Toward Unifying Global Hotspots of Wild and Domesticated Biodiversity
Abstract
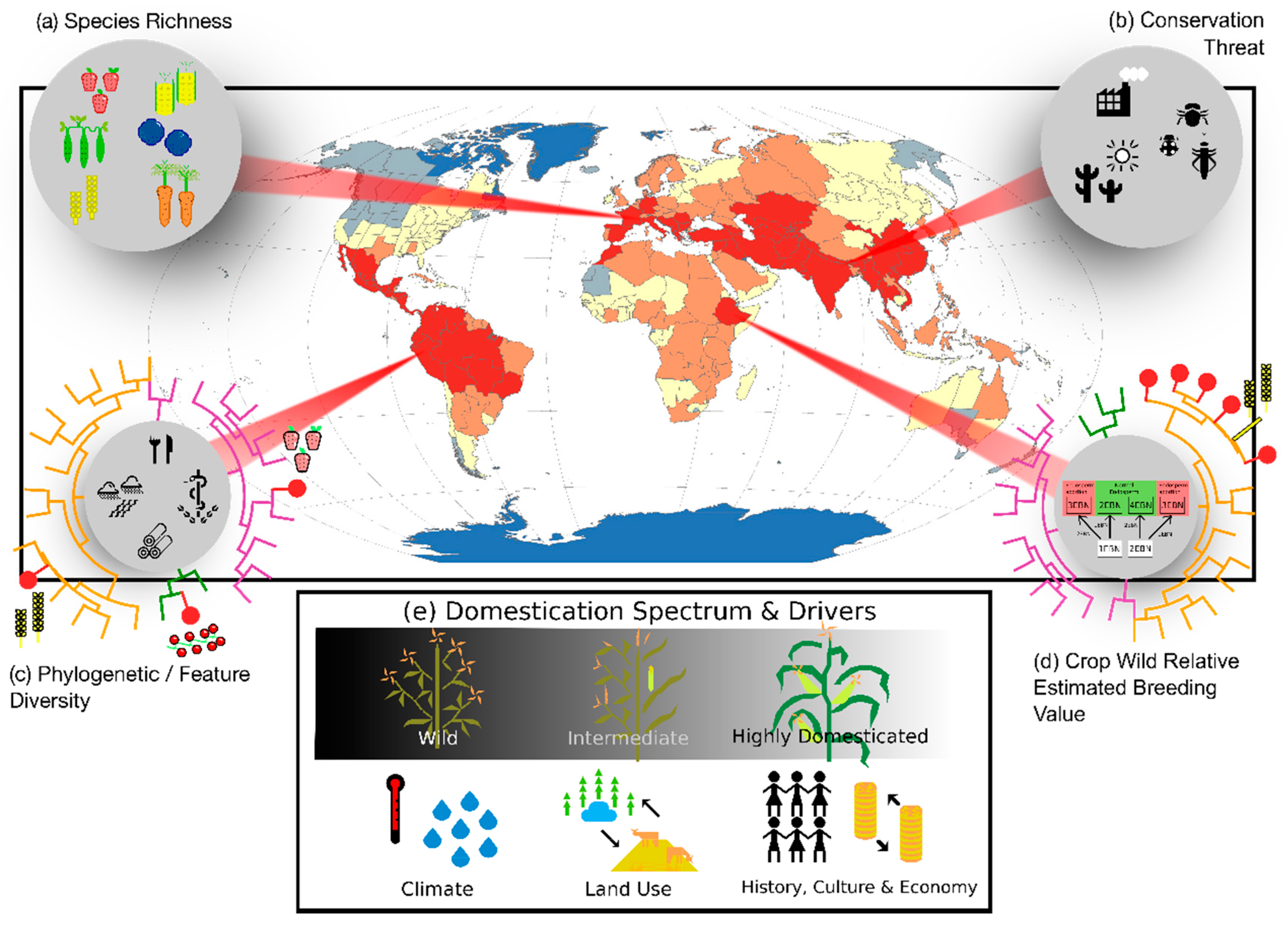
:1. Introduction
2. The History of Diversity Hotspots
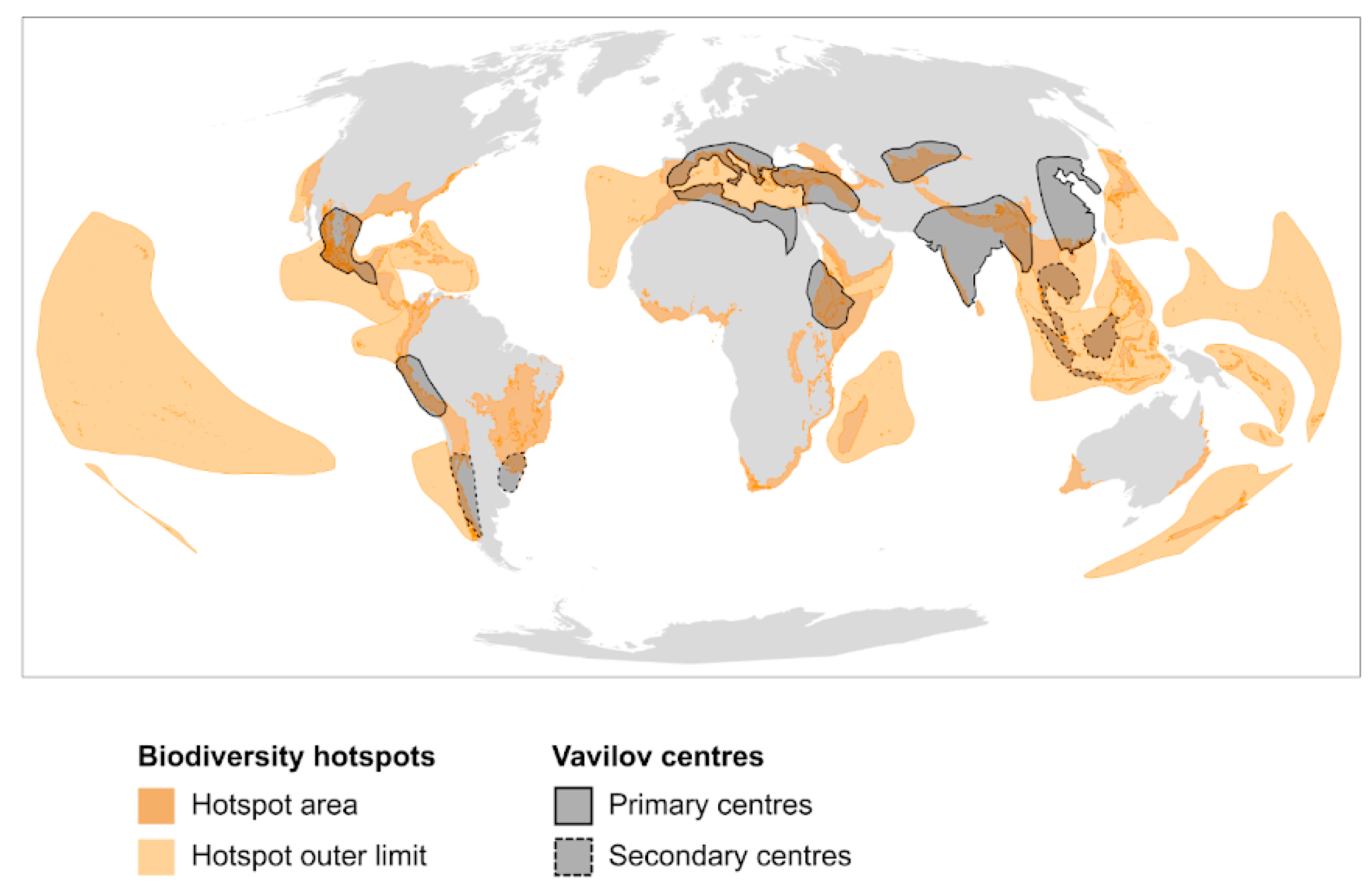
2.1. Biodiversity Hotspots
2.2. Agro-Biodiversity Hotspots
3. From Biodiversity to Agro-Biodiversity Hotspots: A Geographic Continuum
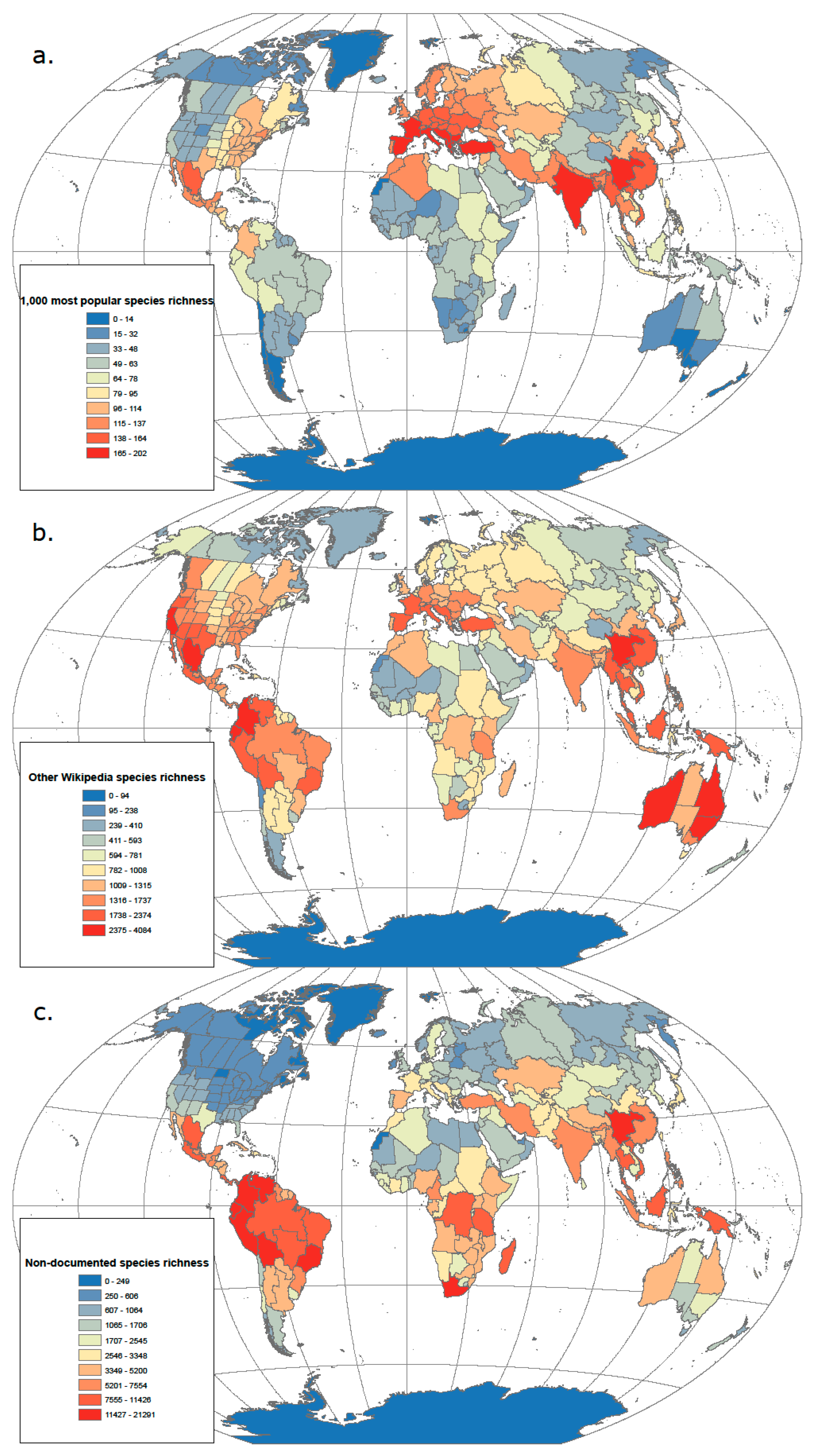
3.1. From Popularity to Anonymity
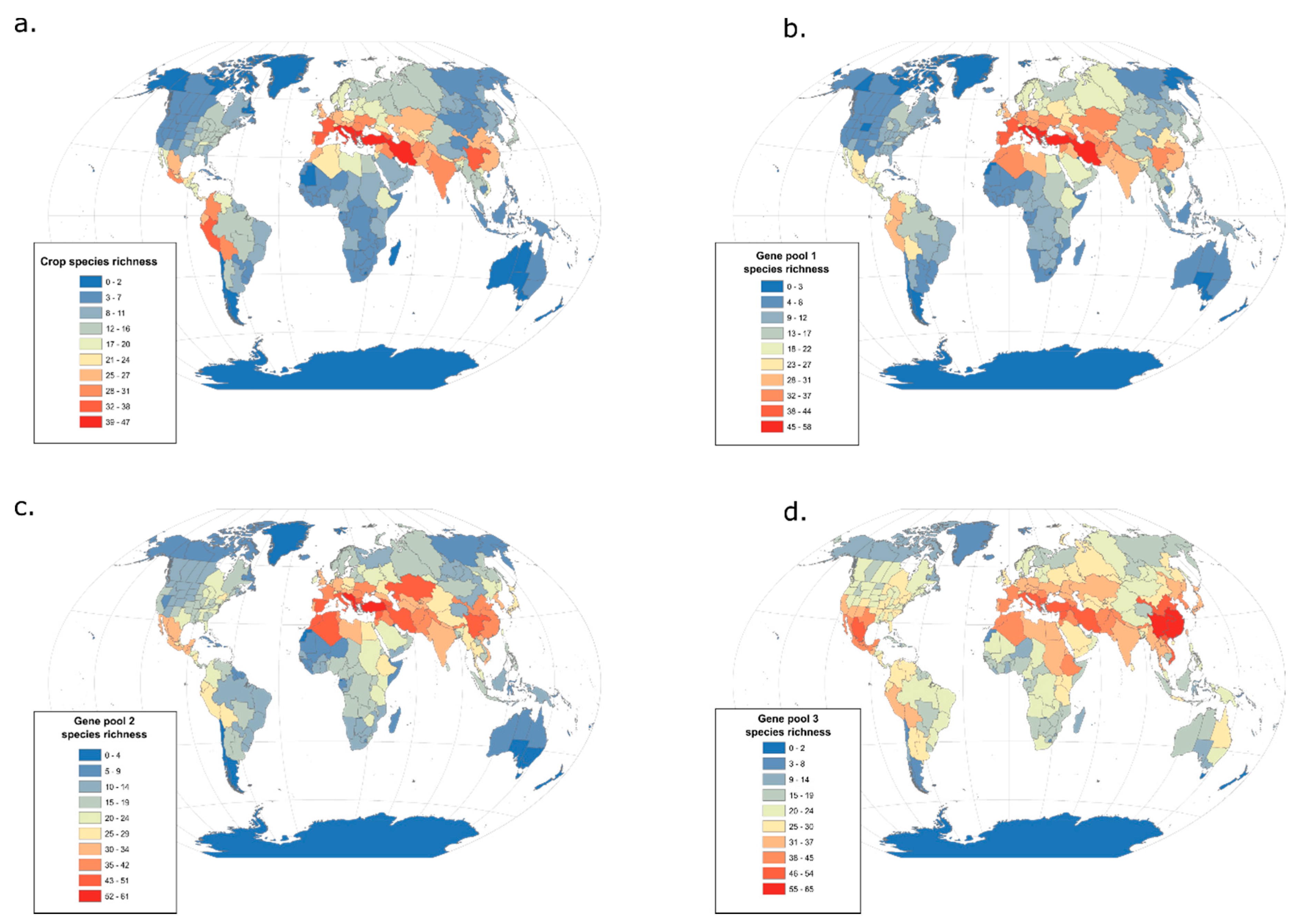
3.2. From Domesticates to Wild Relatives
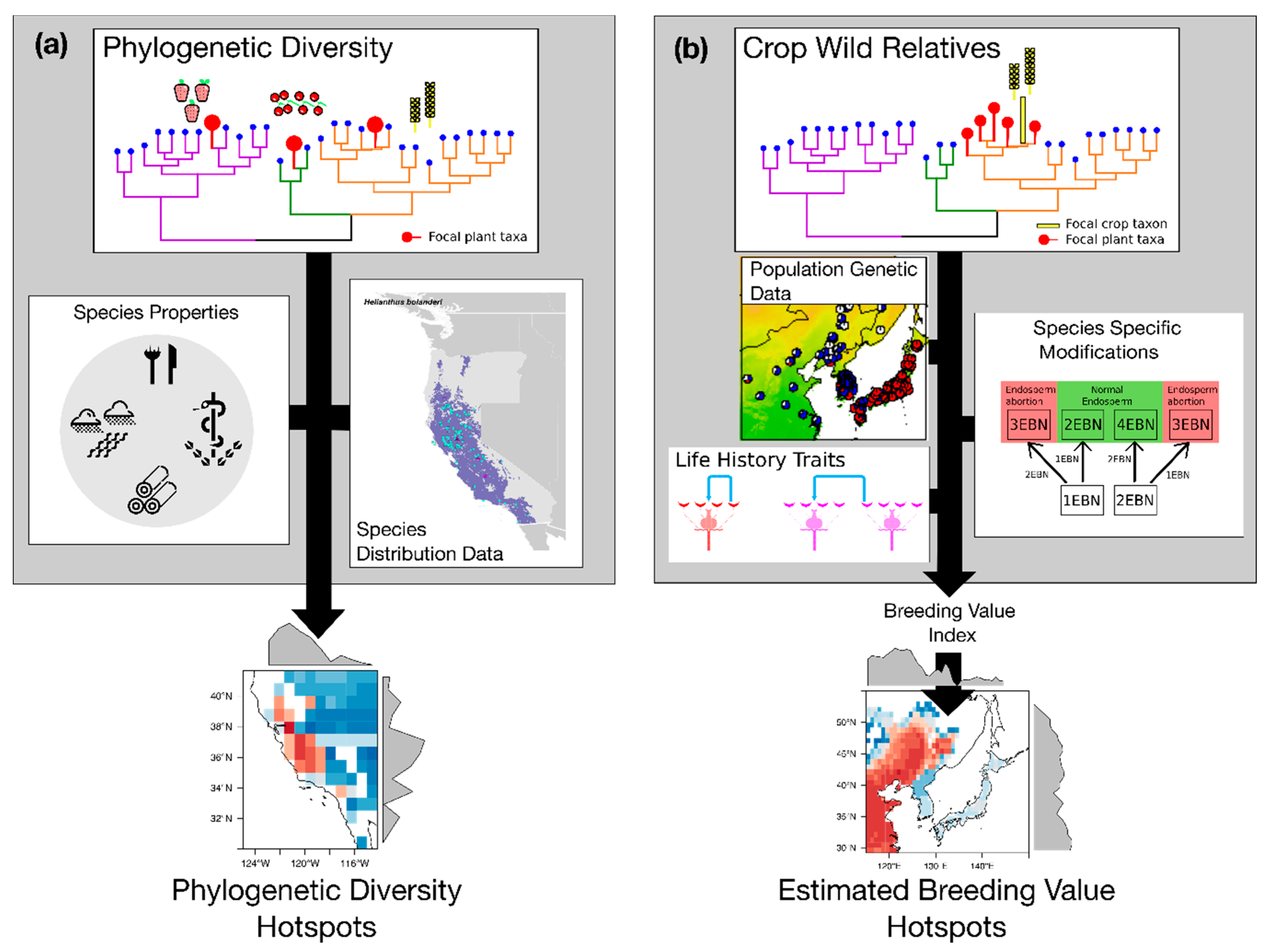
4. Integrating Genetic and Phylogenetic Diversity into Agro-Biodiversity Hotspots
4.1. Hotspots of Phylogenetic Diversity
4.2. Hotspots of Breeding Value
5. Towards a Unified Concept of Agro-Biodiversity Hotspot
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pironon, S.; Papuga, G.; Villellas, J.; Angert, A.L.; García, M.B.; Thompson, J.D. Geographic variation in genetic and demographic performance: New insights from an old biogeographical paradigm: The centre-periphery hypothesis. Biol. Rev. 2017, 92, 1877–1909. [Google Scholar] [CrossRef] [PubMed]
- Chaplin-Kramer, R.; Sharp, R.P.; Weil, C.; Bennett, E.M.; Pascual, U.; Arkema, K.K.; Brauman, K.A.; Bryant, B.P.; Guerry, A.D.; Haddad, N.M.; et al. Global modeling of nature’s contributions to people. Science 2019, 366, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Arnell, A.; de Lamo, X.; García-Rangel, S.; Lewis, M.; Mark, J.; Merow, C.; Miles, L.; Ondo, I.; Pironon, S.; et al. Areas of global importance for terrestrial biodiversity, carbon, and water. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Jetz, W.; McGeoch, M.A.; Guralnick, R.; Ferrier, S.; Beck, J.; Costello, M.J.; Fernandez, M.; Geller, G.N.; Keil, P.; Merow, C.; et al. Essential biodiversity variables for mapping and monitoring species populations. Nat. Ecol. Evol. 2019, 3, 539–551. [Google Scholar] [CrossRef] [Green Version]
- Kier, G.; Kreft, H.; Lee, T.M.; Jetz, W.; Ibisch, P.L.; Nowicki, C.; Mutke, J.; Barthlott, W. A global assessment of endemism and species richness across island and mainland regions. Proc. Natl. Acad. Sci. USA 2009, 106, 9322–9327. [Google Scholar] [CrossRef] [Green Version]
- Khoury, C.K.; Achicanoy, H.A.; Bjorkman, A.D.; Navarro-Racines, C.; Guarino, L.; Flores-Palacios, X.; Engels, J.M.M.; Wiersema, J.H.; Dempewolf, H.; Sotelo, S.; et al. Origins of food crops connect countries worldwide. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160792. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Riddle, B.R.; Brown, J.H. Biogeography; Sinauer Associates: Sunderland, UK, 2006; ISBN 978-0-87893-062-3. [Google Scholar]
- Harlan, J.R.; Hutchinson, J.B.; Clark, G.; Jope, E.M.; Riley, R. Plant and animal distribution in relation to domestication. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1976, 275, 13–25. [Google Scholar] [CrossRef]
- Diamond, J. Evolution, consequences and future of plant and animal domestication. Nature 2002, 418, 700–707. [Google Scholar] [CrossRef]
- Soga, M.; Gaston, K.J. The ecology of human–nature interactions. Proc. R. Soc. B Biol. Sci. 2020, 287, 20191882. [Google Scholar] [CrossRef] [Green Version]
- Hartel, T.; Nita, A.; Rozylowicz, L. Understanding human–nature connections through value networks: The case of ancient wood-pastures of Central Romania. Sustain. Sci. 2020, 15, 1357–1367. [Google Scholar] [CrossRef]
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M.; Chan, K.M.A.; et al. Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 2019, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corlett, R.T. Plant diversity in a changing world: Status, trends, and conservation needs. Plant Divers. 2016, 38, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newbold, T.; Hudson, L.N.; Arnell, A.P.; Contu, S.; Palma, A.D.; Ferrier, S.; Hill, S.L.L.; Hoskins, A.J.; Lysenko, I.; Phillips, H.R.P.; et al. Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science 2016, 353, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Pironon, S.; Etherington, T.R.; Borrell, J.S.; Kühn, N.; Macias-Fauria, M.; Ondo, I.; Tovar, C.; Wilkin, P.; Willis, K.J. Potential adaptive strategies for 29 sub-Saharan crops under future climate change. Nat. Clim. Chang. 2019, 9, 758–763. [Google Scholar] [CrossRef]
- Borrell, J.S.; Dodsworth, S.; Forest, F.; Pérez-Escobar, O.A.; Lee, M.A.; Mattana, E.; Stevenson, P.C.; Howes, M.-J.R.; Pritchard, H.W.; Ballesteros, D.; et al. The climatic challenge: Which plants will people use in the next century? Environ. Exp. Bot. 2020, 170, 103872. [Google Scholar] [CrossRef]
- Cámara-Leret, R.; Fortuna, M.A.; Bascompte, J. Indigenous knowledge networks in the face of global change. Proc. Natl. Acad. Sci. USA 2019, 201821843. [Google Scholar] [CrossRef] [Green Version]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global biodiversity conservation: The critical role of hotspots. In Biodiversity Hotspots; Zachos, F.E., Habel, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–22. ISBN 978-3-642-20991-8. [Google Scholar]
- Jenkins, C.N.; Pimm, S.L.; Joppa, L.N. Global patterns of terrestrial vertebrate diversity and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, E2602–E2610. [Google Scholar] [CrossRef] [Green Version]
- Pollock, L.J.; Thuiller, W.; Jetz, W. Large conservation gains possible for global biodiversity facets. Nature 2017, 546, 141–144. [Google Scholar] [CrossRef]
- Myers, N. Threatened biotas: “Hot spots” in tropical forests. Environmentalist 1988, 8, 187–208. [Google Scholar] [CrossRef]
- Cincotta, R.P.; Wisnewski, J.; Engelman, R. Human population in the biodiversity hotspots. Nature 2000, 404, 990–992. [Google Scholar] [CrossRef]
- Renard, D.; Tilman, D. National food production stabilized by crop diversity. Nature 2019, 571, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, R.; Balmford, A.; Costanza, R.; Fisher, B.; Green, R.E.; Lehner, B.; Malcolm, T.R.; Ricketts, T.H. Global mapping of ecosystem services and conservation priorities. Proc. Natl. Acad. Sci. USA 2008, 105, 9495–9500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, K.; Arrowsmith, N.; Gladis, T. Agrobiodiversity with emphasis on plant genetic resources. Naturwissenschaften 2003, 90, 241–250. [Google Scholar] [CrossRef]
- Jackson, L.E.; Pascual, U.; Hodgkin, T. Utilizing and conserving agrobiodiversity in agricultural landscapes. Agric. Ecosyst. Environ. 2007, 121, 196–210. [Google Scholar] [CrossRef]
- Vavilov, N.I.; Vavylov, M.I.; Vavílov, N.Í.; Dorofeev, V.F. Origin and Geography of Cultivated Plants; Cambridge University Press: Cambridge, UK, 1992; ISBN 978-0-521-40427-3. [Google Scholar]
- Castañeda-Álvarez, N.P.; Khoury, C.K.; Achicanoy, H.A.; Bernau, V.; Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Harker, R.H.; Jarvis, A.; Maxted, N.; et al. Global conservation priorities for crop wild relatives. Nat. Plants 2016, 2, 16022. [Google Scholar] [CrossRef]
- Monfreda, C.; Ramankutty, N.; Foley, J.A. Farming the planet: (2) Geographic distribution of crop areas, yields, physiological types, and net primary production in the year 2000. Glob. Biogeochem. Cycles 2008, 22, GB1022. [Google Scholar] [CrossRef]
- Khoury, C.K.; Amariles, D.; Soto, J.S.; Diaz, M.V.; Sotelo, S.; Sosa, C.C.; Ramírez-Villegas, J.; Achicanoy, H.A.; Velásquez-Tibatá, J.; Guarino, L.; et al. Comprehensiveness of conservation of useful wild plants: An operational indicator for biodiversity and sustainable development targets. Ecol. Indic. 2019, 98, 420–429. [Google Scholar] [CrossRef]
- Attwood, S.J.; Park, S.E.; Marshall, P.; Fanshawe, J.H.; Gaisberger, H. An Argument for Integrating Wild and Agricultural Biodiversity Conservation; Handbooks Online; Routledge: London, UK, 2017; ISBN 978-0-415-74692-2. [Google Scholar]
- Pilling, D.; Bélanger, J.; Hoffmann, I. Declining biodiversity for food and agriculture needs urgent global action. Nat. Food 2020, 1, 144–147. [Google Scholar] [CrossRef]
- de Candolle, A. Géographie Botanique Raisonnée ou Exposition des Faits Principaux et des Lois Concernant la Distribution Géographique des Plantes de l’Époque Actuelle; Masson, V., Ed.; Nabu Press: London, UK, 1855. [Google Scholar]
- Wallace, A.R. The Geographical Distribution of Animals; Harper and Brothers: New York, NY, USA, 1876. [Google Scholar]
- Cailleux, A. Biogéographie Mondiale; Presses Universitaires de France: Paris, France, 1953. [Google Scholar]
- Wulff, E.W. Versuch einer einteilung der vegetation der erde in pflanzengeographische gebiete auf grund der artenzahl. Repertorium Specierum Novarum Regni Vegetabilis 1935, 12, 57–83. [Google Scholar]
- Malyshev, L.I. Quantitative analysis of flora: Spatial diversity, the level of specific richness and representativity of sampling areas. Bot. Zhurnal 1975, 60, 1537–1550. [Google Scholar]
- Frodin, D.G. Guide to Standard Floras of the World; Cambridge University Press: Cambridge, UK, 1984; ISBN 978-1-139-42865-1. [Google Scholar]
- Myers, N. The biodiversity challenge: Expanded hot-spots analysis. Environmentalist 1990, 10, 243–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.D.; Boyer, A.G.; Kim, H.; Pompa-Mansilla, S.; Hamilton, M.J.; Costa, D.P.; Ceballos, G.; Brown, J.H. Drivers and hotspots of extinction risk in marine mammals. Proc. Natl. Acad. Sci. USA 2012, 109, 3395–3400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Righetti, D.; Vogt, M.; Gruber, N.; Psomas, A.; Zimmermann, N.E. Global pattern of phytoplankton diversity driven by temperature and environmental variability. Sci. Adv. 2019, 5, eaau6253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; de Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Marco, M.; Ferrier, S.; Harwood, T.D.; Hoskins, A.J.; Watson, J.E.M. Wilderness areas halve the extinction risk of terrestrial biodiversity. Nature 2019, 573, 582–585. [Google Scholar] [CrossRef]
- Tucker, C.M.; Davies, T.J.; Cadotte, M.W.; Pearse, W.D. On the relationship between phylogenetic diversity and trait diversity. Ecology 2018, 99, 1473–1479. [Google Scholar] [CrossRef] [Green Version]
- Devictor, V.; Mouillot, D.; Meynard, C.; Jiguet, F.; Thuiller, W.; Mouquet, N. Spatial mismatch and congruence between taxonomic, phylogenetic and functional diversity: The need for integrative conservation strategies in a changing world: Spatial mismatch between diversity facets. Ecol. Lett. 2010, 13, 1030–1040. [Google Scholar] [CrossRef]
- Azevedo, J.A.R.; Guedes, T.B.; Nogueira, C.D.C.; Passos, P.; Sawaya, R.J.; Prudente, A.L.C.; Barbo, F.E.; Strüssmann, C.; Franco, F.L.; Arzamendia, V.; et al. Museums and cradles of diversity are geographically coincident for narrowly distributed Neotropical snakes. Ecography 2020, 43, 328–339. [Google Scholar] [CrossRef]
- Barthlott, W.; Lauer, W.; Placke, A. Global distribution of species diversity in vascular plants: Towards a world map of phytodiversity (globale verteilung der artenvielfalt höherer pflanzen: Vorarbeiten zu einer weltkarte der phytodiversität). Erdkunde 1996, 50, 317–327. [Google Scholar] [CrossRef]
- Kier, G.; Mutke, J.; Dinerstein, E.; Ricketts, T.H.; Küper, W.; Kreft, H.; Barthlott, W. Global patterns of plant diversity and floristic knowledge. J. Biogeogr. 2005, 32, 1107–1116. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344. [Google Scholar] [CrossRef] [PubMed]
- WCVP. World Checklist of Vascular Plants, Version 2.0. Facilitated by the Royal Botanic Gardens, Kew. 2020. Published on the Internet. Available online: http://wcvp.science.kew.org/ (accessed on 12 April 2020).
- Darbyshire, I.; Anderson, S.; Asatryan, A.; Byfield, A.; Cheek, M.; Clubbe, C.; Ghrabi, Z.; Harris, T.; Heatubun, C.D.; Kalema, J.; et al. Important plant areas: Revised selection criteria for a global approach to plant conservation. Biodivers. Conserv. 2017, 26, 1767–1800. [Google Scholar] [CrossRef] [Green Version]
- Tropical Important Plant Areas. Available online: https://www.kew.org/science/our-science/projects/tropical-important-plant-areas (accessed on 28 August 2020).
- Kreft, H.; Jetz, W. Global patterns and determinants of vascular plant diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 5925–5930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enquist, B.J.; Feng, X.; Boyle, B.; Maitner, B.; Newman, E.A.; Jørgensen, P.M.; Roehrdanz, P.R.; Thiers, B.M.; Burger, J.R.; Corlett, R.T.; et al. The commonness of rarity: Global and future distribution of rarity across land plants. Sci. Adv. 2019, 5, eaaz0414. [Google Scholar] [CrossRef] [Green Version]
- van Kleunen, M.; Xu, X.; Yang, Q.; Maurel, N.; Zhang, Z.; Dawson, W.; Essl, F.; Kreft, H.; Pergl, J.; Pyšek, P.; et al. Economic use of plants is key to their naturalization success. Nat. Commun. 2020, 11, 3201. [Google Scholar] [CrossRef]
- Olden, J.D.; LeRoy Poff, N.; Douglas, M.R.; Douglas, M.E.; Fausch, K.D. Ecological and evolutionary consequences of biotic homogenization. Trends Ecol. Evol. 2004, 19, 18–24. [Google Scholar] [CrossRef]
- de Candolle, A. Origin of Cultivated Plants; D. Appleton: New York, NY, USA, 1885. [Google Scholar]
- Zhukovsky, P.M. New centres of origin and new gene centres of cultivated plants including specifically endemic microcentres of species closely allied to cultivated species. Bot. J. (Russian Bot. Z.) 1968, 53, 430–460. [Google Scholar]
- Sinskaya, E.N. Historical Geography of Cultivated Floras (at the Dawn of Agriculture); Kolos: Leningrad, Russia, 1969. [Google Scholar]
- Harlan, J.R. Agricultural origins: Centers and noncenters. Science 1971, 174, 468–474. [Google Scholar] [CrossRef]
- Zeven, A.C.; de Wet, J.M.J. Dictionary of Cultivated Plants and Their Centres of Diversity: Excluding Most Ornamentals, Forest Trees and Lower Plants; CAPD: Wageningen, The Netherlands, 1982. [Google Scholar]
- Hawkes, J.G. The Diversity of Crop Plants; Harvard University Press: Cambridge, MA, USA, 1983. [Google Scholar]
- Doebley, J.F.; Gaut, B.S.; Smith, B.D. The molecular genetics of crop domestication. Cell 2006, 127, 1309–1321. [Google Scholar] [CrossRef] [Green Version]
- Price, T.D.; Bar-Yosef, O. The origins of agriculture: New data, new ideas: An Introduction to supplement 4. Curr. Anthropol. 2011, 52, S163–S174. [Google Scholar] [CrossRef] [Green Version]
- Larson, G.; Piperno, D.R.; Allaby, R.G.; Purugganan, M.D.; Andersson, L.; Arroyo-Kalin, M.; Barton, L.; Vigueira, C.C.; Denham, T.; Dobney, K.; et al. Current perspectives and the future of domestication studies. Proc. Natl. Acad. Sci. USA 2014, 111, 6139–6146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chomicki, G.; Renner, S.S. Watermelon origin solved with molecular phylogenetics including Linnaean material: Another example of museomics. New Phytol. 2015, 205, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Purugganan, M.D.; Fuller, D.Q. The nature of selection during plant domestication. Nature 2009, 457, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.S.; DuVal, A.E.; Jensen, H.R. Patterns and processes in crop domestication: An historical review and quantitative analysis of 203 global food crops: Tansley review. New Phytol. 2012, 196, 29–48. [Google Scholar] [CrossRef]
- Romão, R.L. Northeast Brazil: A secondary center of diversity for watermelon (Citrullus lanatus). Genet. Resour. Crop Evol. 2000, 47, 207–213. [Google Scholar] [CrossRef]
- Kearney, J. Food consumption trends and drivers. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2793–2807. [Google Scholar] [CrossRef]
- Khoury, C.K.; Bjorkman, A.D.; Dempewolf, H.; Ramirez-Villegas, J.; Guarino, L.; Jarvis, A.; Rieseberg, L.H.; Struik, P.C. Increasing homogeneity in global food supplies and the implications for food security. Proc. Natl. Acad. Sci. USA 2014, 111, 4001–4006. [Google Scholar] [CrossRef] [Green Version]
- Fader, M.; Gerten, D.; Krause, M.; Lucht, W.; Cramer, W. Spatial decoupling of agricultural production and consumption: Quantifying dependences of countries on food imports due to domestic land and water constraints. Environ. Res. Lett. 2013, 8, 014046. [Google Scholar] [CrossRef]
- MacDonald, G.K.; Brauman, K.A.; Sun, S.; Carlson, K.M.; Cassidy, E.S.; Gerber, J.S.; West, P.C. Rethinking Agricultural Trade Relationships in an Era of Globalization. BioScience 2015, 65, 275–289. [Google Scholar] [CrossRef]
- Badr, A.; M, K.; Sch, R.; Rabey, H.E.; Effgen, S.; Ibrahim, H.H.; Pozzi, C.; Rohde, W.; Salamini, F. On the origin and domestication history of barley (Hordeum vulgare). Mol. Biol. Evol. 2000, 17, 499–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta-Gallegos, J.A.; Kelly, J.D.; Gepts, P. Prebreeding in common bean and use of genetic diversity from wild germplasm. Crop Sci. 2007, 47, S-44–S-59. [Google Scholar] [CrossRef] [Green Version]
- Gepts, P. Plant genetic resources conservation and utilization: The Accomplishments and future of a societal insurance policy. Crop Sci. 2006, 46, 2278–2292. [Google Scholar] [CrossRef]
- Dempewolf, H.; Baute, G.; Anderson, J.; Kilian, B.; Smith, C.; Guarino, L. Past and Future use of wild relatives in crop breeding. Crop Sci. 2017, 57, 1070–1082. [Google Scholar] [CrossRef]
- Das, S.; Lowe, M. Nature read in black and white: Decolonial approaches to interpreting natural history collections. J. Nat. Sci. Collect. 2018, 6, 4–14. [Google Scholar]
- Antonelli, A.; Smith, R.J.; Simmonds, M.S.J. Unlocking the properties of plants and fungi for sustainable development. Nat. Plants 2019, 5, 1100–1102. [Google Scholar] [CrossRef]
- Morueta-Holme, N.; Enquist, B.J.; McGill, B.J.; Boyle, B.; Jørgensen, P.M.; Ott, J.E.; Peet, R.K.; Šímová, I.; Sloat, L.L.; Thiers, B.; et al. Habitat area and climate stability determine geographical variation in plant species range sizes. Ecol. Lett. 2013, 16, 1446–1454. [Google Scholar] [CrossRef]
- Pontarp, M.; Bunnefeld, L.; Cabral, J.S.; Etienne, R.S.; Fritz, S.A.; Gillespie, R.; Graham, C.H.; Hagen, O.; Hartig, F.; Huang, S.; et al. The latitudinal diversity gradient: Novel understanding through mechanistic eco-evolutionary models. Trends Ecol. Evol. 2019, 34, 211–223. [Google Scholar] [CrossRef] [Green Version]
- Brummitt, R.K. World Geographical Scheme for Recording Plant Distributions, Edition 2; Biodiversity Information Standards (TDWG): Geneva, Switzerland, 2001. [Google Scholar]
- Brummitt, N.; Araújo, A.C.; Harris, T. Areas of plant diversity—What do we know? Plants People Planet 2020. [Google Scholar] [CrossRef]
- Vincent, H.; Amri, A.; Castañeda-Álvarez, N.P.; Dempewolf, H.; Dulloo, E.; Guarino, L.; Hole, D.; Mba, C.; Toledo, A.; Maxted, N. Modeling of crop wild relative species identifies areas globally for in situ conservation. Commun. Biol. 2019, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Harlan, J.R.; de Wet, J.M.J. Toward a rational classification of cultivated plants. Taxon 1971, 20, 509–517. [Google Scholar] [CrossRef]
- Maxted, N.; Ford-Lloyd, B.V.; Jury, S.; Kell, S.; Scholten, M. Towards a definition of a crop wild relative. Biodivers. Conserv. 2006, 15, 2673–2685. [Google Scholar] [CrossRef]
- Vincent, H.; Wiersema, J.; Kell, S.; Fielder, H.; Dobbie, S.; Castañeda-Álvarez, N.P.; Guarino, L.; Eastwood, R.; Leόn, B.; Maxted, N. A prioritized crop wild relative inventory to help underpin global food security. Biol. Conserv. 2013, 167, 265–275. [Google Scholar] [CrossRef]
- Morrell, P.L.; Buckler, E.S.; Ross-Ibarra, J. Crop genomics: Advances and applications. Nat. Rev. Genet. 2012, 13, 85–96. [Google Scholar] [CrossRef]
- Winter, M.; Devictor, V.; Schweiger, O. Phylogenetic diversity and nature conservation: Where are we? Trends Ecol. Evol. 2013, 28, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Rodrigues, A.S.L.; Brooks, T.M.; Gaston, K.J. Phylogeny and conservation. In Phylogeny and Conservation; Cambridge University Press: Cambridge, UK, 2005; pp. 101–119. [Google Scholar]
- Brooks, T.M.; Mittermeier, R.A.; da Fonseca, G.A.B.; Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S.L. Global biodiversity conservation priorities. Science 2006, 313, 58–61. [Google Scholar] [CrossRef] [Green Version]
- Forest, F.; Grenyer, R.; Rouget, M.; Davies, T.J.; Cowling, R.M.; Faith, D.P.; Balmford, A.; Manning, J.C.; Procheş, Ş.; van der Bank, M.; et al. Preserving the evolutionary potential of floras in biodiversity hotspots. Nature 2007, 445, 757–760. [Google Scholar] [CrossRef]
- Sechrest, W.; Brooks, T.M.; da Fonseca, G.A.B.; Konstant, W.R.; Mittermeier, R.A.; Purvis, A.; Rylands, A.B.; Gittleman, J.L. Hotspots and the conservation of evolutionary history. Proc. Natl. Acad. Sci. USA 2002, 99, 2067–2071. [Google Scholar] [CrossRef] [Green Version]
- Costion, C.M.; Edwards, W.; Ford, A.J.; Metcalfe, D.J.; Cross, H.B.; Harrington, M.G.; Richardson, J.E.; Hilbert, D.W.; Lowe, A.J.; Crayn, D.M. Using phylogenetic diversity to identify ancient rain forest refugia and diversification zones in a biodiversity hotspot. Divers. Distrib. 2015, 21, 279–289. [Google Scholar] [CrossRef]
- Buerki, S.; Callmander, M.W.; Bachman, S.; Moat, J.; Labat, J.-N.; Forest, F. Incorporating evolutionary history into conservation planning in biodiversity hotspots. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140014. [Google Scholar] [CrossRef] [Green Version]
- Mazel, F.; Pennell, M.W.; Cadotte, M.W.; Diaz, S.; Dalla Riva, G.V.; Grenyer, R.; Leprieur, F.; Mooers, A.O.; Mouillot, D.; Tucker, C.M.; et al. Prioritizing phylogenetic diversity captures functional diversity unreliably. Nat. Commun. 2018, 9, 2888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, F.W.; Turner, W.R.; Brooks, T.M. Conserving critical sites for biodiversity provides disproportionate benefits to people. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Gascon, C.; Brooks, T.M.; Contreras-MacBeath, T.; Heard, N.; Konstant, W.; Lamoreux, J.; Launay, F.; Maunder, M.; Mittermeier, R.A.; Molur, S.; et al. The importance and benefits of species. Curr. Biol. 2015, 25, R431–R438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swenson, N.G. The role of evolutionary processes in producing biodiversity patterns, and the interrelationships between taxonomic, functional and phylogenetic biodiversity. Am. J. Bot. 2011, 98, 472–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardey, P.G.; Chan-Kang, C.; Dehmer, S.P.; Beddow, J.M. Agricultural R&D is on the move. Nat. News 2016, 537, 301–303. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, R. Essentials of Plant Breeding; Stemma Press: Woodbury, MN, USA, 2014. [Google Scholar]
- Hajjar, R.; Hodgkin, T. The use of wild relatives in crop improvement: A survey of developments over the last 20 years. Euphytica 2007, 156, 1–13. [Google Scholar] [CrossRef]
- Nakazato, T.; Warren, D.L.; Moyle, L.C. Ecological and geographic modes of species divergence in wild tomatoes. Am. J. Bot. 2010, 97, 680–693. [Google Scholar] [CrossRef]
- Jarquin, D.; Specht, J.; Lorenz, A. Prospects of genomic prediction in the USDA soybean germplasm collection: Historical data creates robust models for enhancing selection of accessions. G3 Genes Genomes Genet. 2016, 6, 2329–2341. [Google Scholar] [CrossRef] [Green Version]
- Stocks, J.J.; Metheringham, C.L.; Plumb, W.J.; Lee, S.J.; Kelly, L.J.; Nichols, R.A.; Buggs, R.J.A. Genomic basis of European ash tree resistance to ash dieback fungus. Nat. Ecol. Evol. 2019, 3, 1686–1696. [Google Scholar] [CrossRef]
- Wambugu, P.W.; Ndjiondjop, M.-N.; Henry, R.J. Role of genomics in promoting the utilization of plant genetic resources in genebanks. Brief. Funct. Genom. 2018, 17, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Crop Wild Relative—GRIN-Global Web v 1.10.6.2. Available online: https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomysearchcwr.aspx (accessed on 17 July 2020).
- Miller, R.E.; Khoury, C.K. The gene pool concept applied to crop wild relatives: An evolutionary perspective. In North American Crop Wild Relatives, Volume 1: Conservation Strategies; Greene, S.L., Williams, K.A., Khoury, C.K., Kantar, M.B., Marek, L.F., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 167–188. ISBN 978-3-319-95101-0. [Google Scholar]
- Viruel, J.; Kantar, M.B.; Gargiulo, R.; Hesketh-Prichard, P.; Leong, N.; Cockel, C.; Forest, F.; Gravendeel, B.; Perez-Barrales, R.; Leitch, I.J.; et al. Crop wild phylorelatives (CWPs): Phylogenetic distance, cytogenetic compatibility and breeding system data enable estimation of crop wild relative gene pool classification. Bot. J. Linn. Soc. 2020; in press. [Google Scholar]
- Pacicco, L.; Bodesmo, M.; Torricelli, R.; Negri, V. A methodological approach to identify agro-biodiversity hotspots for priority in situ conservation of plant genetic resources. PLoS ONE 2018, 13, e0197709. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.A.; Karp, D.S.; DeClerck, F.; Kremen, C.; Naeem, S.; Palm, C.A. Functional traits in agriculture: Agrobiodiversity and ecosystem services. Trends Ecol. Evol. 2015, 30, 531–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowler, D.E.; Bjorkman, A.D.; Dornelas, M.; Myers-Smith, I.H.; Navarro, L.M.; Niamir, A.; Supp, S.R.; Waldock, C.; Winter, M.; Vellend, M.; et al. Mapping human pressures on biodiversity across the planet uncovers anthropogenic threat complexes. People Nat. 2020, 2, 380–394. [Google Scholar] [CrossRef] [Green Version]
- Royal Botanic Gardens, Kew. State of the World’s Plants; Royal Botanic Gardens: Kew, UK, 2016; ISBN 978-1-84246-628-5.
- Loh, J.; Harmon, D. A global index of biocultural diversity. Ecol. Indic. 2005, 5, 231–241. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pironon, S.; Borrell, J.S.; Ondo, I.; Douglas, R.; Phillips, C.; Khoury, C.K.; Kantar, M.B.; Fumia, N.; Soto Gomez, M.; Viruel, J.; et al. Toward Unifying Global Hotspots of Wild and Domesticated Biodiversity. Plants 2020, 9, 1128. https://doi.org/10.3390/plants9091128
Pironon S, Borrell JS, Ondo I, Douglas R, Phillips C, Khoury CK, Kantar MB, Fumia N, Soto Gomez M, Viruel J, et al. Toward Unifying Global Hotspots of Wild and Domesticated Biodiversity. Plants. 2020; 9(9):1128. https://doi.org/10.3390/plants9091128
Chicago/Turabian StylePironon, Samuel, James S. Borrell, Ian Ondo, Ruben Douglas, Charlotte Phillips, Colin K. Khoury, Michael B. Kantar, Nathan Fumia, Marybel Soto Gomez, Juan Viruel, and et al. 2020. "Toward Unifying Global Hotspots of Wild and Domesticated Biodiversity" Plants 9, no. 9: 1128. https://doi.org/10.3390/plants9091128





