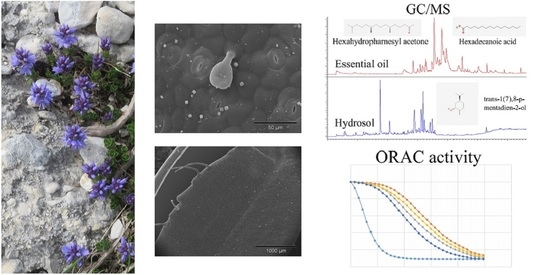
Endemic Veronica saturejoides Vis. ssp. saturejoides–Chemical Composition and Antioxidant Activity of Free Volatile Compounds
Abstract
1. Introduction
2. Results and Discussion
2.1. Gas Chromatography and Mass Spectrometry (GC-MS) Analysis of the Free Volatile Compounds from Essential Oils and Hydrosols
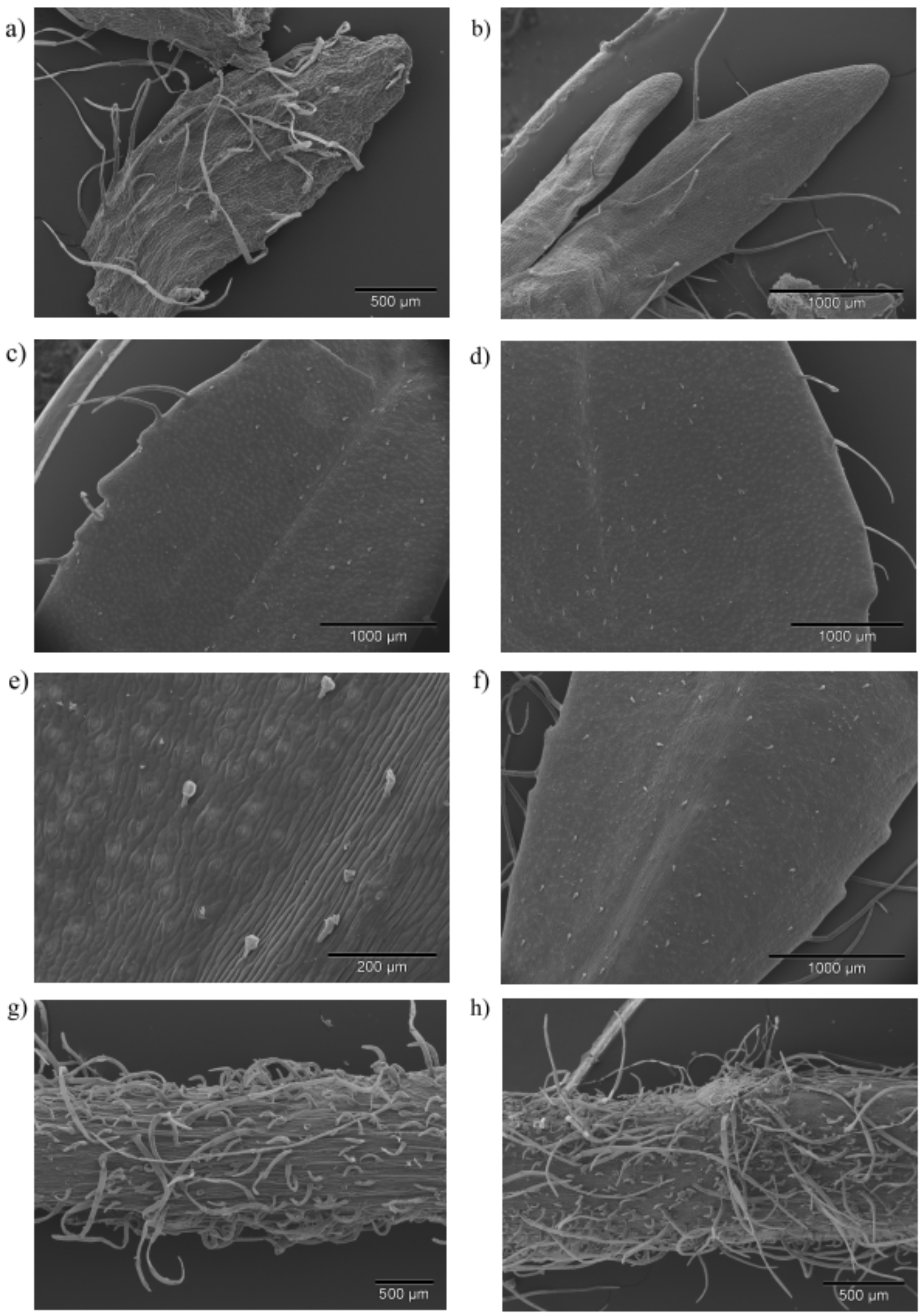
2.2. Micromorphological Traits
2.3. Polyphenol Analysis in Dry Plant Material
2.4. Phenolic Compounds in Hydrosols
2.5. Antioxidant Activity
3. Materials and Methods
3.1. Herbal Material
3.2. GC and GC-MS Analyses
3.3. Micromorphological Traits
3.4. Phenolic Compounds in Hydrosols
3.5. Polyphenol Analysis
3.5.1. Apparatus and Chemicals
3.5.2. Total Polyphenol and Tannin Analysis (Folin–Ciocalteu Phenol Reagent (FCR) Procedure)
3.5.3. Total Flavonoid (TF) Analysis (TF Procedure)
3.5.4. Determination of Total Phenolic Acids (TPA) (TPA Procedure)
3.6. Antioxidant Activity of Essential Oils and Hydrosols
3.6.1. Oxygen Radical Absorbance Capacity Assay (ORAC)
3.6.2. Measurement of the DPPH Radical Scavenging Activity
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barreira, J.C.M.; Dias, M.I.; Zivkovic, J.; Stojkovic, D.; Sokovic, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic profiling of Veronica spp. grown in mountain, urban and sandy soil environments. Food Chem. 2014, 163, 275–283. [Google Scholar] [CrossRef]
- Albach, D.C.; Martínez-Ortega, M.M.; Chase, M.W. Veronica: Parallel morphological evolution and phylogeography in the Mediterranean. Plant Syst. Evol. 2004, 246, 177–194. [Google Scholar] [CrossRef]
- Flora Croatica Database. Available online: http://hirc.botanic.hr/fcd (accessed on 20 January 2019).
- Albach, D.C.; Von Sternburg, M.; Scalone, R.; Bardy, K.E. Phylogenetic analysis and differentiation of Veronica subgenus Stenocarpon in the Balkan Peninsula. Bot. J. Linn. Soc. 2009, 159, 616–636. [Google Scholar] [CrossRef]
- Silic, C. Endemic Plants (Endemične Biljke), 1st ed.; “Svjetlost”, OOUR Zavod za udžbenike i nastavna sredstva: Sarajevo, Bosnia and Herzegovina; Zavod za udžbenike i nastavna sredstva: Belgrade, Serbia, 1984. [Google Scholar]
- Albach, D.C.; Grayer, R.J.; Kite, G.C.; Jensen, S.R. Veronica: Acylated flavone glycosides as chemosystematic markers. Biochem. Syst. Ecol. 2005, 33, 1167–1177. [Google Scholar] [CrossRef]
- Albach, D.C.; Jensen, S.R.; Özgökce, F.; Grayer, R.J. Veronica: Chemical characters for the support of phylogenetic relationships based on nuclear ribosomal and plastid DNA sequence data. Biochem. Syst. Ecol. 2005, 33, 1087–1106. [Google Scholar] [CrossRef]
- Ganzera, M.; Strum, S. Recent advances on HPLC/MS in medicinal plant analysis—An update covering 2011–2016. J. Pharm. Biomed. Anal. 2018, 147, 211–233. [Google Scholar] [CrossRef]
- Dunkic, V.; Kosalec, I.; Kosir, I.J.; Potocnik, T.; Cerenak, A.; Zovko Koncic, M.; Vitali, D.; Dragojevic Muller, I.; Kopricanec, M.; Bezic, N.; et al. Antioxidant and antimicrobial properties of Veronica spicata L. (Plantaginaceae). Curr. Drug Targets 2015, 16, 1660–1670. [Google Scholar] [CrossRef]
- Zivkovic, J.C.; Barreira, J.C.M.; Savikin, K.P.; Alimpic, A.Z.; Stojkovic, D.S.; Dias, M.I.; Santos-Buelga, C.; Duletic-Lausevic, S.N.; Ferreira, I.C.F.R. Chemical profiling and assessment of antineurodegenerative and antioxidant properties of Veronica teucrium L. and Veronica jacquinii Baumg. Chem. Biodivers. 2017, 14, e1700167. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Tayeboon, G.S.; Sharifi-Rad, J.; Iriti, M.; Varoni, E.M.; Razazi, S. Inhibitory activity on type 2 diabetes and hypertension key-enzymes, and antioxidant capacity of Veronica persica phenolic-rich extracts. Cell. Mol. Biol. 2016, 62, 80–85. [Google Scholar] [CrossRef]
- Hamedi, A.; Pasdaran, A.; Zebarjad, Z.; Moein, M. A Survey on Chemical Constituents and Indications of Aromatic Waters Soft Drinks (Hydrosols) Used in Persian Nutrition Culture and Folk Medicine for Neurological Disorders and Mental Health. J. Evid. -Based Complement Altern. Med. 2017, 22, 744–752. [Google Scholar] [CrossRef]
- Rajeswara Rao, B.R. Hydrosols and Water-Soluble Essential Oils: Their Medicinal and Biological Properties. In Recent Progress in Medicinal Plants, 1st ed.; Govil, J.N., Bhattacharya, S., Eds.; Studium Press LLC: New York, NY, USA, 2013; Volume 36, pp. 119–141. [Google Scholar]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Balogun, O.S.; Ajayi, O.S.; Adeleke, A.J. Hexahydrofarnesyl Acetone-Rich Extractives from Hildegardia barteri. J. Herbs Spices Med. Plants 2017, 23, 393–400. [Google Scholar] [CrossRef]
- Silva, R.O.; Sousa, F.B.; Damasceno, S.R.; Carvalho, N.S.; Silva, V.G.; Oliveira, F.R.; Sousa, D.P.; Aragão, K.S.; Barbosa, A.L.; Freitas, R.M.; et al. Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fundam. Clin. Pharmacol. 2014, 28, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Ertas, A.; Boga, M.; Kizil, M.; Ceken, B.; Goren, A.C.; Hasimi, N.; Demirci, S.; Topcu, G.; Kolak, U. Chemical profile and biological activities of Veronica thymoides subsp. pseudocinerea. Pharm. Biol. 2015, 53, 334–339. [Google Scholar] [CrossRef]
- Saikia, D.; Parihar, S.; Chanda, D.; Ojha, S.; Kumar, J.K.; Chanotiya, C.S.; Shanker, K.; Negi, A.S. Antitubercular potential of some semisynthetic analogues of phytol. Bioorg. Med. Chem. Lett. 2010, 20, 508–512. [Google Scholar] [CrossRef]
- Costa, J.P.; Ferreira, P.B.; De Sousa, D.P.; Jordan, J.; Freitas, R.M. Anticonvulsant effect of phytol in a pilocarpine model in mice. Neurosci. Lett. 2012, 523, 115–118. [Google Scholar] [CrossRef]
- Pongprayoon, U.; Baeckström, P.; Jacobsson, U.; Lindström, M.; Bohlin, L. Antispasmodic Activity of β-Damascenone and E-Phytol Isolated from Ipomoea pes-caprae. Planta Med. 1992, 58, 19–21. [Google Scholar] [CrossRef]
- Pejin, B.; Kojic, V.; Bogdanovic, G. An insight into the cytotoxic activity of phytol at in vitro conditions. Nat. Prod. Res. 2014, 28, 2053–2056. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R. Methyl eugenol: Its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. J. Insect Sci. 2012, 12, 56. [Google Scholar] [CrossRef]
- Li, F. Analysis of chemical constituents of essential oil in Veronica linariifolia by gas chromatography-mass spectrometry. Chin. J. Anal. Chem. 2002, 30, 822–825. [Google Scholar]
- Çelik, E.; Yuvali Çelik, G.; Meysun, A. Essential Oil Composition and Antibacterial Activity of Some Plant Species. J. Appl. Biol. Sci. 2010, 1, 45–48. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4.1 ed.; Allured Publishing: Carol Stream, IL, USA, 2017. [Google Scholar]
- Kurer, G.A. Kutikularfalten und Protuberanzen an Haaren und Epidermen; Druck, Gebr: Leemann, Germany, 1917. [Google Scholar]
- Kraehmer, H.; Baur, P. Weed Anatomy; Wiley-Blackwell: Oxford, UK, 2013. [Google Scholar]
- Haratym, W.; Weryszko-Chmielewska, E. Ultrastructural and histochemical analysis of glandular trichomes of Marrubium vulgare L. (Lamiaceae). Flora 2017, 231, 11–20. [Google Scholar] [CrossRef]
- Giuliani, C.; Pellegrino, R.; Tirillini, B.; Bini, L.M. Micromorphological and chemical characterisation of Stachys recta L. subsp. serpentini (Fiori) Arrigoni in comparison to Stachys recta L. subsp. recta (Lamiaceae). Flora Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 376–385. [Google Scholar] [CrossRef]
- Dunkic, V.; Kremer, D.; Grubesic, R.J.; Rodriguez, J.V.; Ballian, D.; Bogunic, F.; Stesevic, D.; Kosalec, I.; Bezic, N.; Stabentheiner, E. Micromorphological and phytochemical traits of four Clinopodium L. species (Lamiaceae). S. Afr. J. Bot. 2017, 111, 232–241. [Google Scholar] [CrossRef]
- Kristen, U.; Lockhausen, J. The leaf glands of Veronica beccabunga L.: Ultrastructure and a possible pathway of secretion. Israel J. Bot. 1985, 34, 147–156. [Google Scholar] [CrossRef]
- Bilusic Vundac, V.; Stabentheiner, E.; Brantner, A.; Plazibat, M. Morphology and distribution of trichomes on leaves in seven Croatian taxa of the genus Stachys (Lamiaceae). Phyton 2011, 51, 161–176. [Google Scholar]
- Hanlidou, E.; Kokkini, S.; Bosabalidis, A.M.; Bessière, J.M. Glandular trichomes and essential oil constituents of Calamintha menthifolia (Lamiaceae). Plant Syst. Evol. 1991, 177, 17–26. [Google Scholar] [CrossRef]
- Kremer, D.; Stabentheiner, E.; Dunkic, V.; Müller, I.D.; Vujic, L.; Kosalec, I.; Ballian, D.; Bogunic, F.; Bezic, N. Micromorphological and chemotaxonomical traits of Micromeria croatica (Pers.) Schott. Chem. Biodivers. 2012, 9, 755–768. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities and synthesis. Angew. Chem. -Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Harput, U.S.; Karadeniz, A.; Genç, Y.; Saraçoglu, I. Comparative Bioactivity Studies on Four Veronica species. FABAD J. Pharm. Sci. 2009, 34, 67–72. [Google Scholar]
- Beara, I.; Zivkovic, J.; Lesjak, M.; Nadpal, J.; Savikin, K.; Maksimovic, Z.A.; Jankovic, T. Phenolic profile and anti-inflammatory activity of three Veronica species. Ind. Crop. Prod. 2015, 63, 276–280. [Google Scholar] [CrossRef]
- Stojkovic, D.S.; Zivkovic, J.; Sokovic, M.; Glamoclija, J.; Ferreira, I.C.F.R.; Jankovic, T.; Maksimovic, Z. Antibacterial activity of Veronica montana L. extract and of protocatechuic acid incorporated in a food system. Food Chem. Toxicol. 2013, 55, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Aazza, S.; Lyoussi, B.; Miguel, M.G. Antioxidant activity of some Morrocan hydrosols. J. Med. Plants Res. 2011, 5, 6688–6696. [Google Scholar] [CrossRef]
- Bentayeb, K.; Vera, P.; Rubio, K.; Nerín, K. The additive properties of Oxygen Radical Absorbance Capacity (ORAC) assay: The case of essential oils. Food Chem. 2014, 148, 204–208. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Navajas, Y.R.; Zapata, E.S.; Fernández-López, J.; Perez-Alvarez, J.A. Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour Fragr. J. 2010, 25, 13–19. [Google Scholar] [CrossRef]
- Harput, U.Ş.; Genç, Y.; Khan, N.; Saracoglu, I. Radical scavenging effects of different Veronica species. Rec. Nat. Prod. 2011, 5, 100–107. [Google Scholar]
- Mocan, A.; Vodnar, D.C.; Vlase, L.; Crișan, O.; Gheldiu, A.M.; Crișan, G. Phytochemical characterization of Veronica officinalis L., V. teucrium L. and V. orchidea Crantz from Romania and their antioxidant and antimicrobial properties. Int. J. Mol. Sci. 2015, 16, 21109–21127. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kim, H.J.; Lee, K.H.; Kang, S.C.; Zee, O.P. Antioxidative iridoid glycosides and phenolic compounds from Veronica peregrina. Arch. Pharm. Res. 2009, 32, 207–213. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Kremer, D.; Dragojevic, I.; Dunkic, V.; Vitali Cepo, D.; Stabentheiner, E.; Oberländer, A.; Bezic, N.; Kosalec, I. Chemical traits and antimicrobial activity of endemic Teucrium arduini L. from Mt Biokovo (Croatia). Cent. Eur. J. Biol. 2012, 7, 941–947. [Google Scholar] [CrossRef]
- Payne, W.W. A glossary of plant hair thermonology. Brittonia 1978, 30, 239–255. [Google Scholar] [CrossRef]
- Jukic Spika, M.; Zanetić, M.; Kraljic, K.; Soldo, B.; Ljubenkov, I.; Politeo, O.; Skevin, D. Differentiation between Unfiltered and Filtered Oblica and Leccino cv. Virgin Olive Oils. J. Food Sci. 2019, 84, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.V.; Grubesic, R.J.; Kremer, D.; Kokot, V. Quality Assessment of Two Spectrophotometric Procedures for Polyphenol Determination and Application in Moltkia petraea Species. J. Chin. Chem. Soc. 2016, 63, 677–687. [Google Scholar] [CrossRef]
- Schneider, G. To determine the tannin with casein. Zur Bestimmung der Gerbstroffe mit Casein. Arch. Pharm. 1976, 309, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Christ, B.; Mueller, K.H. On the serial determination of the content of flavonol derivatives in drugs. Arch. Pharm. ber Dtsch. Pharm. Ges. 1960, 293, 1033–1042. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 9th ed.; Council of Europe: Strasbourg, France, 2016; Volume 2.
- Fredotovic, Z.; Sprung, M.; Soldo, B.; Ljubenkov, I.; Budic-Leto, I.; Bilusic, T.; Cikes-Culic, V.; Puizina, J. Chemical composition and biological activity of Allium cepa L. and Allium × cornutum (Clementi ex Visiani 1842) methanolic extracts. Molecules 2017, 22, 448. [Google Scholar] [CrossRef]
- Payet, B.; Sing, A.S.C.; Smadja, J. Assessment of antioxidant activity of cane brown sugars by ABTS and DPPH radical scavenging assays: Determination of their polyphenolic and volatile constituents. J. Agric. Food Chem. 2005, 53, 10074–10079. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D. Scavenging Effect of Methanolic Extracts of Peanuts Hulls on Free-Radical and Active-Oxygen Species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
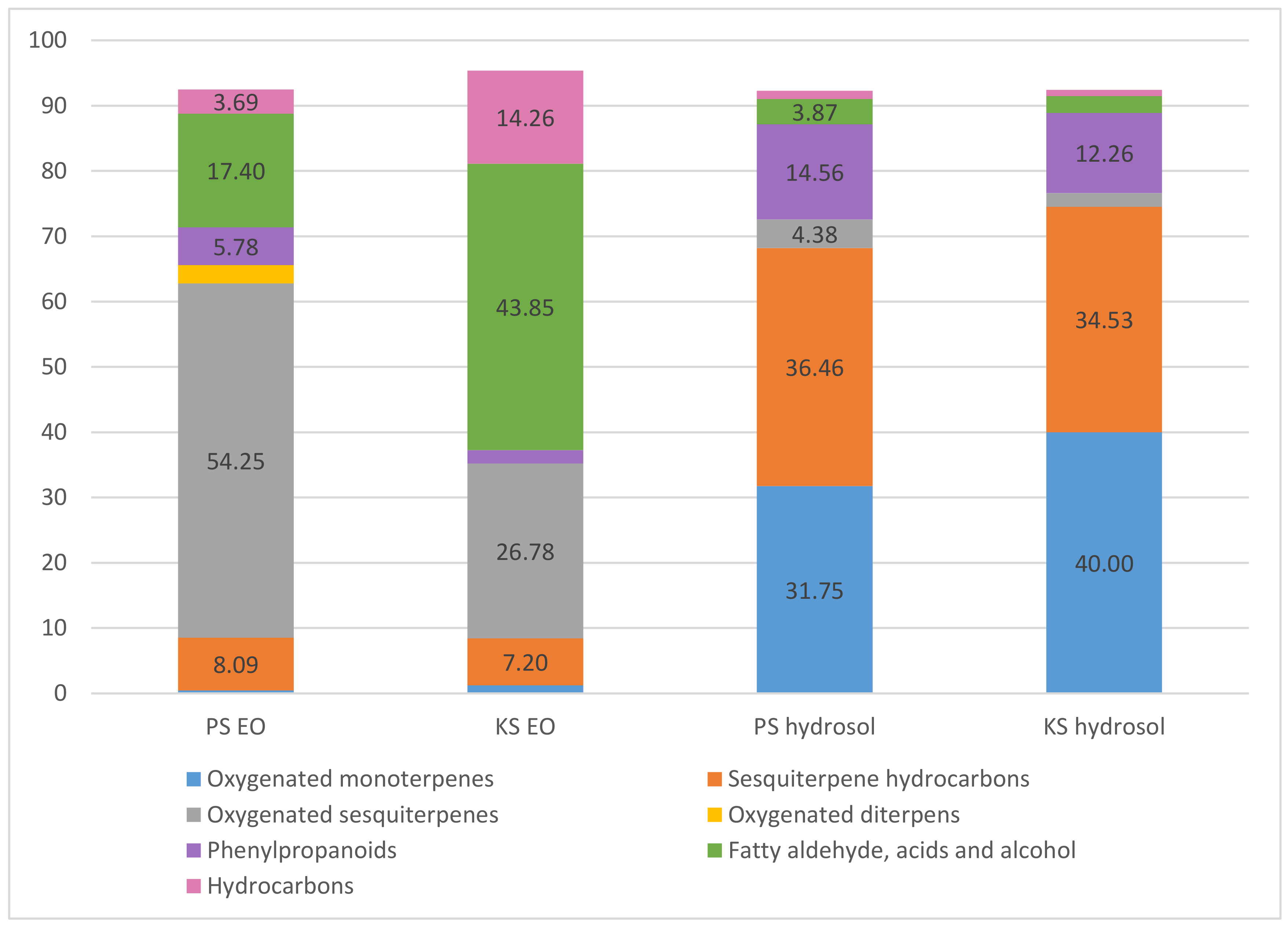
| Essential Oil Samples (%) | Hydrosol Samples (%) | |||||
|---|---|---|---|---|---|---|
| Component | RI a | RI b | PS | KS | PS | KS |
| Oxygenated monoterpenes | 0.44 | 1.23 | 31.75 | 40 | ||
| trans-p-Mentha-1(7),8-dien-2-ol | 1187 | 1803 | - | 1.23 ± 0.01 | 31.75 ± 0.01 b | 36.63 ± 0.01 a |
| Verbenone | 1204 | 1705 | - | - | - | 0.76 ± 0.01 |
| endo-Fenchyl acetate | 1218 | - | - | - | - | 2.61 ± 0.01 |
| Piperitone oxide | 1365 | - | 0.44 ± 0.01 | - | - | - |
| Sesquiterpene hydrocarbons | 8.09 | 7.2 | 36.46 | 34.53 | ||
| (E)-Caryophyllene * | 1424 | 1585 | 0.94 ± 0.01 b | 2.46 ± 0.01 a | 24.52 ± 0.01 a | 12.35 ± 0.01 b |
| Z-Methyl isoeugenol | 1451 | - | - | - | 1.25 ± 0.01 b | 4.16 ± 0.01 a |
| allo-Aromadendrene | 1465 | 1662 | 2.35 ± 0.01 a | 0.68 ± 0.01 b | 8.13 ± 0.01 b | 11.53 ± 0.01 a |
| β-Chamigrene | 1476 | 1724 | - | 0.44 ± 0.01 | - | 1.17 ± 0.01 |
| γ-Muurolene | 1478 | 1685 | 0.39 ± 0.01 b | 0.87 ± 0.01 a | - | 0.65 ± 0.03 |
| Germacrene D | 1482 | 1692 | 2.78 ± 0.01 a | 1.77 ± 0.01 b | 2.56 ± 0.01 b | 4.67 ± 0.01 a |
| δ-Cadinene | 1517 | 1745 | 1.63 ± 0.01 a | 0.98 ± 0.01 b | - | - |
| Oxygenated sesquiterpenes | 54.25 | 26.78 | 4.38 | 2.1 | ||
| Spathulenol | 1577 | 2101 | 0.22 ± 0.01 b | 0.87 ± 0.01 a | - | - |
| Caryophyllene oxide * | 1581 | 1955 | 20.25 ± 0.01 a | 2.34 ± 0.01 b | 0.26 ± 0.01 b | 0.84 ± 0.05 a |
| γ-Eudesmol | 1632 | 2175 | 0.74 ± 0.01 a | 0.33 ± 0.01 b | 0.28 ± 0.01 | - |
| α-Muurolol | 1645 | 2181 | 2.91 ± 0.01 | - | - | - |
| α-Bisabolol | 1685 | 2210 | - | - | 0.32 ± 0.05 | - |
| Hexahydrofarnesyl acetone | 1839 | 2113 | 30.13 ± 0.01 a | 23.24 ± 0.01b | 3.52 ± 0.01 b | 1.26 ± 0.01 a |
| Oxygenated diterpens | 2.82 | - | - | - | ||
| Phytol | 1942 | 2610 | 2.82 ± 0.03 | - | - | - |
| Phenylpropanoids | 5.78 | 2.06 | 14.56 | 12.26 | ||
| Benzaldehyde | 964 | 1513 | - | - | 0.75 ± 0.01 a | 0.34 ± 0.01 b |
| 2-Methoxy-4-vinylphenol | 1294 | 2178 | 2.56 ± 0.01 | - | 0.46 ± 0.01 | - |
| Methyl eugenol | 1403 | 2005 | 1.28 ± 0.01 a | 1.13 ± 0.01b | 13.35 ± 0.01 a | 11.92 ± 0.01 b |
| Benzyl benzoate | 1754 | - | 1.94 ± 0.01 a | 0.93 ± 0.01b | - | - |
| Fatty aldehyde, acids and alcohol | 17.4 | 43.85 | 3.87 | 2.59 | ||
| n-Nonanal | 1100 | 1389 | - | - | 0.16 ± 0.01 | - |
| Hexyl 2-methyl butanoate | 1233 | 1425 | - | - | 1.13 ± 0.01 b | 1.82 ± 0.01 a |
| Nonanoic acid | 1267 | 2149 | 0.12 ± 0.01 | - | - | - |
| Dodecanoic acid | 1564 | 2480 | 0.59 ± 0.01 b | 1.67 ± 0.01 a | - | - |
| 1-Hexadecanol | 1874 | 2371 | 7.73 ± 0.01 a | 4.53 ± 0.01 b | 1.32 ± 0.01 | - |
| Hexadecanoic acid | 1959 | 2912 | 7.88 ± 0.01 b | 37.31 ± 0.01 a | 1.26 ± 0.01 a | 0.77 ± 0.01 b |
| Oleic acid | 2133 | - | 1.08 ± 0.01 a | 0.34 ± 0.03 b | - | - |
| Hydrocarbons | 3.69 | 14.26 | 1.27 | 0.95 | ||
| Heneicosane * | 2100 | 2100 | 0.42 ± 0.02 b | 0.68 ± 0.07 a | - | - |
| Docosane * | 2200 | 2200 | 0.73 ± 0.01 b | 3.27 ± 0.01 a | 1.27 ± 0.01 a | 0.95 ± 0.01 b |
| Tricosane * | 2300 | 2300 | 1.27 ± 0.01 b | 4.03 ± 0.01 a | - | - |
| Tetracosane * | 2400 | 2400 | 0.59 ± 0.05 | - | - | - |
| Pentacosane * | 2500 | 2500 | 0.68 ± 0.01 b | 6.28 ± 0.01 a | - | - |
| Total (%) | 92.47 | 95.38 | 92.29 | 92.43 | ||
| Sample | Trichome | Leaf | Calyx | Stem | |
|---|---|---|---|---|---|
| Type | Adaxial | Abaxial | |||
| Prenj | Attenuate * | ± | ± | + | ++ |
| capitate C1 | ±/+ | ±/+ | –/± | ±/+ | |
| Kamešnica | attenuate | ± | ± | + | +/++ |
| capitate C1 | ±/+ | ±/+ | –/± | ± | |
| Species | TP (mg/g DW) | T (mg/g DW) | TF (mg/g DW) | TPA (505 nm) (mg/g DW) | TPA (525 nm) (mg/g DW) |
|---|---|---|---|---|---|
| V. saturejoides (KS) | 86.9 ±1.4 a | 2.3 ± 1.3 | 0.8 ± 0.00 | 33.1 ± 1.7 a | 65.6 ± 0.2 a |
| V. saturejoides (PS) | 70.9 ± 0.9 b | 1.7 ± 0.5 | 0.8 ± 0.00 | 19.5 ± 0.2 b | 45.4 ± 2.1 b |
| Samples | Vanillin | Cinnamic Acid | Protocatechuic Acid |
|---|---|---|---|
| V. saturejoides (KS) | 0.22 ± 0.01 | 0.12 ± 0.02 | 7.33 ± 0.35 |
| V. saturejoides (PS) | - | - | - |
| Essential Oil | Hydrosols | Hexahydropharnesyl Acetone | |||
|---|---|---|---|---|---|
| Antioxidant Assay | PS | KS | PS | KS | |
| ORAC (Trolox eq) | 255.1 ± 2.54 | 256.5 ± 5.73 | 0.559 ± 0.059 | 0.679 ± 0.036 | - |
| DPPH (Trolox eq) | 20.73 ± 0.21 b | 44.32 ± 0.13 a | 0.225 ± 0.062 | 0.323 ± 0.014 | - |
| DPPH (% inhibition) | 46.23 ± 4.37 b | 66.99 ± 2.98 a | 34.84 ± 0.89 b | 49.26 ± 1.7 a | - |
| DPPH (IC 50) | 10.88 ± 1.24 b | 7.16 ± 0.12 a | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazlić, M.; Kremer, D.; Grubešić, R.J.; Soldo, B.; Vuko, E.; Stabentheiner, E.; Ballian, D.; Bogunić, F.; Dunkić, V. Endemic Veronica saturejoides Vis. ssp. saturejoides–Chemical Composition and Antioxidant Activity of Free Volatile Compounds. Plants 2020, 9, 1646. https://doi.org/10.3390/plants9121646
Nazlić M, Kremer D, Grubešić RJ, Soldo B, Vuko E, Stabentheiner E, Ballian D, Bogunić F, Dunkić V. Endemic Veronica saturejoides Vis. ssp. saturejoides–Chemical Composition and Antioxidant Activity of Free Volatile Compounds. Plants. 2020; 9(12):1646. https://doi.org/10.3390/plants9121646
Chicago/Turabian StyleNazlić, Marija, Dario Kremer, Renata Jurišić Grubešić, Barbara Soldo, Elma Vuko, Edith Stabentheiner, Dalibor Ballian, Faruk Bogunić, and Valerija Dunkić. 2020. "Endemic Veronica saturejoides Vis. ssp. saturejoides–Chemical Composition and Antioxidant Activity of Free Volatile Compounds" Plants 9, no. 12: 1646. https://doi.org/10.3390/plants9121646
APA StyleNazlić, M., Kremer, D., Grubešić, R. J., Soldo, B., Vuko, E., Stabentheiner, E., Ballian, D., Bogunić, F., & Dunkić, V. (2020). Endemic Veronica saturejoides Vis. ssp. saturejoides–Chemical Composition and Antioxidant Activity of Free Volatile Compounds. Plants, 9(12), 1646. https://doi.org/10.3390/plants9121646