Abstract
Coreopsis is a flowering plant belonging to the Asteraceae family. It is an ornamental plant native to the Americas, Asia and Oceania and its flower is used as a raw material for tea and food manufacture in China. In this study, new cultivars of C. rosea (“golden ring”) were developed via radiation-induced mutation of the original cultivar, “pumpkin pie”. The chemical composition and antioxidant activities of flowers belonging to three different Coreopsis cultivars were evaluated: “golden ring”, “pumpkin pie” and “snow chrysanthemum” (coreopsis tea; C. tinctoria). The volatile compounds were characterized via gas chromatography-mass spectrometry (GC-MS) and 50–59 oils representing 95.3–96.8% of the total volatile compounds in these flower materials were identified. ”Golden ring” contained a high amount of fatty acids (38.13%), while “pumpkin pie” and “snow chrysanthemum” teas were rich in aliphatic amides (43.01%) and esters (67.22%), respectively. The antioxidant activities of the volatile oils of these cultivars were evaluated using 1,1-diphenyl-2-picrylhydraxyl (DPPH) and 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging assays. The volatile extract of “golden ring” showed higher antioxidant activities compared with the extracts of the other cultivars. Therefore, “golden ring” can be used for further development as a raw material for tea manufacture or as a dietary supplement.
1. Introduction
Approximately 80 species of Coreopsis (Asteraceae) are native to North America and are currently distributed throughout the Americas, Asia and Oceania [1]. Coreopsis thrives as annual and perennial types, and is an ornamental plant used to decorate gardens and roadsides [1,2]. In addition, the ethnopharmacological use of this plant’s flower has been reported [3]. It has been traditionally used to treat diarrhea, vomiting, and bleeding in North America [3]. The Coreopsis flower has been consumed as a drink to control diabetes in China and Portugal [3]. Currently, the flowers of C. tinctoria, known as “snow chrysanthemum”, are used as tea, with detoxifying and cooling effects, in China [4,5]. In a previous phytochemical study of Coreopsis species, phenolics, flavonoids (aurones, chalcones, and flavones), acetylenes and volatile oils were reported [2,3,4,5,6,7,8,9]. The plant’s extracts or constituents have been found to exhibit diverse biological activities such as antileukemic [2], antidiabetic [3,7], antioxidant [5,6], anti-inflammatory [7,8] and chemopreventive effects [9].
Hybridization between plants and mutations has yielded numerous varieties with high crop productivity and improved quality [10,11]. Mutation breeding via spontaneous mutation, ultraviolet light, chemical mutagenesis and ionizing radiation can be used effectively to induce genetic diversity [11]. Approximately 50% of the mutant varieties registered in the joint Food and Agriculture Organization/International Atomic Energy Agency (FAO/IAEA) mutant variety database are mutants induced by gamma irradiation [12]. Coreopsis species are of little commercial importance, but gardeners’ preferences for them are increasing due to their cold resistance, attractive flowers and leaves and tolerance of various environmental conditions [13]. Therefore, our research group has developed a new mutant cultivar of C. rosea (“golden ring”) bred via the exposure of stem cuttings of the original cultivar, “pumpkin pie”, to gamma irradiation and registered it in the Korea Seed and Variety Service [14]. C. rosea has been mainly studied for breeding and culture growth [15]; however, no studies have reported its chemical constituents and pharmacological activity until now.
In this study, we analyzed the volatile oils of flowers obtained from a new cultivar of C. rosea (“golden ring”) compared with those of its original cultivar (“pumpkin pie”) and the commercially available coreopsis tea (“snow chrysanthemum”) as control groups (Figure 1), via gas chromatography-mass spectrometry (GC-MS). Their antioxidant activities were also evaluated using 1,1-diphenyl-2-picrylhydraxyl (DPPH) and 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging assays.

Figure 1.
The flowers of (a) the gamma-irradiated mutant cultivar, “golden ring”; (b) its original cultivar, “pumpkin pie”; (c) the commercially available tea material, “snow chrysanthemum” (photographed by authors, Y.G.J. and B.-R.K.).
2. Results and Discussion
2.1. Chemical Composition of Dichloromethane Extracts of Three Different Coreopsis Cultivars
The flowers of “pumpkin pie”, “golden ring” and “snow chrysanthemum” were extracted with dichloromethane. The volatile components of these flowers were identified based on their molecular formula, retention index (RI) and National Institute of Standards and Technology (NIST) library similarity index. The GC-MS analysis revealed a total of 89 components in these flowering materials, which led to the identification of 50 components in “snow chrysanthemum”, 58 in “pumpkin pie” and 59 in “golden ring” (Table 1).

Table 1.
Composition of volatile compounds of the dichloromethane extracts obtained from “golden ring”, “pumpkin pie” and “snow chrysanthemum”.
The volatile compounds were classified into seven classes: hydrocarbons, fatty acids, alcohols, ketones, esters, terpenoids and aliphatic amides (Figure 2). Table 2 summarizes the ten most abundant constituents among the volatile compounds detected via GC-MS. The volatile oils of “golden ring” and “pumpkin pie” comprised a large proportion of aliphatic amides, fatty acids and hydrocarbons. “Golden ring” contained total volatile oils constituting 96.84% of its total volatile compounds, including 38.13% fatty acids, 33.93% aliphatic amides and 11.263% hydrocarbons, while “pumpkin pie” contained 43.01% aliphatic amides, 22.21% fatty acids and 15.44% hydrocarbons (Figure 2). The extract of “snow chrysanthemum” comprised total volatile compounds up to 96.29%, including esters (67.22%), aliphatic amides (14.6%) and acids (3.16%) (Figure 2). Table 3 summarizes the ten most abundant compounds among the volatile components of these cultivars detected via GC-MS. The major compound in the “golden ring” extract was (Z)-9-octadecenamide constituting 28.38% of the total composition (Figure 3), followed by (Z,Z)-9,12-octadecadienoic acid (17.36%), n-hexadecanoic acid (7.83%), (Z,Z,Z)-9,12,15-octadecatrienoic acid (6.86%) and nonacosane (3.88%) (Table 2). The major compound in the “pumpkin pie” extract was also (Z)-9-octadecenamide (37.48%), followed by (Z,Z)-9,12-octadecadienoic acid (8.69%), heptacosane (5.06%), nonacosane (4.67%) and (Z,Z,Z)-9,12,15-octadecatrienoic acid (4.65%). In contrast to the volatile oils of “golden ring” and “pumpkin pie”, the main constituent of “snow chrysanthemum” was ethyl oleate (28.53%) (Figure 3), followed by linoleic acid ethyl ester (16.89%), hexadecenoic acid ethyl ester (14.0%), (Z)-9-octadecenamide (12.57%) and (E)-9-octadecenoic acid ethyl ester (2.07%) (Table 2).
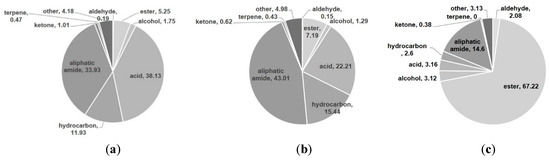
Figure 2.
Relative content (%) of volatile compounds in (a) “golden ring”; (b) “pumpkin pie”; (c) “snow chrysanthemum”.

Table 2.
Top ten volatile compounds in “golden ring”, “pumpkin pie” and “snow chrysanthemum”.

Table 3.
Antioxidant activities of the dichloromethane extract of three different Coreopsis cultivars.
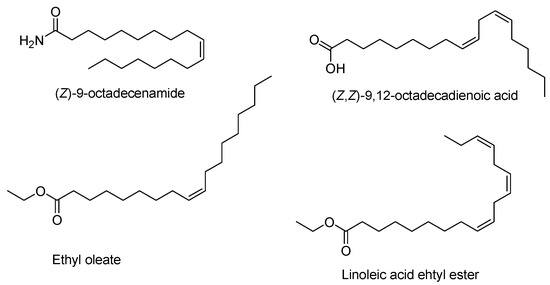
Figure 3.
The chemical structures of the most intense components in “golden ring”, “pumpkin pie” and “snow chrysanthemum”.
Reports have suggested that (Z)-9-octadecenamide is predominant in the natural volatile oil derived from “golden ring” and “pumpkin pie”, with strong antioxidant and antimicrobial properties [16]. In addition, extracts containing (Z,Z)-9,12-octadecadienoic acid (linoleic acid) and (Z,Z,Z)-9,12,15-ocatadecadienoic acid (linolenic acid), which are abundant in “golden ring” and “pumpkin pie” but less abundant in “snow chrysanthemum”, have also been reported to exhibit antioxidant activities [17,18]. It has been reported that n-hexadecanoic acid (palmitic acid), which is relatively high in “golden ring”, has anti-inflammatory [19,20] and anti-cancer effects [21]. The high content of volatile esters in “snow chrysanthemum” may contribute to the strong flavor of the tea.
2.2. Antioxidant Activities of Dichloromethane Extracts Obtained from Three Different Coreopsis Cultivars
The antioxidant activity of dichloromethane extracts of the three different Coreopsis cultivars was evaluated via ABTS and DPPH radical scavenging assays. These are rapid and sensitive methods used to evaluate antioxidant effects due to the hydrogen donor properties of the substance. The “golden ring” extract showed higher DPPH radical scavenging activity—with a 50% inhibitory concentration (IC50) value of 602.1 μg/mL—than did “pumpkin pie” (IC50 2291.3 μg/mL) or “snow chrysanthemum” (IC50 3166.2 μg/mL). As in the DPPH free radical scavenging activities, the “golden ring” extract showed optimal antioxidant activity with an IC50 of 137.0 μg/mL in the ATBS radical scavenging assay (Table 3). Previous studies have reported the biological activities of the fatty acids in “golden ring”, such as anti-oxidant activities [22,23,24], cellular reactive oxygen species generation [25] and anti-cancer activity [26]. Thus, the potent antioxidant activity of the volatile extract of “golden ring” is attributed to the twofold higher fatty acid content compared with that of “pumpkin pie”.
3. Materials and Methods
3.1. Plant Material
Radiation mutants of C. resae, “golden ring”, were generated via the treatment of stem cuttings of the original cultivars, “pumpkin pie”, with gamma (60Co) irradiation (150 TBq capacity; AECL, Ottawa, ON, Canada). These mutants were selected from variants in floral size and showed stable inheritance of the phenotype for four years. The radiation mutant cultivars were grown by Uriseed Group Corporation (Icheon-si, Gyeonggi-do, Korea) and registered as new plant varieties in the Korea Seed and Variety Service. The flowers were handpicked and randomly collected at the flowering stage in the same plantation. They were freeze-dried and stored at −20 °C in polyethylene plastic bags until further analysis. Voucher specimens were deposited at the Uriseed Group Corporation. “Snow chrysanthemum” was purchased in China as a commercially available tea material.
3.2. Sample Preparation
Fresh flowers of “golden ring” and “pumpkin pie” were freeze-dried and ground into powder. The dried tea material from “snow chrysanthemum” was ground into powder. Samples (200 mg each) were directly extracted with 20 mL of dichloromethane in an ultrasonic bath for 60 min. Subsequently, the extracts were dehydrated over 0.5 g of anhydrous sodium sulfate and filtered through a polyvinylidene fluoride syringe filter (0.45 µm) for GC-MS analysis.
3.3. GC-MS Analysis
Dichloromethane extracts were analyzed using a Shimadzu QP-2010 Ultra (Shimadzu, Kyoto, Japan). The compounds were separated on the DB-5MS capillary column (30 m × 0.25 mm × 0.25 µm; Agilent Technologies Co., Santa Clara, CA, USA). The carrier gas was 99.99% high-purity helium with a column flow rate of 1.53 mL/min and the injection port in splitless mode. The oven temperature was 50 °C, which was gradually increased to 100 °C at a rate of 5 °C/min and held steady for 5 min, then increased to 230 °C at a rate of 5 °C/min. After holding steady for 20 min again, it was finally increased to 250 °C at a rate of 10 °C/min, and held steady for 5 min. The MS parameters were electron ionization (EI) mode with ionization voltage 70 eV, ion source temperature 230 °C and scan range 45–450 m/z.
The retention indices of all GC peaks were calculated using the retention times of C7–C30 n-alkane standards under the same chromatographic conditions (Figures S1 and S2). Each peak was identified by comparing with the mass spectra database (National Institute of Standards and Technology, Mass Spectra Libraries, Gaithersburg, MD, USA) [27,28].
In Equation (1) above, I denotes the retention index of the components to be tested and i is the adjusted retention time (min) of the components to be tested; n and n + 1 represent the carbon amounts of n-alkanes before and after the diffusion of unknown substances, respectively. The values of Tn and Tn+1 denote the carbon retention times of n-alkanes.
3.4. Measurement of DPPH Free Radical and ABTS Radical Cation Scavenging Activities
The DPPH of each sample was determined using Brand-Williams’ method [29]. Briefly, the 2 mM DPPH solution was diluted with ethanol to an absorbance of less than 1.0 at 517 nm before the analysis. Each dichloromethane extract was evaporated in vacuo and initially dissolved in dimethyl sulfoxide (DMSO) to produce 5 mg/mL stock solution. Subsequently, the final concentrations of the samples were diluted to 125, 250, 1000 and 5000 μg/mL using DMSO. Next, each sample was suspended in DMSO and 40 μL of the sample was made to react with 160 μL of 0.2 mM DPPH solution. After 6 min, absorbance was measured at 517 nm using an ELISA reader (Benchmark Plus, Bio-Rad, Hercules, CA, USA).
The ABTS of each sample was determined using the method published by Re et al. [30]. In brief, the ABTS was measured with a pre-formed radical monocation. The mixtures, along with 7.4 mM ABTS solution and 2.6 mM potassium persulfate, were incubated at room temperature in the dark for 24 h. The ABTS solution was diluted with phosphate-buffered saline (pH 7.4) to achieve an absorbance of 0.7 ± 0.02 at 734 nm. The final concentrations of the extracts were diluted to 25, 125, 250 and 500 μg/mL using DMSO and 40 μL of the sample was made to react with 160 μL of the ABTS solution. Absorbance was measured 6 min after the reaction at 734 nm using an ELISA reader (Benchmark Plus, Bio-Rad, Hercules, CA, USA). The DPPH free radical and ABTS radical scavenging activities (%) were calculated as follows:
where Asample and Acontrol represent the absorbance of the sample and control, respectively.
The 50% inhibitory concentration (IC50) of the extract was calculated using a linear standard curve, and the IC50 of ascorbic acid, which was used as a positive control, was determined on the basis of its molecular mass.
4. Conclusions
The volatile compounds obtained from the dichloromethane extract of the gamma-irradiated mutant cultivar of C. rosea (“golden ring”), its original cultivar (“pumpkin pie”) and the tea commercially available in China (C. tinctoria; “snow chrysanthemum”) were successfully identified by GC-MS. The major components of “golden ring” and “pumpkin pie” varied slightly, although along with “snow chrysanthemum” they showed significant differences in chemical composition. Meanwhile, the antioxidant effect of “golden ring” extract was higher compared with that of “pumpkin pie” and “snow chrysanthemum”. Despite phytochemical and pharmacological studies of C. tinctoria, the chemical composition and antioxidant activity of C. rosea was reported for the first time in this study. The chemical composition and strong antioxidant efficacy of “golden ring” favor the development of tea materials such as “snow chrysanthemum”.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/6/717/s1: Figure S1: GC-MS chromatograms of the dichloromethane extract of (a) “golden ring”, (b) “pumpkin pie” and (c) “snow chrysanthemum”; Figure S2: GC-MS chromatograms of n-alkane standard.
Author Contributions
Conceptualization, A.-R.H., S.-Y.K. and K.Y.P.; methodology, A.-R.H. and C.H.J.; software, B.-R.K. and H.M.K.; validation, B.-R.K. and H.M.K.; formal analysis, B.-R.K. and H.M.K.; investigation, B.-R.K., H.M.K. and Y.G.J.; resources, Y.G.J. and K.Y.P.; data curation, B.-R.K., C.H.J. and A.-R.H.; writing—original draft preparation, B.-R.K.; writing—review and editing, B.-R.K., C.H.J. and A.-R.H.; visualization, B.-R.K. and Y.G.J.; supervision, A.-R.H. and I.-S.L.; project administration, J.-B.K.; funding acquisition, J.-B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Korea Atomic Energy Research Institute, grant number 523320-20.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, S.-C.; Crawford, D.J.; Tadesse, M.; Berbee, M.; Ganders, F.R.; Pirseyedi, M.; Esselman, E.J. ITS sequences and phylogenetic relationships in Bidens and Coreopsis (Asteraceae). Syst. Bot. 1999, 24, 480–493. [Google Scholar] [CrossRef]
- Pardede, A.; Mashita, K.; Ninomiya, M.; Tanaka, K.; Koketsu, M. Flavonoid profile and antileukemic activity of Coreopsis lanceolata flowers. Bioorg. Med. Chem. Lett. 2016, 26, 2784–2787. [Google Scholar] [CrossRef] [PubMed]
- Dias, T.; Bronze, M.R.; Houghton, P.J.; Mota-Filipe, H.; Paulo, A. The flavonoid-rich fraction of Coreopsis tinctoria promotes glucose tolerance regain through pancreatic function recovery in streptozotocin-induced glucose-intolerant rats. J. Ethnopharmacol. 2010, 132, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, X.; Liu, J.; Kang, L.; Chen, S.; Ma, B.; Guo, B. Quantitative and qualitative analysis of flavonoids and phenolic acids in snow chrysanthemum (Coreopsis tinctoria Nutt.) by HPLC-DAD and UPLC-ESI-QTOF-MS. Molecules 2016, 21, 1307. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xi, M.; Guo, Q.; Shen, Z. Chemical components and antioxidant activity of volatile oil of a Compositae tea (Coreopsis tinctoria Nutt.) from Mt. Kunlun. Ind. Crop. Prod. 2015, 67, 318–323. [Google Scholar] [CrossRef]
- Ma, Z.; Zheng, S.; Han, H.; Meng, J.; Yang, X.; Zeng, S.; Zhou, H.; Jiang, H. The bioactive components of Coreopsis tinctoria (Asteraceae) capitual: Antioxidant activity in vitro and profile in rat plasma. J. Funct. Foods. 2016, 20, 575–586. [Google Scholar] [CrossRef]
- Yao, L.; Zhaang, Y.; Li, L.; Zhang, R.; Wang, J.; Li, X.; Li, H. The anti-infalmmatory and antifibrotic effects of Coreopsis tinctoria Nutt on high-glucose-fat diet and streptozotocin-induced diabetic renal damage in rats. BMC Complement. Altern. Med. 2015, 15, 314. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, S.; Zhao, M.; Chai, X.; Tu, P. Coreosides A-D,C14-polyacetylene glycosides from the capitula of Coreopsis tinctoria and its anti-inflammatory activity against COX-2. Fioterapia 2013, 87, 93–97. [Google Scholar] [CrossRef]
- Kim, H.-G.; Oh, H.-J.; Ko, J.-H.; Song, H.S.; Lee, Y.-G.; Kang, S.C.; Lee, D.Y.; Baek, N.-I. Lanceolein A-G, hydroxychalcones, from the flowers of Coreopsis lanceolate and their chemopreventive effects against human colon cancer cells. Bioor. Chem. 2019, 85, 274–281. [Google Scholar] [CrossRef]
- Tanaka, A.; Shikazono, N.; Hase, Y. Studies on biological effects of ion beams on lethality, molecular nature of mutation, mutation rate, and spectrum of mutation phenotype for mutation breeding in higher plants. J. Radiat. Res. 2010, 51, 223–233. [Google Scholar] [CrossRef]
- Ali, H.; Ghori, Z.; Sheikh, S.; Gul, A. Effects of gamma radiation on crop production. In Crop Production and Global Environmental Issues; Hakeem, K., Ed.; Springer: Cham, Switzerland, 2016; pp. 27–78. [Google Scholar]
- Mutant Varieties Database. Available online: https://www.iaea.org/resources/databases/mutant-varieties-database (accessed on 7 May 2020).
- Damann, M.P.; Lyons, R.E. Juvenility, flowering, and the effects of a limited inductive photoperiod in Coreopsis grandiflora and C. lanceolata. J. Amer. Soc. Hort. Sci. 1993, 118, 503–518. [Google Scholar] [CrossRef]
- Korea Seed & Variety Service. Available online: http://www.seed.go.kr/sites/seed_eng/index..do (accessed on 7 May 2020).
- Burnett, S.E.; Keever, G.J.; Kessler, J.R.; Cilliam, C.H. Foliar application of plant growth retardants to Coreopsis rosea ‘American dream’. J. Environ. Hort. 2000, 18, 39–62. [Google Scholar]
- Olaoluwa, O.; Moronkola, D.; Taiwo, O.; Iganboh, P. Volatile oil composition, antioxidant and antimicrobial properties of Boerhavia erecta L. and Euphorbia hirta L. Trends Phytochem. Res. 2018, 2, 171–178. [Google Scholar]
- Krishna, A.N.V.; Raman, B.V.; Babu, K.R.; Apparao, C. Antioxidant activity and GC-MS analysis of Phragmytes vallatoria leaf ethanoic extract. Int. Res. J. Pharm. 2012, 3, 252–254. [Google Scholar]
- Tian, C.; Gao, X.; Yang, J.; Gua, Y.; Wang, H.; Liu, M. Chemical compositions, extraction technology and antioxidant activity of petroleum ether extract from Abutilon theophrasti Medic. leaves. Int. J. Food. Prop. 2018, 21, 1789–1799. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug. Des. 2012, 80, 434–439. [Google Scholar] [CrossRef]
- Korbecki, J.; Bajdak-Rusinek, K. The effect of palmitic acid on inflammatory response in macrophages: An overview of molecular mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef]
- Harada, H.; Yamashita, U.; Kurihara, H.; Fukushi, E.; Kawabata, J.; Kamei, Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002, 22, 2587–2590. [Google Scholar]
- Nengroo, Z.R.; Rauf, A. Fatty acid composition and antioxidant activities of five medicinal plants from Kashmir. Ind. Crops Prod. 2019, 140, 111596. [Google Scholar] [CrossRef]
- Karimi, E.; Jaafar, H.Z.E.; Ghasemxadeh, A.; Ebrahimi, M. Fatty acid composition, antioxidant and antibacterial properties of the microwave aqueous extract of three varieties of Labisia pumila Benth. Biol. Res. 2015, 48. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Assessment of the antioxidant potential and fatty acid composition of four Centaurea L. taxa from Turkey. Food Chem. 2013, 141, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic. Biol. Med. 2008, 45, 231–241. [Google Scholar]
- Pacheco, B.S.; Dos Santos, M.A.Z.; Schultze, E.; Martins, R.M.; Lund, R.G.; Seixas, F.K.; Colepicolo, P.; Collares, T.; Paula, F.R.; De Pereira, C.M.P. Cytotoxic activity of fatty acids from Antarctic macroalgae on the growth of human breast cancer cells. Front Bioeng. Biotechnol. 2018, 6, 185. [Google Scholar] [CrossRef] [PubMed]
- Babushok, V.L.; Linstrom, P.J.; Zenkevich, I.G. Retention induices for frequently reported compounds of plant essential olis. J. Phys. Chem. Ref. Data 2011, 40, 1–46. [Google Scholar] [CrossRef]
- Aobuli, A.; Matiusong, J.; Bakri, M.; Lu, X.; Maiwulanjing, M.; Aisa, H.A. The effects of volatile oil from Vernonia anthelmintica seeds on melanin synthesis in B16 cells and its chemical analysis by GC-QTOF-MS. Evid. Based. Complement. Alternat. Med. 2018, 6291281, 1–8. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 28–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, M.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).