Combined Effects of UV-B and Drought on Native and Exotic Populations of Verbascum thapsus L.
Abstract
1. Introduction
2. Results
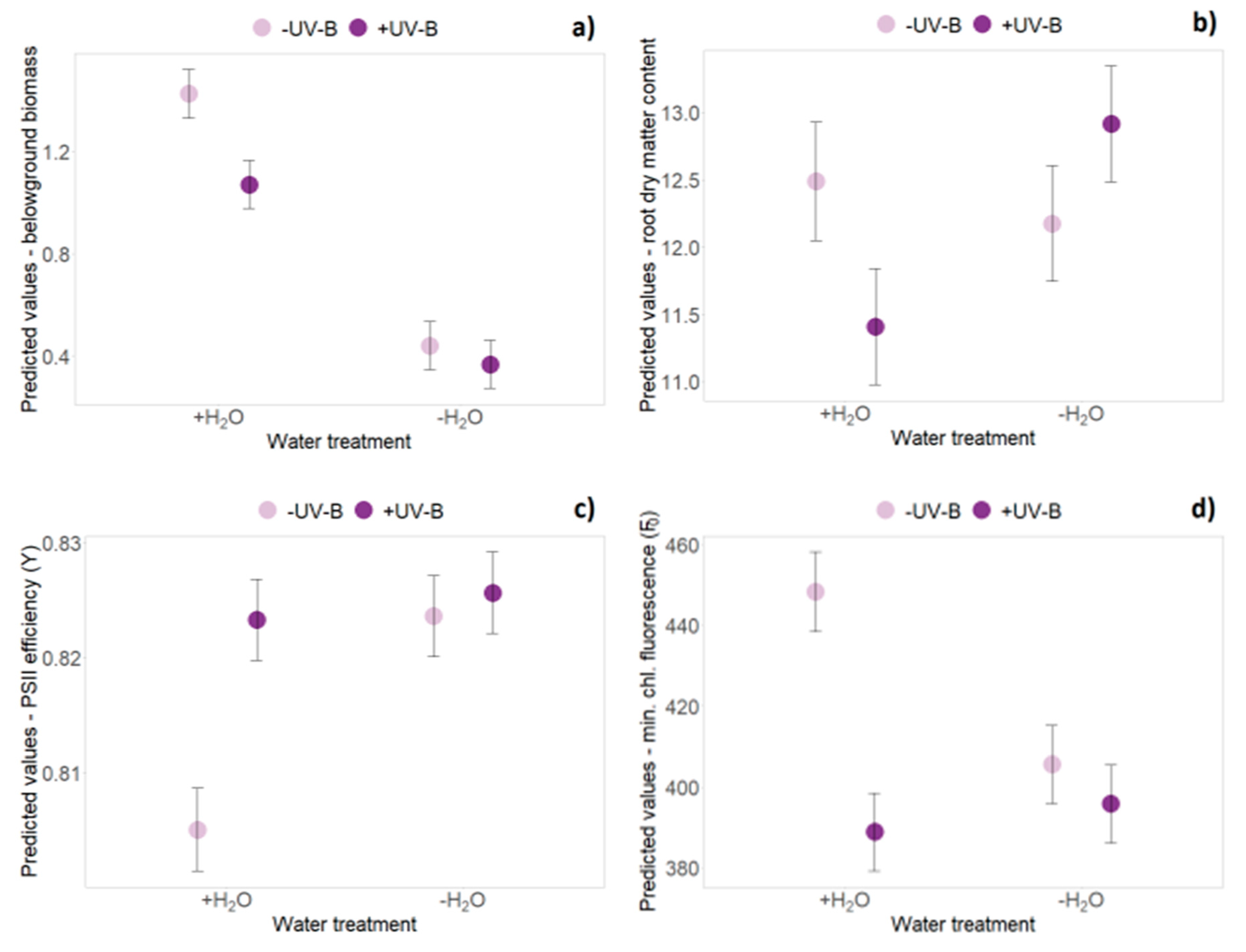
2.1. Biomass Data (Harvest Data Hx)
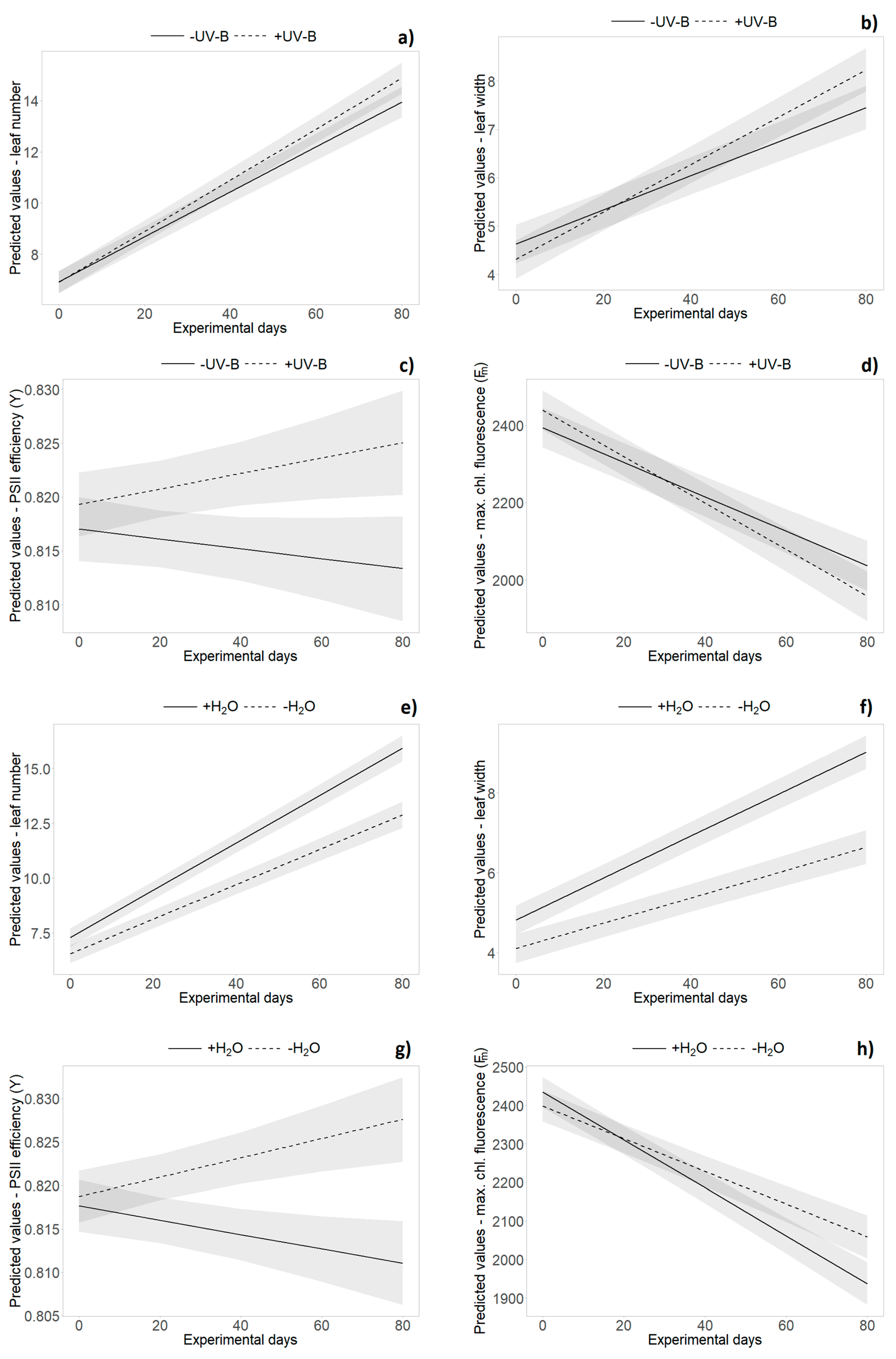
2.2. Growth Data (Monitoring Data)
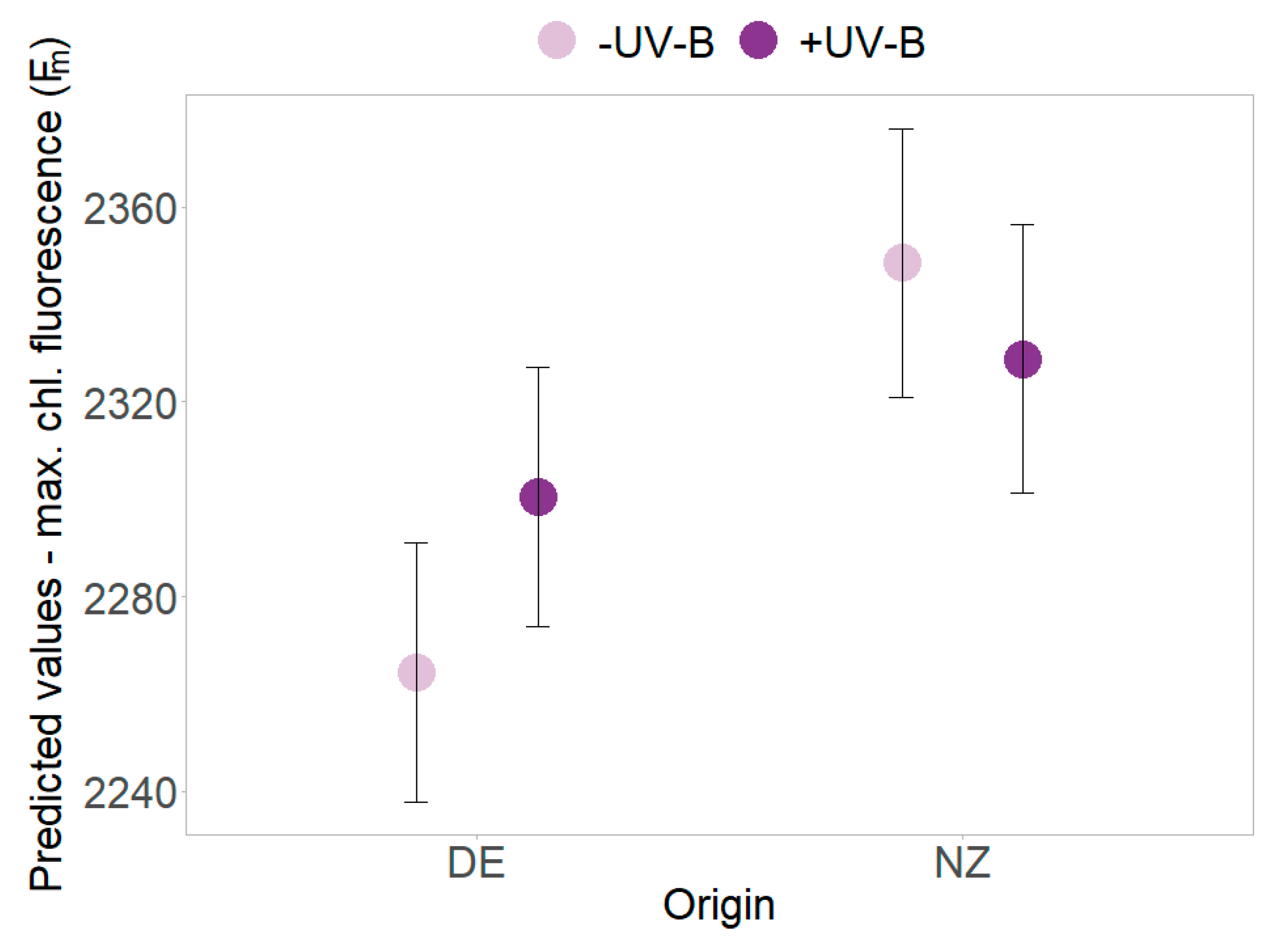
2.3. Functional Leaf Traits and Physiology
3. Discussion
3.1. Single and Combined Effects of UV-B and Drought
3.2. Origin Differentiation and Origin-Specific Response to UV-B and Drought
4. Materials and Methods
4.1. Study Species
4.2. Experimental Design
4.3. Data Collection
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pereira, M.G.; Calado, T.J.; DaCamara, C.C.; Calheiros, T. Effects of regional climate change on rural fires in Portugal. Clim. Res. 2013, 57, 187–200. [Google Scholar] [CrossRef]
- Keane, R.M.; Crawley, M.J. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Theoharides, K.A.; Dukes, J.S. Plant invasion across space and time: Factors affecting nonindigenous species success during four stages of invasion. New Phytol. 2007, 176, 256–273. [Google Scholar] [CrossRef] [PubMed]
- Erfmeier, A. Constraints and release at different scales—The role of adaptation in biological invasions. Basic Appl. Ecol. 2013, 14, 281–288. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Pyšek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jarošík, V.; Wilson, J.R.U.; Richardson, D.M. A proposed unified framework for biological invasions. Trends Ecol. Evol. 2011, 26, 333–339. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pyšek, P.; Rejmánek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and invasion of alien plants: Concepts and definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- Sharma, G.P.; Esler, K.J. Phenotypic plasticity among Echium plantagineum populations in different habitats of Western Cape, South Africa. S. Afr. J. Bot. 2008, 74, 746–749. [Google Scholar] [CrossRef]
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef]
- Schlaepfer, D.R.; Glättli, M.; Fischer, M.; van Kleunen, M. A multi-species experiment in their native range indicates pre-adaptation of invasive alien plant species. New Phytol. 2010, 185, 1087–1099. [Google Scholar] [CrossRef]
- Colautti, R.I.; Eckert, C.G.; Barrett, S.C.H. Evolutionary constraints on adaptive evolution during range expansion in an invasive plant. Proc. R. Soc. B Biol. Sci. 2010, 277, 1799–1806. [Google Scholar] [CrossRef]
- Buswell, J.M.; Moles, A.T.; Hartley, S. Is rapid evolution common in introduced plant species? J. Ecol. 2011, 99, 214–224. [Google Scholar] [CrossRef]
- Broennimann, O.; Treier, U.A.; Müller-Schärer, H.; Thuiller, W.; Peterson, A.T.; Guisan, A. Evidence of climatic niche shift during biological invasion. Ecol. Lett. 2007, 10, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Petitpierre, B.; Kueffer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic niche shifts are rare among terrestrial plant invaders. Science 2012, 335, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Phoenix, G.K.; Gwynn-Jones, D.; Callaghan, T.V.; Sleep, D.; Lee, J.A. Effects of global change on a sub-Arctic heath: Effects of enhanced UV-B radiation and increased summer precipitation. J. Ecol. 2001, 89, 256–267. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Yeung, E.C.; Reid, D.M. Growth and physiological responses of an invasive alien species, Silene noctiflora, during two developmental stages to four levels of ultraviolet-B radiation. Ecoscience 2008, 15, 150–159. [Google Scholar] [CrossRef]
- Beckmann, M.; Hock, M.; Bruelheide, H.; Erfmeier, A. The role of UV-B radiation in the invasion of Hieracium pilosella—A comparison of German and New Zealand plants. Environ. Exp. Bot. 2012, 75, 173–180. [Google Scholar] [CrossRef]
- Hock, M.; Hofmann, R.W.; Müller, C.; Erfmeier, A. Exotic plant species are locally adapted but not to high ultraviolet-B radiation: A reciprocal multispecies experiment. Ecology 2019, 100, e02665. [Google Scholar] [CrossRef]
- Norby, R.J.; Luo, Y. Evaluating ecosystem responses to rising atmospheric CO2 and global warming in a multi-factor world. New Phytol. 2004, 162, 281–293. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Bornman, J.F.; Ballaré, C.L.; Flint, S.D.; Kulandaivelu, G. Terrestrial ecosystems, increased solar ultraviolet radiation, and interactions with other climate change factors. Photochem. Photobiol. Sci. 2007, 6, 252–266. [Google Scholar] [CrossRef]
- Alba, C.; Fahey, C.; Flory, S.L. Global change stressors alter resources and shift plant interactions from facilitation to competition over time. Ecology 2019, 100, e02859. [Google Scholar] [CrossRef]
- Côté, I.M.; Darling, E.S.; Brown, C.J. Interactions among ecosystem stressors and their importance in conservation. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152592. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Galic, N.; Sullivan, L.L.; Grimm, V.; Forbes, V.E. When things don’t add up: Quantifying impacts of multiple stressors from individual metabolism to ecosystem processing. Ecol. Lett. 2018, 21, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Dicke, M. Plant interactions with microbes and insects: From molecular mechanisms to ecology. Trends Plant Sci. 2007, 12, 564–569. [Google Scholar] [CrossRef]
- Mauro, R.P.; Monaco, A.L.; Lombardo, S.; Restuccia, A.; Mauromicale, G. Eradication of Orobanche/Phelipanche spp. seedbank by soil solarization and organic supplementation. Sci. Hortic. 2015, 193, 62–68. [Google Scholar] [CrossRef]
- McKenzie, R.L.; Aucamp, P.J.; Bais, A.F.; Björn, L.O.; Ilyas, M. Changes in biologically-active ultraviolet radiation reaching the Earth’s surface. Photochem. Photobiol. Sci. 2007, 6, 218–231. [Google Scholar] [CrossRef]
- Li, F.R.; Peng, S.L.; Chen, B.M.; Hou, Y.P. A meta-analysis of the responses of woody and herbaceous plants to elevated ultraviolet-B radiation. Acta Oecol. 2010, 36, 1–9. [Google Scholar] [CrossRef]
- Watanabe, S.; Sudo, K.; Nagashima, T.; Takemura, T.; Kawase, H.; Nozawa, T. Future projections of surface UV-B in a changing climate. J. Geophys. Res. Atmos. 2011, 116. [Google Scholar] [CrossRef]
- Jansen, M.A.K.; Gaba, V.; Greenberg, B.M. Higher plants and UV-B radiation: Balancing damage, repair and acclimation. Trends Plant Sci. 1998, 3, 131–135. [Google Scholar] [CrossRef]
- Hofmann, R.W.; Campbell, B.D.; Fountain, D.W.; Jordan, B.R.; Greer, D.H.; Hunt, D.Y.; Hunt, C.L. Multivariate analysis of intraspecific responses to UV-B radiation in white clover (Trifolium repens L.). Plant Cell Environ. 2001, 24, 917–927. [Google Scholar] [CrossRef]
- Watermann, L.Y.; Hock, M.; Blake, C.; Erfmeier, A. Plant invasions into high elevations implies adaptation to high UV-B environments—A multi-species experiment. Biol. Invasions 2019. [Google Scholar] [CrossRef]
- Holmes, M.G.; Keiller, D.R. Effects of pubescence and waxes on the reflectance of leaves in the ultraviolet and photosynthetic wavebands: A comparison of a range of species. Plant Cell Enviorn. 2002, 25, 85–93. [Google Scholar] [CrossRef]
- Manetas, Y. The importance of being hairy: The adverse effects of hair removal on stem photosynthesis of Verbascum speciosum are due to solar UV-B radiation. New Phytol. 2003, 158, 503–508. [Google Scholar] [CrossRef]
- Micco, V.D.; Aronne, G. Morpho-Anatomical Traits for Plant Adaptation to Drought. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 37–61. [Google Scholar]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Ren, J.; Dai, W.; Xuan, Z.; Yao, Y.; Korpelainen, H.; Li, C. The effect of drought and enhanced UV-B radiation on the growth and physiological traits of two contrasting poplar species. For. Ecol. Manag. 2007, 239, 112–119. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, Y.; Xu, G.; Li, C. Growth and physiological responses to drought and elevated ultraviolet-B in two contrasting populations of Hippophae rhamnoides. Physiol. Plant. 2005, 124, 431–440. [Google Scholar] [CrossRef]
- Sullivan, J.H.; Teramura, A.H. Field Study of the Interaction between Solar Ultraviolet-B Radiation and Drought on Photosynthesis and Growth in Soybean. Plant Physiol. 1990, 92, 141–146. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Björn, L.O.; Callaghan, T.V.; Johnsen, I.; Lee, J.A.; Manetas, Y.; Paul, N.D.; Sonesson, M.; Wellburn, A.R.; Coop, D.; Heide-Jørgensen, H.S.; et al. The effects of UV-B radiation on European heathland species. In UV-B and Biosphere. Advances in Vegetation Science; Rozema, J., Gieskes, W.W.C., Van De Geijn, S.C., Nolan, C., De Boois, H., Eds.; Springer: Dordrecht, The Netherlands, 1997; Volume 17, pp. 252–264. [Google Scholar]
- Manetas, Y.; Petropoulou, Y.; Stamatakis, K.; Nikolopoulos, D.; Levizou, E.; Psaras, G.; Karabourniotis, G. Beneficial effects of enhanced UV-B radiation under field conditions: Improvement of needle water relations and survival capacity of Pinus pinea L. seedlings during the dry Mediterranean summer. In UV-B and Biosphere. Advances in Vegetation Science; Rozema, J., Gieskes, W.W.C., Van De Geijn, S.C., Nolan, C., De Boois, H., Eds.; Springer: Dordrecht, The Netherlands, 1997; Volume 17, pp. 100–108. [Google Scholar]
- Poulson, M.E.; Donahue, R.A.; Konvalinka, J.; Boeger, M.R.T. Enhanced tolerance of photosynthesis to high-light and drought stress in Pseudotsuga menziesii seedlings grown in ultraviolet-B radiation. Tree Physiol. 2002, 22, 829–838. [Google Scholar] [CrossRef]
- Hofmann, R.W.; Campbell, B.D.; Bloor, S.J.; Swinny, E.E.; Markham, K.R.; Ryan, K.G.; Fountain, D.W. Responses to UV-B radiation in Trifolium repens L—Physiological links to plant productivity and water availability. Plant Cell Enviorn. 2003, 26, 603–612. [Google Scholar] [CrossRef]
- Bandurska, H.; Niedziela, J.; Chadzinikolau, T. Separate and combined responses to water deficit and UV-B radiation. Plant Sci. 2013, 213, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, R.W.; Campbell, B.D. Leaf-level responses to ultraviolet-B radiation in Trifolium repens populations under defoliation pressure. Enviorn. Exp. Bot. 2012, 78, 64–69. [Google Scholar] [CrossRef]
- Bacelar, E.; Moutinho-Pereira, J.; Ferreira, H.; Correia, C. Enhanced ultraviolet-B radiation affect growth, yield and physiological processes on triticale plants. Procedia Enviorn. Sci. 2015, 29, 219–220. [Google Scholar] [CrossRef]
- Newsham, K.K.; Robinson, S.A. Responses of plants in polar regions to UVB exposure: A meta-analysis. Glob. Chang. Biol. 2009, 15, 2574–2589. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Juurola, E.; Nikinmaa, E.; Berninger, F.; Ensminger, I.; Hari, P. Seasonal acclimation of photosystem II in Pinus sylvestris. I. Estimating the rate constants of sustained thermal energy dissipation and photochemistry. Tree Physiol. 2008, 28, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Ranjbarfordoei, A.; Samson, R.; Van Damme, P. Photosynthesis performance in sweet almond [Prunus dulcis (Mill) D. Webb] exposed to supplemental UV-B radiation. Photosynthetica 2011, 49, 107. [Google Scholar] [CrossRef]
- Osório, J.; Osório, M.L.; Correia, P.J.; de Varennes, A.; Pestana, M. Chlorophyll fluorescence imaging as a tool to understand the impact of iron deficiency and resupply on photosynthetic performance of strawberry plants. Sci. Hortic. 2014, 165, 148–155. [Google Scholar] [CrossRef]
- Anjum, S.; Xie, X.; Wang, L.; Saleem, M.S.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Li, R.H.; Guo, P.G.; Michael, B.; Stefania, G.; Salvatore, C. Evaluation of chlorophyll content and fluorescence parameters as indicators of drought tolerance in barley. Agric. Sci. China 2006, 5, 751–757. [Google Scholar] [CrossRef]
- Yao, J.; Sun, D.; Cen, H.; Xu, H.; Weng, H.; Yuan, F.; He, Y. Phenotyping of Arabidopsis drought stress response using kinetic chlorophyll fluorescence and multicolor fluorescence imaging. Front Plant Sci. 2018, 9, 603. [Google Scholar] [CrossRef]
- Alba, C.; Hufbauer, R. Exploring the potential for climatic factors, herbivory, and co-occurring vegetation to shape performance in native and introduced populations of Verbascum thapsus. Biol. Invasions 2012, 14, 2505–2518. [Google Scholar] [CrossRef]
- Hideg, É.; Nagy, T.; Oberschall, A.; Dudits, D.; Vass, I. Detoxification function of aldose/aldehyde reductase during drought and ultraviolet-B (280–320 nm) stresses. Plant Cell Enviorn. 2003, 26, 513–522. [Google Scholar] [CrossRef]
- Rinnan, R.; Keinänen, M.M.; Kasurinen, A.; Asikainen, J.; Kekki, T.K.; Holopainen, T.; Ro-Poulsen, H.; Mikkelsen, T.N.; Michelsen, A. Ambient ultraviolet radiation in the Arctic reduces root biomass and alters microbial community composition but has no effects on microbial biomass. Glob. Chang. Biol. 2005, 11, 564–574. [Google Scholar] [CrossRef]
- Jouili, H.; El Ferjani, E. Changes in antioxidant and lignifying enzyme activities in sunflower roots (Helianthus annuus L.) stressed with copper excess. C. R. Biol. 2003, 326, 639–644. [Google Scholar] [CrossRef]
- Claussen, W. Proline as a measure of stress in tomato plants. Plant Sci. 2005, 168, 241–248. [Google Scholar] [CrossRef]
- Lachmuth, S.; Durka, W.; Schurr, F.M. The making of a rapid plant invader: Genetic diversity and differentiation in the native and invaded range of Senecio inaequidens. Mol. Ecol. 2010, 19, 3952–3967. [Google Scholar] [CrossRef] [PubMed]
- Colautti, R.I.; Maron, J.L.; Barrett, S.C. Common garden comparisons of native and introduced plant populations: Latitudinal clines can obscure evolutionary inferences. Evol. Appl. 2009, 2, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Hodgins, K.A.; Rieseberg, L. Genetic differentiation in life-history traits of introduced and native common ragweed (Ambrosia artemisiifolia) populations. J. Evol. Biol. 2011, 24, 2731–2749. [Google Scholar] [CrossRef]
- Dieskau, J.; Bruelheide, H.; Gutknecht, J.; Erfmeier, A. Biogeographic differences in plant-soil biota relationships contribute to the invasion of Verbascum thapsus. Ecol. Evol. under review.
- Alba, C.; Bowers, D.M.; Blumenthal, D.M.; Hufbauer, R. Evolution of growth but not structural or chemical defense in Verbascum thapsus (common mullein) following introduction to North America. Biol. Invasions 2011, 13, 2379–2389. [Google Scholar] [CrossRef]
- Endriss, S.B.; Alba, C.; Norton, A.P.; Pyšek, P.; Hufbauer, R.A. Breakdown of a geographic cline explains high performance of introduced populations of a weedy invader. J. Ecol. 2018, 106, 699–713. [Google Scholar] [CrossRef]
- Alpert, P.; Bone, E.; Holzapfel, C. Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspect Plant Ecol. 2000, 3, 52–66. [Google Scholar] [CrossRef]
- te Beest, M.; Elschot, K.; Olff, H.; Etienne, R.S. Invasion success in a marginal habitat: An experimental test of competitive ability and drought tolerance in Chromolaena odorata. PLoS ONE 2013, 8, e68274. [Google Scholar] [CrossRef] [PubMed]
- Ashbacher, A.C.; Cleland, E.E. Native and exotic plant species show differential growth but similar functional trait responses to experimental rainfall. Ecosphere 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Antunes, C.; Pereira, A.J.; Fernandes, P.; Ramos, M.; Ascensão, L.; Correia, O.; Máguas, C. Understanding plant drought resistance in a Mediterranean coastal sand dune ecosystem: Differences between native and exotic invasive species. J. Plant Ecol. 2018, 11, 26–38. [Google Scholar] [CrossRef]
- Erfmeier, A.; Böhnke, M.; Bruelheide, H. Secondary invasion of Acer negundo: The role of phenotypic responses versus local adaptation. Biol. Invasions 2011, 13, 1599–1614. [Google Scholar] [CrossRef]
- Kumschick, S.; Hufbauer, R.A.; Alba, C.; Blumenthal, D.M. Evolution of fast-growing and more resistant phenotypes in introduced common mullein (Verbascum thapsus). J. Ecol. 2013, 101, 378–387. [Google Scholar] [CrossRef]
- Hubbard, J.C.; Wilson, J.B. A survey of the lowland vegetation of the Upper Clutha District of Otago, New Zealand. N. Z. J. Bot. 1988, 26, 21–35. [Google Scholar] [CrossRef]
- Turker, A.U.; Gurel, E. Common mullein (Verbascum thapsus L.): Recent advances in research. Phytother. Res. 2005, 19, 733–739. [Google Scholar] [CrossRef]
- Gross, K.L.; Werner, P.A. The biology of Canadian weeds: 28. Verbascum thapsus L. and V. blattaria L. Can. J. Plant Sci. 1978, 58, 401–413. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82. [Google Scholar] [CrossRef]
- Alexieva, V.; Ivanov, S.; Sergiev, I.; Karanov, E. Interaction between stresses. Bulg. J. Plant Physiol. 2003, 29, 1–17. [Google Scholar]
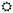 −UV-B/
−UV-B/ +UV-B) and water treatment (water levels:
+UV-B) and water treatment (water levels:  low/
low/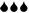 well-watered). The table provides information on the dates of monitoring (Mx), physiological measurements (Px) and biomass harvests (Hx) during the 12-week experimental period, and adds information on the treatment intensity applied. Note that levels of water addition were adjusted after 4 weeks to account for rapid initial increase in plant size.
well-watered). The table provides information on the dates of monitoring (Mx), physiological measurements (Px) and biomass harvests (Hx) during the 12-week experimental period, and adds information on the treatment intensity applied. Note that levels of water addition were adjusted after 4 weeks to account for rapid initial increase in plant size.
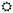 −UV-B/
−UV-B/ +UV-B) and water treatment (water levels:
+UV-B) and water treatment (water levels:  low/
low/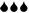 well-watered). The table provides information on the dates of monitoring (Mx), physiological measurements (Px) and biomass harvests (Hx) during the 12-week experimental period, and adds information on the treatment intensity applied. Note that levels of water addition were adjusted after 4 weeks to account for rapid initial increase in plant size.
well-watered). The table provides information on the dates of monitoring (Mx), physiological measurements (Px) and biomass harvests (Hx) during the 12-week experimental period, and adds information on the treatment intensity applied. Note that levels of water addition were adjusted after 4 weeks to account for rapid initial increase in plant size.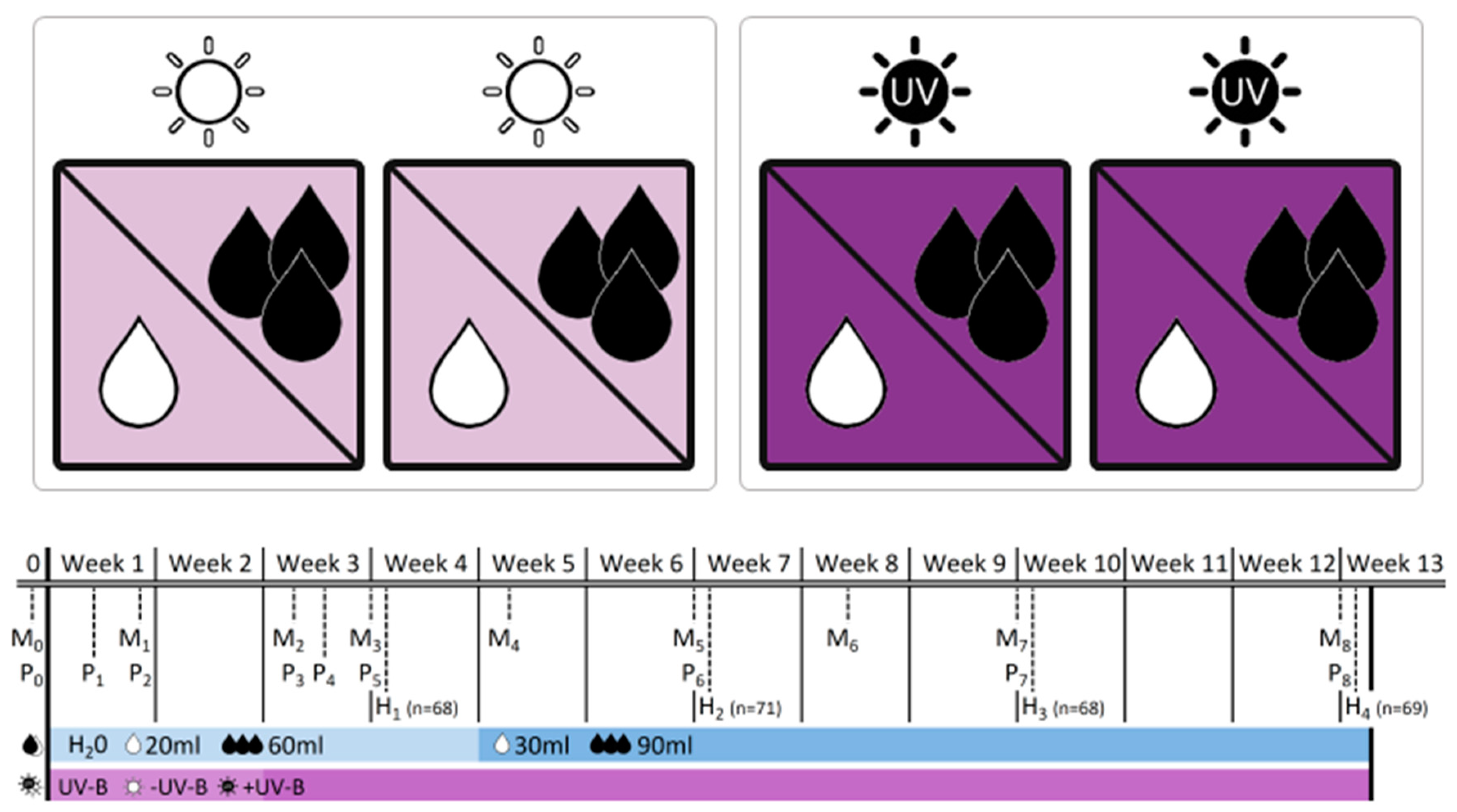

| Variable/Source | dfN | 1st Harvest (3 Weeks) | 2nd Harvest (6 Weeks) | 3rd Harvest (9 Weeks) | 4th Harvest (12 Weeks) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dfD | F | p | dfD | F | p | dfD | F | p | dfD | F | p | ||||||
| Total biomass | |||||||||||||||||
| Origin | 1 | 57.1 | 5.449 | 0.023 | * | 17.9 | 2.415 | 0.138 | 23.2 | 0.146 | 0.706 | 18.9 | 2.929 | 0.103 | |||
| UV-B | 1 | 1.3 | 0.001 | 0.984 | 2.0 | 0.483 | 0.560 | 44.5 | 2.448 | 0.125 | 45.2 | 0.064 | 0.801 | ||||
| Water | 1 | 58.3 | 30.522 | <0.001 | *** | 43.6 | 156.263 | <0.001 | *** | 44.7 | 85.480 | <0.001 | *** | 45.3 | 123.432 | <0.001 | *** |
| Initial leaf number (Covariate) | 1 | 57.2 | 30.682 | <0.001 | *** | 34.6 | 18.697 | <0.001 | *** | 58.6 | 14.589 | <0.001 | *** | 59.4 | 16.040 | <0.001 | *** |
| Origin × UV-B | 1 | 57.0 | 0.185 | 0.669 | 44.2 | 0.047 | 0.829 | 44.6 | 0.250 | 0.619 | 45.1 | 0.318 | 0.575 | ||||
| Origin × Water | 1 | 26.6 | 0.797 | 0.380 | 44.0 | 3.692 | 0.061 | . | 44.6 | 0.164 | 0.688 | 45.2 | 4.241 | 0.045 | * | ||
| UV-B × Water | 1 | 58.3 | 0.001 | 0.975 | 44.5 | 0.809 | 0.373 | 44.7 | 0.762 | 0.387 | 45.1 | 0.617 | 0.436 | ||||
| Origin × UV-B × Water | 1 | 26.2 | 0.238 | 0.629 | 43.3 | 0.098 | 0.755 | 44.6 | 0.020 | 0.888 | 46.7 | 0.195 | 0.661 | ||||
| Aboveground biomass | |||||||||||||||||
| Origin | 1 | 57.1 | 5.281 | 0.025 | * | 18.5 | 1.777 | 0.199 | 23.2 | 0.064 | 0.802 | 18.5 | 1.430 | 0.247 | |||
| UV-B | 1 | 1.3 | 0.036 | 0.875 | 43.6 | 0.118 | 0.733 | 44.7 | 0.628 | 0.432 | 44.8 | 0.075 | 0.786 | ||||
| Water | 1 | 58.3 | 26.053 | <0.001 | *** | 45.3 | 82.856 | <0.001 | *** | 44.8 | 58.462 | <0.001 | *** | 44.9 | 49.639 | <0.001 | *** |
| Initial leaf number (Covariate) | 1 | 57.2 | 28.713 | <0.001 | *** | 37.8 | 12.078 | 0.001 | ** | 58.2 | 16.458 | <0.001 | *** | 59.3 | 7.827 | 0.007 | ** |
| Origin × UV-B | 1 | 57.0 | 0.504 | 0.480 | 46.2 | 0.000 | 0.988 | 44.7 | 0.316 | 0.577 | 44.7 | 0.441 | 0.510 | ||||
| Origin × Water | 1 | 25.4 | 0.297 | 0.590 | 46.3 | 0.926 | 0.341 | 44.7 | 0.078 | 0.782 | 44.8 | 0.088 | 0.768 | ||||
| UV-B × Water | 1 | 58.3 | 0.004 | 0.952 | 46.3 | 0.000 | 0.988 | 44.8 | 0.175 | 0.678 | 44.8 | 1.862 | 0.179 | ||||
| Origin × UV-B × Water | 1 | 25.1 | 0.464 | 0.502 | 45.7 | 0.011 | 0.918 | 44.7 | 0.055 | 0.816 | 46.1 | 0.051 | 0.823 | ||||
| Belowground biomass | |||||||||||||||||
| Origin | 1 | 57.1 | 4.137 | 0.047 | * | 17.8 | 2.915 | 0.105 | 23.9 | 0.100 | 0.755 | 18.9 | 4.035 | 0.059 | . | ||
| UV-B | 1 | 1.2 | 0.471 | 0.602 | 2.0 | 2.905 | 0.232 | 1.7 | 1.413 | 0.374 | 1.7 | 0.353 | 0.620 | ||||
| Water | 1 | 58.6 | 33.416 | <0.001 | *** | 44.3 | 250.101 | <0.001 | *** | 44.8 | 83.539 | <0.001 | *** | 45.9 | 135.924 | <0.001 | *** |
| Initial leaf number (Covariate) | 1 | 57.3 | 26.224 | <0.001 | *** | 28.5 | 29.625 | <0.001 | *** | 57.0 | 9.803 | 0.003 | ** | 51.3 | 16.662 | <0.001 | *** |
| Origin × UV-B | 1 | 57.0 | 0.160 | 0.691 | 45.0 | 0.574 | 0.453 | 42.8 | 0.109 | 0.742 | 43.8 | 0.126 | 0.724 | ||||
| Origin × Water | 1 | 16.5 | 3.488 | 0.080 | . | 45.0 | 12.119 | 0.001 | ** | 44.8 | 2.071 | 0.157 | 45.7 | 11.764 | 0.001 | ** | |
| UV-B × Water | 1 | 58.6 | 0.084 | 0.773 | 45.0 | 6.936 | 0.012 | * | 44.9 | 0.347 | 0.559 | 45.7 | 0.015 | 0.903 | |||
| Origin × UV-B × Water | 1 | 16.2 | 0.091 | 0.766 | 44.3 | 0.462 | 0.500 | 44.9 | 0.008 | 0.928 | 47.6 | 0.649 | 0.424 | ||||
| Shoot:mass ratio | |||||||||||||||||
| Origin | 1 | 17.1 | 0.195 | 0.664 | 20.0 | 0.946 | 0.342 | 57.4 | 0.837 | 0.364 | 16.2 | 0.870 | 0.365 | ||||
| UV-B | 1 | 44.9 | 3.788 | 0.058 | . | 1.9 | 8.108 | 0.108 | 1.8 | 1.405 | 0.368 | 1.7 | 6.740 | 0.141 | |||
| Water | 1 | 45.9 | 4.413 | 0.041 | * | 45.8 | 69.580 | <0.001 | *** | 59.0 | 95.446 | <0.001 | *** | 42.8 | 463.820 | <0.001 | *** |
| Initial leaf number (Covariate) | 1 | 47.2 | 1.465 | 0.232 | 38.8 | 0.421 | 0.520 | 57.9 | 0.0225 | 0.637 | 59.2 | 14.310 | <0.001 | *** | |||
| Origin × UV-B | 1 | 44.8 | 0.765 | 0.386 | 46.2 | 0.007 | 0.933 | 57.0 | 0.217 | 0.643 | 39.6 | 1.700 | 0.200 | ||||
| Origin × Water | 1 | 44.9 | 2.113 | 0.153 | 46.0 | 0.028 | 0.868 | 59.0 | 0.319 | 0.575 | 42.3 | 4.660 | 0.037 | * | |||
| UV-B × Water | 1 | 45.5 | 0.005 | 0.944 | 46.7 | 0.714 | 0.402 | 59.0 | 0.045 | 0.833 | 42.6 | 0.040 | 0.845 | ||||
| Origin × UV-B × Water | 1 | 44.7 | 0.437 | 0.512 | 45.3 | 0.086 | 0.771 | 59.0 | 0.281 | 0.598 | 44.2 | 3.670 | 0.062 | . | |||
| Root dry matter content | |||||||||||||||||
| Origin | 1 | 57.1 | 0.085 | 0.772 | 61.2 | 4.562 | 0.037 | * | 59.0 | 0.912 | 0.344 | 58.1 | 0.011 | 0.919 | |||
| UV-B | 1 | 1.2 | 0.856 | 0.503 | 1.9 | 0.057 | 0.835 | 59.0 | 0.964 | 0.330 | 59.7 | 3.427 | 0.069 | . | |||
| Water | 1 | 58.5 | 17.623 | <0.001 | *** | 61.7 | 1.998 | 0.162 | 59.0 | 4.820 | 0.032 | * | 59.7 | 0.791 | 0.377 | ||
| Initial leaf number (Covariate) | 1 | 57.3 | 5.020 | 0.029 | * | 61.9 | 0.002 | 0.961 | 59.0 | 0.602 | 0.441 | 59.7 | 5.606 | 0.021 | * | ||
| Origin × UV-B | 1 | 57.0 | 0.893 | 0.349 | 60.4 | 1.354 | 0.249 | 59.0 | 0.234 | 0.630 | 59.7 | 0.562 | 0.456 | ||||
| Origin × Water | 1 | 17.6 | 0.002 | 0.966 | 60.0 | 0.037 | 0.848 | 59.0 | 3.678 | 0.060 | . | 59.7 | 0.823 | 0.368 | |||
| UV-B × Water | 1 | 58.5 | 0.001 | 0.974 | 61.7 | 4.828 | 0.032 | * | 59.0 | 0.610 | 0.438 | 59.7 | 0.832 | 0.365 | |||
| Origin × UV-B × Water | 1 | 17.2 | 0.929 | 0.349 | 60.0 | 0.002 | 0.961 | 59.0 | 0.004 | 0.984 | 59.8 | 1.349 | 0.250 | ||||
| Leaf dry matter content | |||||||||||||||||
| Origin | 1 | 56.1 | 5.683 | 0.021 | * | 62.0 | 14.119 | <0.001 | *** | 21.3 | 0.044 | 0.836 | 16.9 | 0.623 | 0.441 | ||
| UV-B | 1 | 1.0 | 4.180 | 0.294 | 62.0 | 0.977 | 0.327 | 1.2 | 0.000 | 0.994 | 1.7 | 0.004 | 0.957 | ||||
| Water | 1 | 57.4 | 56.591 | <0.001 | *** | 62.0 | 19.869 | <0.001 | *** | 31.4 | 16.113 | <0.001 | *** | 44.0 | 8.474 | 0.006 | ** |
| Initial leaf number (Covariate) | 1 | 55.9 | 44.973 | <0.001 | *** | 62.0 | 5.653 | 0.021 | * | 54.2 | 4.123 | 0.047 | * | 52.7 | 4.903 | 0.031 | * |
| Origin × UV-B | 1 | 56.1 | 0.577 | 0.451 | 62.0 | 0.030 | 0.863 | 41.8 | 0.173 | 0.680 | 42.8 | 0.050 | 0.824 | ||||
| Origin × Water | 1 | 10.3 | 0.102 | 0.756 | 62.0 | 0.405 | 0.527 | 31.8 | 0.321 | 0.576 | 43.8 | 2.840 | 0.099 | . | |||
| UV-B × Water | 1 | 57.4 | 0.907 | 0.345 | 62.0 | 2.773 | 0.101 | 30.2 | 0.452 | 0.506 | 43.8 | 0.656 | 0.422 | ||||
| Origin × UV-B × Water | 1 | 10.1 | 1.161 | 0.306 | 62.0 | 0.173 | 0.679 | 30.0 | 0.818 | 0.373 | 46.0 | 0.037 | 0.849 | ||||
| Specific leaf area | |||||||||||||||||
| Origin | 1 | 59.0 | 0.402 | 0.528 | 20.4 | 0.080 | 0.780 | 20.8 | 3.013 | 0.097 | . | 18.1 | 1.193 | 0.289 | |||
| UV-B | 1 | 59.0 | 0.110 | 0.741 | 46.6 | 1.327 | 0.255 | 1.8 | 0.560 | 0.540 | 44.4 | 0.005 | 0.944 | ||||
| Water | 1 | 59.0 | 2.638 | 0.110 | 46.6 | 4.553 | 0.038 | * | 42.5 | 6.955 | 0.012 | * | 44.5 | 3.259 | 0.078 | . | |
| Initial leaf number (Covariate) | 1 | 59.0 | 19.424 | <0.001 | *** | 42.1 | 0.762 | 0.388 | 52.9 | 5.081 | 0.028 | * | 59.3 | 0.157 | 0.694 | ||
| Origin × UV-B | 1 | 59.0 | 0.062 | 0.805 | 47.5 | 0.003 | 0.957 | 41.0 | 1.401 | 0.243 | 44.3 | 0.993 | 0.324 | ||||
| Origin × Water | 1 | 59.0 | 0.165 | 0.686 | 47.6 | 0.552 | 0.461 | 42.6 | 0.405 | 0.528 | 44.4 | 10.525 | 0.002 | ** | |||
| UV-B × Water | 1 | 59.0 | 0.569 | 0.454 | 47.6 | 0.099 | 0.755 | 42.8 | 1.647 | 0.206 | 44.4 | 2.822 | 0.100 | . | |||
| Origin × UV-B × Water | 1 | 59.0 | 1.626 | 0.207 | 47.0 | 0.059 | 0.810 | 42.9 | 0.520 | 0.475 | 46.1 | 1.662 | 0.204 | ||||
| Source | dfN | Leaf Number | Leaf Length | Leaf Width | Proportion of Dead Leaves | ||||||||||||
| dfD | F | p | dfD | F | p | dfD | F | p | dfD | F | p | ||||||
| Origin | 1 | 15.61 | 10.01 | 0.0062 | ** | 34.3 | 56.710 | <0.001 | *** | 18.601 | 20.91 | <0.001 | *** | 15.9 | 3.120 | 0.096 | . |
| UV-B | 1 | 2.911 | 0.05 | 0.8427 | 4.9 | 2.240 | 0.196 | 3.059 | 2.33 | 0.2228 | 2.7 | 0.990 | 0.401 | ||||
| Water | 1 | 236.17 | 26.89 | <0.001 | *** | 231.8 | 46.890 | <0.001 | *** | 248.68 | 41.32 | <0.001 | *** | 252.9 | 11.510 | <0.001 | *** |
| Time | 1 | 200.54 | 1524.66 | <0.001 | *** | 115.5 | 558.000 | <0.001 | *** | 147.23 | 686.36 | <0.001 | *** | 169.2 | 2727.47 | <0.001 | *** |
| Origin × UV-B | 1 | 236.96 | 0.19 | 0.6646 | 232.4 | 0.160 | 0.693 | 249.35 | 1.77 | 0.185 | 253.6 | 0.360 | 0.547 | ||||
| Origin × Water | 1 | 236.14 | 0.27 | 0.6069 | 231.8 | 0.410 | 0.520 | 248.55 | 0.06 | 0.812 | 252.9 | 1.230 | 0.2684 | ||||
| UV-B × Water | 1 | 236.7 | 0.06 | 0.8051 | 232.2 | 0.750 | 0.389 | 249.18 | 0.82 | 0.3659 | 253.3 | 0.770 | 0.3811 | ||||
| Origin × Time | 1 | 200.54 | 0.2 | 0.657 | 115.5 | 38.860 | <0.001 | *** | 147.24 | 13.99 | <0.001 | *** | 169.2 | 0.040 | 0.8368 | ||
| UV-B × Time | 1 | 200.55 | 6.4 | 0.0122 | * | 115.5 | 0.070 | 0.7947 | 147.18 | 15.7 | <0.001 | *** | 169.2 | 0.320 | 0.570 | ||
| Water × Time | 1 | 200.55 | 33.43 | <0.001 | *** | 115.6 | 36.070 | <0.001 | *** | 147.3 | 42.43 | <0.001 | *** | 169.2 | 20.530 | <0.001 | *** |
| Origin × UV-B × Water | 1 | 236.66 | 2.77 | 0.0975 | . | 232.2 | 0.530 | 0.467 | 249.05 | 0.09 | 0.7702 | 253.4 | 0.310 | 0.577 | |||
| Origin × UV-B × Time | 1 | 200.55 | 0.04 | 0.8459 | 115.5 | 0.510 | 0.478 | 147.2 | 2.16 | 0.1435 | 169.2 | 2.930 | 0.089 | . | |||
| Origin × Water × Time | 1 | 200.54 | 1.3 | 0.2554 | 116.1 | 1.700 | 0.195 | 147.96 | 2.03 | 0.1564 | 169.2 | 0.150 | 0.703 | ||||
| UV-B × Water × Time | 1 | 200.55 | 0.05 | 0.8263 | 115.6 | 0.050 | 0.823 | 147.29 | 2.97 | 0.0871 | . | 169.2 | 0.070 | 0.785 | |||
| Origin × UV-B × Water × Time | 1 | 200.54 | 0.01 | 0.9082 | 116.1 | 0.870 | 0.353 | 147.95 | 0.17 | 0.6786 | 169.2 | 0.050 | 0.817 | ||||
| Source | dfN | Rosette Area | PSII Efficiency (Y) | Min. Chlorophyll Fluorescence | Max. Chlorophyll Fluorescence | ||||||||||||
| dfD | F | p | dfD | F | p | dfD | F | p | dfD | F | p | ||||||
| Origin | 1 | 20.841 | 26.55 | <0.001 | *** | 21.6 | 10.259 | 0.004 | ** | 20.5 | 10.079 | 0.005 | ** | 27.5 | 1.770 | 0.195 | |
| UV-B | 1 | 3.879 | 1.51 | 0.2889 | 217.0 | 2.368 | 0.125 | 3.0 | 0.115 | 0.756 | 2.3 | 1.240 | 0.367 | ||||
| Water | 1 | 255 | 43.67 | <0.001 | *** | 215.9 | 0.461 | 0.498 | 226.5 | 5.354 | 0.022 | * | 234.6 | 5.690 | 0.018 | * | |
| Time | 1 | 202.01 | 1351.2 | <0.001 | *** | 138.7 | 0.670 | 0.414 | 148.1 | 257.314 | <0.001 | *** | 170.9 | 420.720 | <0.001 | *** | |
| Origin × UV-B | 1 | 255.72 | 0.08 | 0.7768 | 217.2 | 1.239 | 0.267 | 228.1 | 0.52 | 0.472 | 235.9 | 6.490 | 0.011 | * | |||
| Origin × Water | 1 | 254.9 | 0.35 | 0.5528 | 215.9 | 0.472 | 0.493 | 226.3 | 2.293 | 0.131 | 234.2 | 2.330 | 0.128 | ||||
| UV-B × Water | 1 | 255.53 | 2.79 | 0.0961 | . | 216.5 | 0.015 | 0.903 | 227.2 | 2.295 | 0.131 | 235.2 | 3.040 | 0.082 | . | ||
| Origin × Time | 1 | 202.02 | 21.99 | <0.001 | *** | 138.7 | 2.588 | 0.110 | 148.1 | 2.839 | 0.361 | 170.9 | 8.300 | 0.004 | ** | ||
| UV-B × Time | 1 | 202.01 | 0.18 | 0.6752 | 138.6 | 5.691 | 0.018 | * | 148.0 | 15.478 | <0.001 | *** | 170.8 | 9.030 | 0.003 | ** | |
| Water × Time | 1 | 202.06 | 176.111 | <0.001 | *** | 138.6 | 16.625 | <0.001 | *** | 148.0 | 0.222 | 0.638 | 170.8 | 14.080 | <0.001 | *** | |
| Origin × UV-B × Water | 1 | 255.44 | 0.44 | 0.5059 | 216.5 | 2.947 | 0.087 | . | 227.0 | 0.823 | 0.365 | 234.8 | 0.820 | 0.367 | |||
| Origin × UV-B × Time | 1 | 202.01 | 0.69 | 0.4085 | 138.6 | 0.067 | 0.797 | 148.0 | 0.004 | 0.949 | 170.8 | 0.400 | 0.530 | ||||
| Origin × Water × Time | 1 | 202.3 | 3.82 | 0.0519 | . | 138.6 | 1.301 | 0.256 | 148.0 | 3.853 | 0.052 | . | 170.7 | 1.450 | 0.230 | ||
| UV-B × Water × Time | 1 | 202.06 | 0.12 | 0.733 | 138.6 | 1.778 | 0.185 | 148.0 | 6.593 | 0.011 | * | 170.8 | 2.120 | 0.147 | |||
| Origin × UV-B × Water × Time | 1 | 202.3 | 1.01 | 0.3152 | 138.6 | 0.434 | 0.511 | 148.0 | 1.119 | 0.292 | 170.7 | 0.170 | 0.679 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hock, M.; Plos, C.; Sporbert, M.; Erfmeier, A. Combined Effects of UV-B and Drought on Native and Exotic Populations of Verbascum thapsus L. Plants 2020, 9, 269. https://doi.org/10.3390/plants9020269
Hock M, Plos C, Sporbert M, Erfmeier A. Combined Effects of UV-B and Drought on Native and Exotic Populations of Verbascum thapsus L. Plants. 2020; 9(2):269. https://doi.org/10.3390/plants9020269
Chicago/Turabian StyleHock, Maria, Carolin Plos, Maria Sporbert, and Alexandra Erfmeier. 2020. "Combined Effects of UV-B and Drought on Native and Exotic Populations of Verbascum thapsus L." Plants 9, no. 2: 269. https://doi.org/10.3390/plants9020269
APA StyleHock, M., Plos, C., Sporbert, M., & Erfmeier, A. (2020). Combined Effects of UV-B and Drought on Native and Exotic Populations of Verbascum thapsus L. Plants, 9(2), 269. https://doi.org/10.3390/plants9020269





