W.; El-Feraly, F.S.; Hufford, C.D. Sesquiterpene lactones from pyrethrum flowers. Can. J. Chem. 1971, 49, 2103–2110. [Google Scholar] [CrossRef]
- Ramirez, A.M.; Saillard, N.; Yang, T.; Franssen, M.C.R.; Bouwmeester, H.J.; Jongsma, M.A. Biosynthesis of sesquiterpene lactones in pyrethrum (Tanacetum cinerariifolium). PLoS ONE 2013, 8, e65030. [Google Scholar] [CrossRef] [PubMed]
- Ikezawa, N.; Göpfert, J.C.; Nguyen, D.T.; Kim, S.-U.; O’Maille, P.E.; Spring, O.; Ro, D.-K. Lettuce costunolide synthase (CYP71BL2) and its homolog (CYP71BL1) from sunflower catalyze distinct regio- and stereoselective hydroxylations in sesquiterpene lactone metabolism. J. Biol. Chem. 2011, 286, 21601–21611. [Google Scholar] [CrossRef] [PubMed]
- Rees, S.B.; Harborne, J.B. The role of sesquiterpene lactones and phenolics in the chemical defence of the chicory plant. Phytochemistry 1985, 24, 2225–2231. [Google Scholar] [CrossRef]
- Arnason, J.T.; Isman, M.B.; Philogène, B.J.R.; Waddell, T.G. Mode of action of the sesquiterpene lactone, tenulin, from Helenium amarum against herbivorous insects. J. Nat. Prod. 1987, 50, 690–695. [Google Scholar] [CrossRef]
- Rossiter, M.; Gershenzon, J.; Mabry, T.J. Behavioral and growth responses of specialist herbivore, Homoeosoma electellum, to major terpenoid of its host, Helianthus spp. J. Chem. Ecol. 1986, 12, 1505–1521. [Google Scholar] [CrossRef]
- Cis, J.; Nowak, G.; Kisiel, W. Antifeedant properties and chemotaxonomic implications of sesquiterpene lactones and syringin from Rhaponticum pulchrum. Biochem. Syst. Ecol. 2006, 34, 862–867. [Google Scholar] [CrossRef]
- Khajehei, F.; Hartung, J.; Graeff-Hönninger, S. Total phenolic content and antioxidant activity of yacon (Smallanthus Sonchifolius Poepp. and Endl.) chips: Effect of cultivar, pre-treatment and drying. Agriculture 2018, 8, 183. [Google Scholar] [CrossRef]
- Oliveira, R.B.; Chagas-Paula, D.A.; Secatto, A.; Gasparoto, T.H.; Faccioli, L.H.; Campanelli, A.P.; Da Costa, F.B. Topical anti-inflammatory activity of yacon leaf extracts. Rev. Bras. De Farmacogn. 2013, 23, 497–505. [Google Scholar] [CrossRef]
- Lachman, J.; Fernández, E.C.; Orsák, M. Yacon [Smallanthus sonchifolia (Poepp. et Endl.) H. Robinson] chemical composition and use—A review. Plant Soil Environ. 2003, 49, 283–290. [Google Scholar] [CrossRef]
- Stipanovic, R.D. Function and Chemistry of Plant Trichomes and Glands in Insect Resistance. In Plant Resistance to Insects; Hedin, P.A., Ed.; American Chemical Society: Washington, DC, USA, 1983; Volume 208, pp. 69–100. [Google Scholar]
- Sletvold, N.; Huttunen, P.; Handley, R.; Kärkkäinen, K.; Ågren, J. Cost of trichome production and resistance to a specialist insect herbivore in Arabidopsis lyrata. Evol Ecol 2010, 24, 1307–1319. [Google Scholar] [CrossRef]
- Li, C.-H.; Liu, Y.; Hua, J.; Luo, S.-H.; Li, S.-H. Peltate glandular trichomes of Colquhounia seguinii harbor new defensive clerodane diterpenoids. J. Integr. Plant Biol. 2014, 56, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Recent progress in secondary metabolism of plant glandular trichomes. Plant Biotechnol. 2014, 31, 353–361. [Google Scholar] [CrossRef]
- Tian, D.; Tooker, J.; Peiffer, M.; Chung, S.H.; Felton, G.W. Role of trichomes in defense against herbivores: Comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta 2012, 236, 1053–1066. [Google Scholar] [CrossRef]
- Morimoto, M. Chemical defense against insects in Heterotheca subaxillaris and three Orobanchaceae species using exudates from trichomes. Pest Manag. Sci. 2019, 75, 2474–2481. [Google Scholar] [CrossRef]
- Morimoto, M.; Cantrell, C.L.; Libous-Bailey, L.; Duke, S.O. Phytotoxicity of constituents of glandular trichomes and the leaf surface of camphorweed, Heterotheca subaxillaris. Phytochemistry 2009, 70, 69–74. [Google Scholar] [CrossRef]
- Lopes, A.A.; Pina, E.S.; Silva, D.B.; Pereira, A.M.S.; da Silva, M.F.D.G.; Da Costa, F.B.; Lopes, N.P.; Pupo, M.T. A biosynthetic pathway of sesquiterpene lactones in Smallanthus sonchifolius and their localization in leaf tissues by MALDI imaging. Chem. Commun. 2013, 49, 9989–9991. [Google Scholar] [CrossRef]
- Ambrósio, S.R.; Oki, Y.; Heleno, V.C.G.; Chaves, J.S.; Nascimento, P.G.B.D.; Lichston, J.E.; Constantino, M.G.; Varanda, E.M.; Da Costa, F.B. Constituents of glandular trichomes of Tithonia diversifolia: Relationships to herbivory and antifeedant activity. Phytochemistry 2008, 69, 2052–2060. [Google Scholar] [CrossRef]
- Inoue, A.; Tamogami, S.; Kato, H.; Nakazato, Y.; Akiyama, M.; Kodama, O.; Akatsuka, T.; Hashidoko, Y. Antifungal melampolides from leaf extracts of Smallanthus sonchifolius. Phytochemistry 1995, 39, 845–848. [Google Scholar] [CrossRef]
- Srivastava, R.P.; Proksch, P.; Wray, V. Toxicity and antifeedant activity of a sesquiterpene lactone from Encelia against Spodoptera littoralis. Phytochemistry 1990, 29, 3445–3448. [Google Scholar] [CrossRef]
- Galindo, J.C.G.; Hernández, A.; Dayan, F.E.; Tellez, M.R.; Macías, F.A.; Paul, R.N.; Duke, S.O. Dehydrozaluzanin C, a natural sesquiterpenolide, cause rapid plasma membrane leakage. Phytochemistry 1999, 52, 805–813. [Google Scholar] [CrossRef]
- Chen, F.; Hao, F.; Li, C.; Gou, J.; Lu, D.; Gong, F.; Tang, H.; Zhang, Y. Identifying three ecological chemotypes of Xanthium strumarium glandular trichomes using a combined NMR and LC-MS method. PLoS ONE 2013, 8, e76621. [Google Scholar] [CrossRef] [PubMed]
- Ahern, J.R.; Whitney, K.D. Sesquiterpene lactone stereochemistry influences herbivore resistance and plant fitness in the field. Ann. Bot. 2014, 113, 731–740. [Google Scholar] [CrossRef]
- Ali, E.; Dastidar, P.P.G.; Pakrashi, S.C.; Durham, L.J.; Duffield, A.M. Studies on Indian medicinal plants—XXVIII: Sesquiterpene lactones of Enhydra fluctuans Lour. Structures of enhydrin, fluctuanin and fluctuadin. Tetrahedron 1972, 28, 2285–2298. [Google Scholar] [CrossRef]
- Herz, W.; Bhat, S.V. Isolation and structure of two new germacranolides from Polymnia uvedalia. J. Org. Chem. 1970, 35, 2605–2611. [Google Scholar] [CrossRef]
- Morimoto, H.; Oshio, H. Isolation of deacetylviguiestenin and erioflorin from Helianthus tuberosus. J. Nat. Prod. 1981, 44, 748–749. [Google Scholar] [CrossRef]
- Escoubas, P.; Lajide, L.; Mizutani, J. An improved leaf-disk antifeedant bioassay and its application for the screening of Hokkaido plants. Entomol. Exp. Appl. 1993, 66, 99–107. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 2nd ed.; Cambridge Univ. Press: London, UK, 1952; p. 318. [Google Scholar]


| Antifeedant Activity against S. litura Larvae | |||
|---|---|---|---|
| Compounds | ED50 (mg/cm2) | ED50 (µmol/cm2) | 95% CI (mg/cm2) |
| 1 | 0.0080 | 0.018 | 0.0062–0.012 |
| 2 | 0.0042 | 0.0090 | 0.0026–0.0086 |
| 3 | 0.030 | 0.072 | 0.022–0.043 |
| 4 | 0.040 | 0.058 | 0.015–0.070 |
| 5 | 0.027 | 0.088 | 0.0076–0.053 |
| 6 | 0.25 | 7.3 | 0.15–0.69 |
| 7 | 0.20 | 7.9 | 0.18–0.21 |
| 8 (Aza) | 0.00023 | 0.00032 | 0.00021–0.00027 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsunaki, K.; Morimoto, M. Chemical Defense of Yacón (Smallanthus sonchifolius) Leaves against Phytophagous Insects: Insect Antifeedants from Yacón Leaf Trichomes. Plants 2020, 9, 848. https://doi.org/10.3390/plants9070848
Tsunaki K, Morimoto M. Chemical Defense of Yacón (Smallanthus sonchifolius) Leaves against Phytophagous Insects: Insect Antifeedants from Yacón Leaf Trichomes. Plants. 2020; 9(7):848. https://doi.org/10.3390/plants9070848
Chicago/Turabian StyleTsunaki, Kaisei, and Masanori Morimoto. 2020. "Chemical Defense of Yacón (Smallanthus sonchifolius) Leaves against Phytophagous Insects: Insect Antifeedants from Yacón Leaf Trichomes" Plants 9, no. 7: 848. https://doi.org/10.3390/plants9070848
APA StyleTsunaki, K., & Morimoto, M. (2020). Chemical Defense of Yacón (Smallanthus sonchifolius) Leaves against Phytophagous Insects: Insect Antifeedants from Yacón Leaf Trichomes. Plants, 9(7), 848. https://doi.org/10.3390/plants9070848