Development of an In Vitro Propagation Protocol and a Sequence Characterized Amplified Region (SCAR) Marker of Viola serpens Wall. ex Ging
Abstract
1. Introduction
2. Results
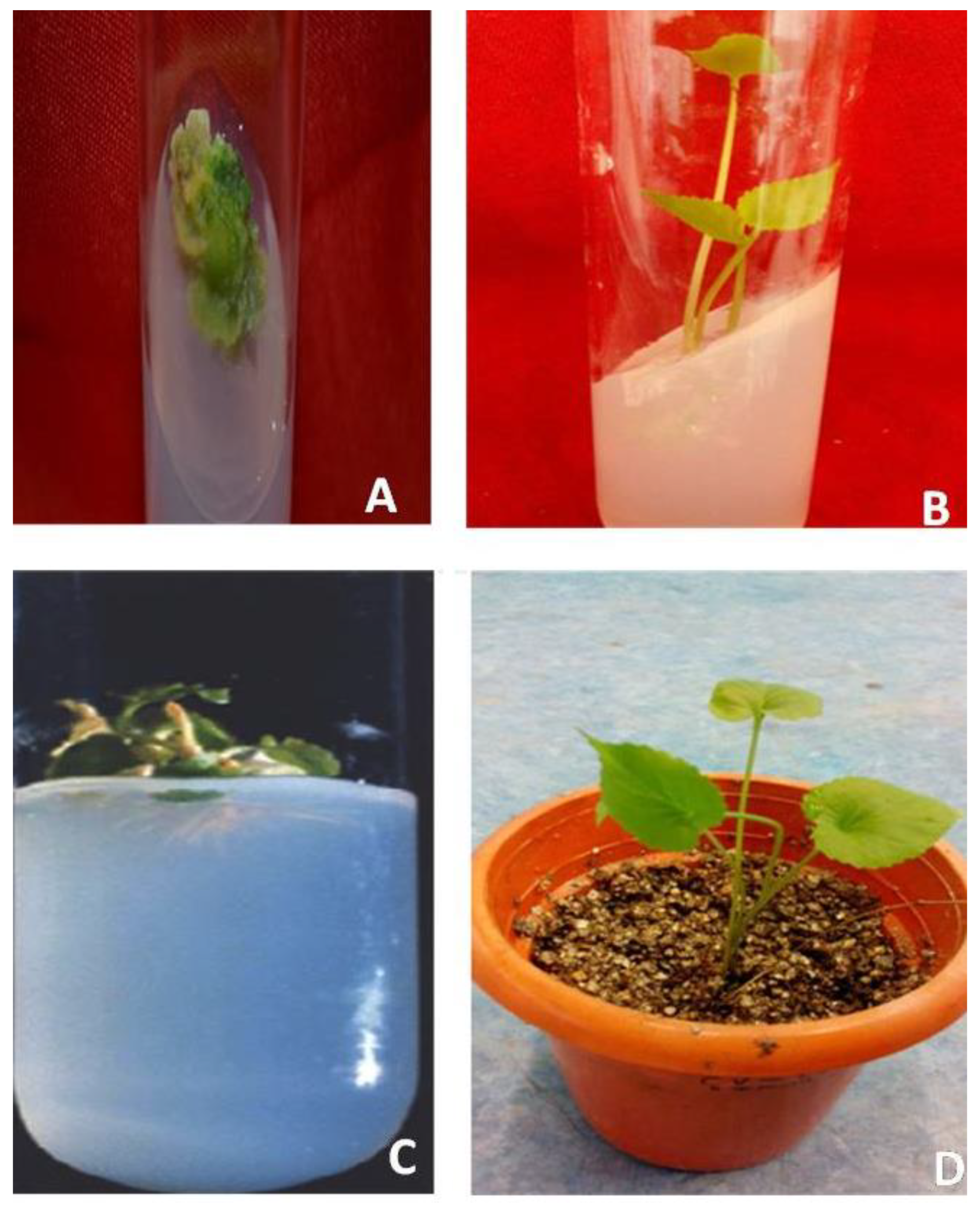
2.1. Callus Induction
2.2. Induction of Multiple Shoots
2.3. In Vitro Rooting as Influenced by Plant Growth Regulators
2.4. Acclimatization
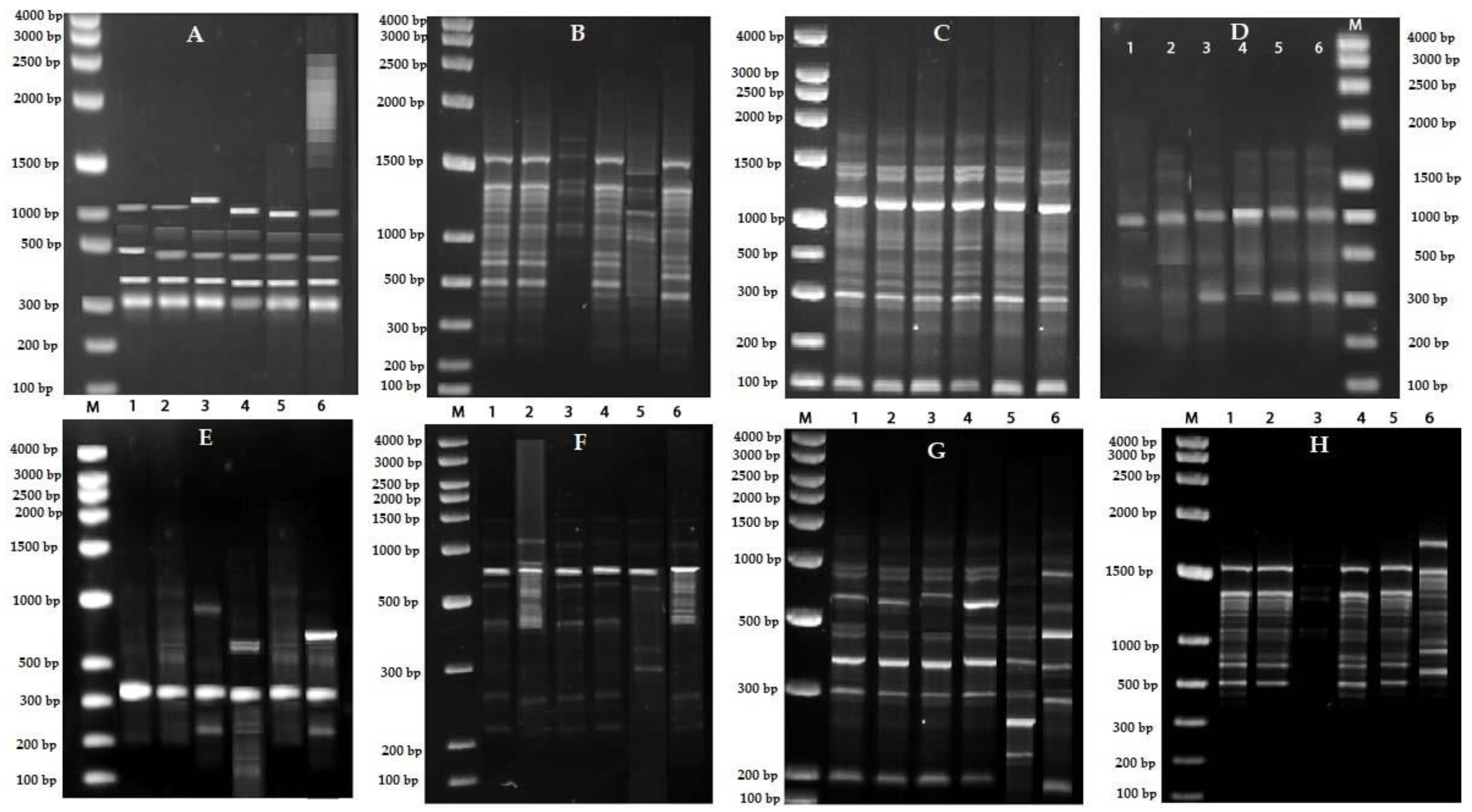
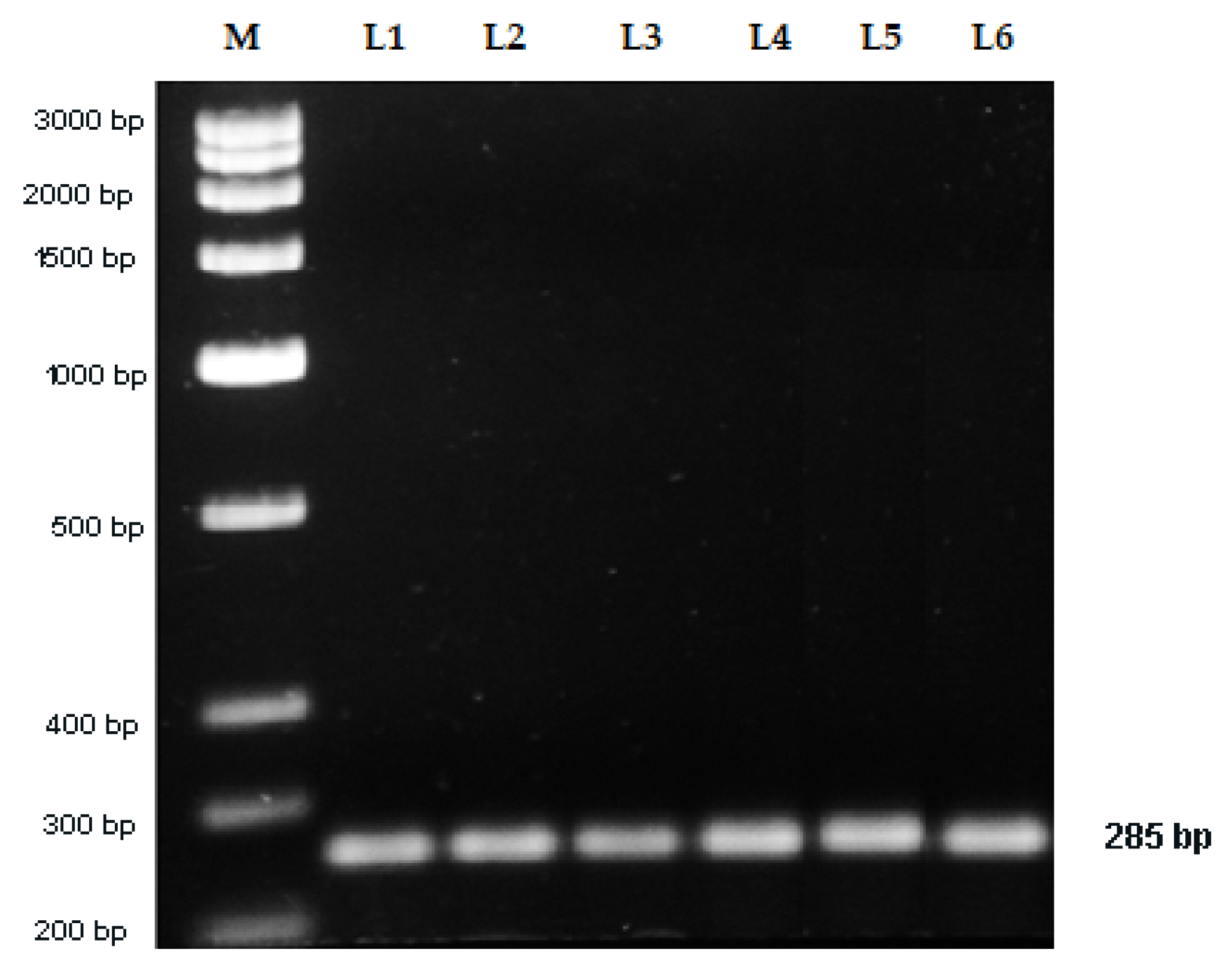
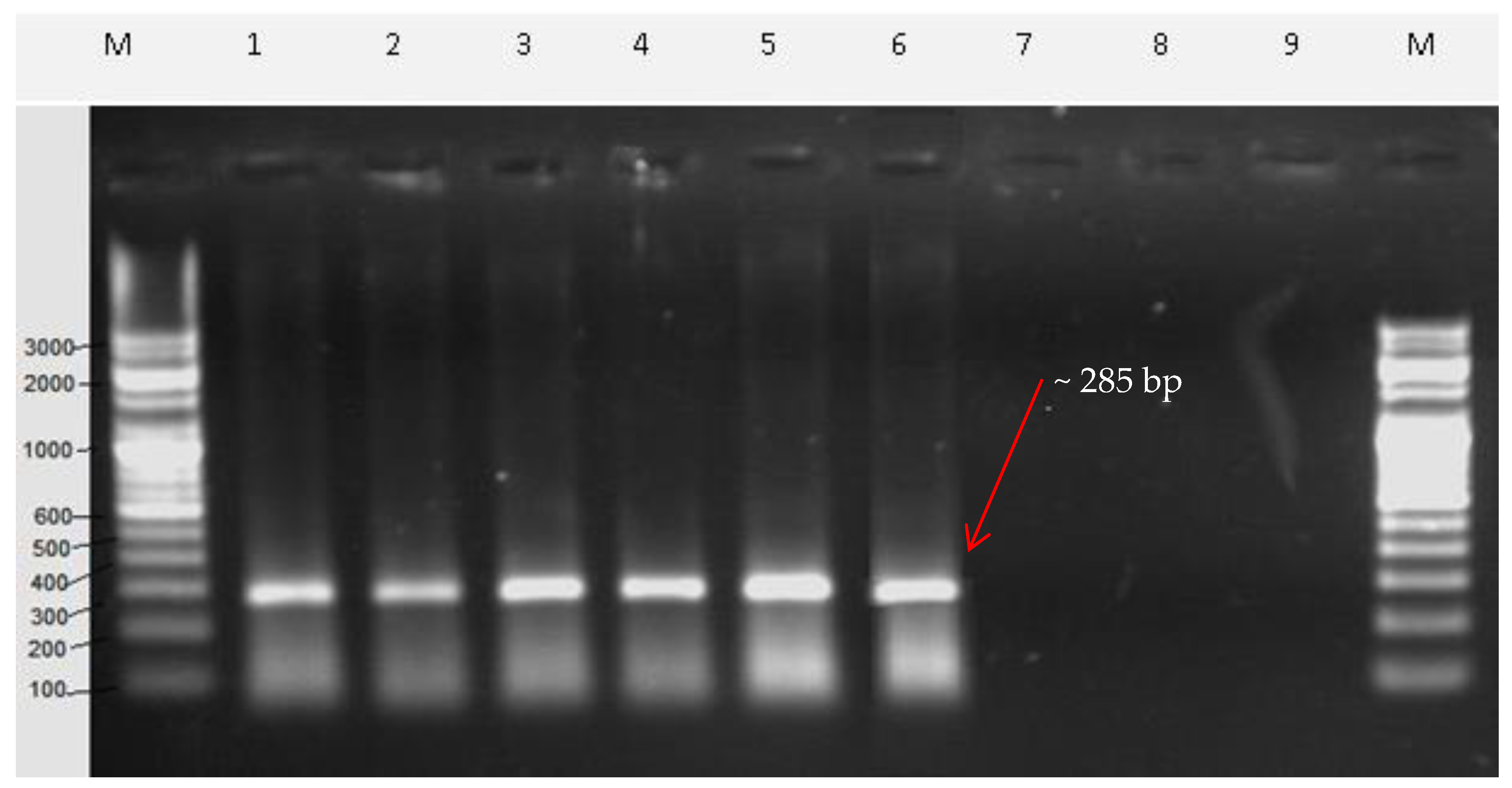
2.5. Sequence Characterized Amplified Region (SCAR) Development
3. Discussion
4. Materials and Methods
4.1. Collection of Plant Materials
4.2. Micropropagation of V. serpens
4.3. Development of Sequence Characterized Amplified Region (SCAR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anonymous. Wealth of India; Council of Scientific and Industrial Research: New Delhi, India, 1978; pp. 333–335. [Google Scholar]
- Kurup, P.N.; Ramadas, V.N.; Joshi, P. Hand Book of Medicinal Plants; Central Council for Research in Ayurveda and Siddha: New Delhi, India, 1979; pp. 188–189. [Google Scholar]
- Shastri, A.D. Bhaishajya Ratnavali of Govindadasa; Chawkhambha Sanskrit Sansthan: Varanasi, India, 2001; p. 858. [Google Scholar]
- Vishwakarma, U.R.; Gurav, A.M.; Sharma, P.C. Regeneration of multiple shoots from petiole callus of Viola serpens Wall. Pharm. Res. 2013, 5, 86–92. [Google Scholar]
- Dhar, U.; Kachroo, P. Alpine Flora of Kashmir Himalaya; Scientific Publishers: Jodhpur, India, 1983; pp. 86–87. [Google Scholar]
- Chandra, D.; Kohli, G.; Prasad, K.; Bisht, G.; Punetha, V.D.; Pandey, H.K. Chemical composition of the essential oil of Viola serpens from Bageshwar (Shama), Uttarakhad, India. J. Med. Plants Res. 2017, 11, 513–517. [Google Scholar]
- Kumar, A.; Kumari, M.; Mazumdar, R.S.; Dhewa, T. In vitro antibacterial activity of ethanolic extracts of Viola serpens and Morus nigra against pathogens isolated from patients suffering from jaundice. World J. Pharm. Res. 2015, 4, 889–898. [Google Scholar]
- Kumar, P.; Digvijay, S. Assessment of genetic diversity of Viola serpens Wall. In Himachal Pradesh using molecular markers. World. J. Pharm. Res. 2014, 3, 2716–2726. [Google Scholar]
- Hooker, J.D. The Flora of British India, Ranunculaceae to Sapindaceae; Reeve and Co. Ltd.: Kent, UK, 1875; p. 134. [Google Scholar]
- Tandon, P.; Kumaria, S.; Nongrum, L. Conservation and management of plant genetic resources of Northeast India. Ind. J. Trad. Knowl. 2009, 8, 29–34. [Google Scholar]
- Joshi, K.; Chavan, P.; Warude, D.; Patwardhan, B. Molecular markers in herbal drug technology. Curr. Sci. 2004, 87, 159–165. [Google Scholar]
- Tharachand, C.; Immanuel, S.C.; Mythili, M.N. Molecular markers in characterization of medicinal plants: An overview. Res. Plant Biol. 2012, 2, 1–12. [Google Scholar]
- Leela, T.; Suhas, P.W.; Seetha, K.; Naresh, B.; Thakur, K.S.; David, A.H.; Devi, P.; Rajeev, K.V. AFLP-based molecular characterization of an elite germplasm collection of Jatropha curcas L., a biofuel plant. Plant Sci. 2009, 176, 505–513. [Google Scholar]
- Haberlandt, G. Kulturversuche mit isolierten Pflanzenzellen. Sitzungsber K Preuss Akad Wiss Wien. Math Nat. 1902, 111, 69–92. [Google Scholar]
- Ahmed, M.R.; Anis, M.; Alatar, A.A.; Faisal, M. In vitro clonal propagation and evaluation of genetic fidelity using RAPD and ISSR marker in micropropagated plants of Cassia alata L.: A potential medicinal plant. Agrofor. Syst. 2017, 91, 637–647. [Google Scholar] [CrossRef]
- Javed, S.B.; Alatar, A.A.; Anis, M.; Faisal, M. Synthetic seeds production and germination studies, for short term storage and long distance transport of Erythrina variegata L.: A multipurpose tree legume. Ind. Crop. Prod. 2017, 105, 41–46. [Google Scholar] [CrossRef]
- Khan, M.I.; Ahmad, N.; Anis, M.; Alatar, A.A.; Faisal, M. In vitro conservation strategies for the Indian willow (Salix tetrasperma Roxb.), a vulnerable tree species via propagation through synthetic seeds. Biocatal. Agric. Bio. 2018, 16, 17–21. [Google Scholar] [CrossRef]
- Chalageri, G.; Babu, U.V. In vitro plant regeneration via petiole callus of Viola patrinii and genetic fidelity assessment using RAPD markers. Turk. J. Bot. 2013, 36, 358–368. [Google Scholar]
- Tadahiko, S.; Kwon, O.C.; Miyake, H.; Taniguchi, T.; Maeda, E. Regeneration of Plantlets from petiole callus of wild Viola (Viola patrinii DC.). Plant Cell Rep. 1995, 14, 768–772. [Google Scholar]
- Slazak, B.; Sliwinska, E.; Saługa, M.; Ronikier, M.; Bujak, J.; Słomka, A.; Go¨ransson, U.; Kuta, E. Micropropagation of Viola uliginosa (Violaceae) for endangered species conservation and for somaclonal variation-enhanced cyclotide biosynthesis. Plant Cell Tissue Organ Cult. 2015, 120, 179. [Google Scholar] [CrossRef]
- Soni, M.; Kaur, R. Rapid in vitro propagation, conservation and analysis of genetic stability of Viola pilosa. Physiol. Mol. Biol. Plant. 2014, 20, 95–101. [Google Scholar] [CrossRef][Green Version]
- Wijowska, M.; Kuta, E.; Przywara, L. In vitro culture of unfertilized ovules of Viola odorata L. Acta. Biol. Crac. Ser. Bot. 1999, 17, 1–11. [Google Scholar]
- Moon, B.C.; Choo, B.K.; Cheon, M.S.; Yoon, T.; Ji, Y.; Kim, B.B.; Lee, A.Y.; Kim, H.K. Rapid molecular authentication of three medicinal plant species, Cynanchum wilfordii, Cynanchum auriculatum, and Polygonum multiflorum (Fallopia multiflorum) by the development of RAPD-derived SCAR markers and multiplex-PCR. Plant Bio. Rep. 2010, 4, 1–7. [Google Scholar] [CrossRef]
- Adinolfi, B.; Chicca, A.; Martinotti, E.; Breschi, M.C.; Nieri, P. Sequence Characterized amplified region (SCAR) analysis on DNA from three medicinal Echinacea species. Fitoterapia 2007, 78, 43–45. [Google Scholar] [CrossRef]
- Devaiah, K.M.; Venkatasubramaniam, P. Development of SCAR marker for authentication of Pueraria tuberosa (Roxb. ex. Wild) DC. Curr. Sci. 2008, 94, 1306–1309. [Google Scholar]
- Mei, Z.; Zhou, B.; Wei, C.; Cheng, J.; Imani, S.; Chen, H.; Fu, J. Genetic authentication of Gardenia jasminoides Ellis var. grandiflora Nakai by improved RAPD-derived DNA markers. Molecules 2015, 20, 20219–20229. [Google Scholar] [CrossRef] [PubMed]
- Theerakulpisut, P.; Kanawpee, N.; Maensiri, D.; Bunnag, S.; Chantaranothai, P. Development of species-specific SCAR markers for identification of three medicinal species of Phyllanthus. J. Sys. Evol. 2008, 46, 614–621. [Google Scholar]
- Dhanya, K.; Syamkumar, S.; Siju, B.; Sasikumar, B. SCAR markers for adulterant detection in ground chilli. Br. Food J. 2011, 113, 656–668. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, Y.; Sun, W.; Wen, X.; Milne, R. High genetic diversity and low differentiation of Michelia coriacea (Magnoliaceae), a critically endangered endemic in southeast Yunnan, China. Int. J. Mol. Sci. 2012, 13, 4396–4411. [Google Scholar] [CrossRef]
- Sairkar, P.K.; Sharma, A.; Shukla, N.P. SCAR Marker for identification and discrimination of Commiphora wightii and C. myrrha. Mol. Biol. Int. 2016, 2016, 1482796. [Google Scholar] [CrossRef][Green Version]
- Feng, S.; Zhu, Y.; Yu, C.; Jiao, K.; Jiang, M.; Lu, J.; Shen, C.; Ying, Q.; Wang, H. Development of species-specific SCAR markers, based on a SCoT analysis to authenticate Physalis (Solanaceae) species. Front. Genet. 2018, 9, 192. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- McClelland, M.; Mathieu, D.F.; Welsh, J. RNA fingerprinting and differential display using arbitrarily primed PCR. Trends Gene. 1995, 11, 242–246. [Google Scholar] [CrossRef]


| Plant Growth Regulators | Concentrations (mg/L) | Callusing | No. of Days | Percentage of Explants Responded (%) |
|---|---|---|---|---|
| MS Medium | - | - | 50–60 Days | 0 |
| MS + NAA | 0.25 0.50 0.75 0.85 1.00 | - - - - - | 50–60 days | 0 |
| MS + BAP | 0.10 0.25 0.50 0.75 1.00 | - - - - - | 50–60 days | 0 |
| MS + 2,4-D | 0.10 0.11 0.25 0.35 0.40 0.50 | - - - + - | 25 days | 10 |
| MS + KIN | 0.10 0.25 0.50 0.75 1.00 | - - - - - | 50–60 days | 0 |
| MS + BAP + 2,4-D | 2.0 + 0.11 2.0 + 0.12 2.0 + 0.13 2.0 + 0.14 2.0 + 0.15 | + - + + + | 45 days | 20 - 88.5 75 60 |
| MS + BAP + IAA | 2.0 + 0.25 2.0 + 0.50 2.0 + 0.50 2.0 + 0.75 2.0 + 1.00 | - - - - + | 25 days | - - - - 12 |
| MS + BAP + 2,4- D + KIN | 2.0 + 1.0 + 0.1 2.0 + 1.0 + 0.2 2.0 + 1.0 + 0.3 2.0 + 1.0 + 0.4 2.0 + 1.0 + 0.5 | + + - - + | 50–60 days | 40 35 - - 45 |
| MS + NAA + KIN | 2.0 + 0.8 2.2 + 0.8 2.3 + 0.9 2.0 + 0.5 2.5 + 0.5 | + + - - - | 40 days | 45 38 - - - |
| Plant Growth Regulators | Concentrations (mg/L) | Number of Shoots after Fifth Subculture (Mean ± SE) | Shoot Length (cm) (Mean ± SE) | Frequency of Shoots (%) |
|---|---|---|---|---|
| MS + NAA | 0.10 | - | - | - |
| 0.20 | - | - | - | |
| 0.25 | - | - | - | |
| 0.50 | 1.03 ± 0.34 | 1.02 ± 0.05 | 6 | |
| MS + KIN | 1.00 | - | - | - |
| 1.50 | - | - | - | |
| 1.75 | - | - | - | |
| 2.50 | - | - | - | |
| MS + BAP | 1.00 | - | - | - |
| 1.25 | - | - | - | |
| 1.50 | 2.15 ± 0.48 | 1.85 ± 0.20 | 8 | |
| 2.00 | 1.06 ± 0.67 | 2.07 ± 0.31 | 6 | |
| MS + NAA + BAP | 0.50 + 1.00 | 3.33 ± 0.33 | 1.05 ± 0.18 | 10 |
| 0.50 + 1.50 | 5.60 ± 0.48 | 3.34 ± 0.27 | 49 | |
| 0.50 + 1.75 | 3.50 ± 0.22 | 2.45 ± 0.14 | 15 | |
| 0.50 + 2.00 | 3.98 ± 0.43 | 2.67 ± 0.20 | 17 | |
| MS + NAA + KIN | 0.50 + 1.50 | 3.25 ± 0.20 | 2.25 ± 0.12 | 12 |
| 0.50 + 1.75 | 3.80 ± 0.29 | 2.56 ± 0.11 | 23 | |
| 0.50 + 2.00 | 4.20 ± 0.32 | 2.80 ± 0.12 | 36 | |
| 0.50 + 2.25 | 12.08 ± 0.79 | 5.96 ± 0.25 | 86 |
| Plant Growth Regulators | Concentration | Number of Roots/Explants (Mean ± SE) | Root Length (cm) (Mean ± SE) | Rooting Response (%) |
|---|---|---|---|---|
| MS plain media | - | - | - | - |
| IAA | 0.5 | - | - | - |
| 1.0 | - | - | - | |
| 1.5 | - | - | - | |
| 2.0 | 1.85 ± 0.37 | 0.71 ± 0.05 | 34 | |
| 2.5 | 1.50 ± 0.21 | 1.370 ± 0.10 | 29 | |
| IBA | 0.5 | - | - | - |
| 1.0 | 1.19 ± 0.49 | 0.51 ± 0.05 | 64 | |
| 1.5 | 2.80 ± 0.50 | 1.06 ± 0.08 | 56 | |
| 2.0 | 5.67 ± 3.70 | 1.87 ± 0.32 | 81 | |
| 2.5 | 2.50 ± 0.29 | 0.65 ± 0.33 | 65 |
| Plant Name | Collections | Source | Altitude (m) |
|---|---|---|---|
| V. serpens | V1 | Jammu, Jammu and Kashmir | 2530 |
| V. serpens | V2 | Joginder Nagar, Himachal Pradesh | 2286 |
| V. serpens | V3 | Kumaon, Uttarakhand | 1789 |
| V. serpens | V4 | Kashmir, Jammu and Kashmir | 2530 |
| V. serpens | V5 | Tehri Garhwal, Uttarakhand | 1500 |
| V. serpens | V6 | Palampur, Himachal Pradesh | 1472 |
| SCAR Primers | No. of Base | Sequences (5ʹ–3ʹ) | G + C Content (%) | Annealing Temperature (°C) | Expected Product Size (bp) |
|---|---|---|---|---|---|
| Vio F | 20 | TAGCCTACAGTGCCCTTACG | 55 | 58.89 | 285 |
| Vio R | 20 | AATCGCACAGTCACCCAGTA | 50 | 59.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jha, S.R.; Naz, R.; Asif, A.; Okla, M.K.; Soufan, W.; Al-Ghamdi, A.A.; Ahmad, A. Development of an In Vitro Propagation Protocol and a Sequence Characterized Amplified Region (SCAR) Marker of Viola serpens Wall. ex Ging. Plants 2020, 9, 246. https://doi.org/10.3390/plants9020246
Jha SR, Naz R, Asif A, Okla MK, Soufan W, Al-Ghamdi AA, Ahmad A. Development of an In Vitro Propagation Protocol and a Sequence Characterized Amplified Region (SCAR) Marker of Viola serpens Wall. ex Ging. Plants. 2020; 9(2):246. https://doi.org/10.3390/plants9020246
Chicago/Turabian StyleJha, Shipra Rani, Ruphi Naz, Ambreen Asif, Mohammad K. Okla, Walid Soufan, Abdullah A. Al-Ghamdi, and Altaf Ahmad. 2020. "Development of an In Vitro Propagation Protocol and a Sequence Characterized Amplified Region (SCAR) Marker of Viola serpens Wall. ex Ging" Plants 9, no. 2: 246. https://doi.org/10.3390/plants9020246
APA StyleJha, S. R., Naz, R., Asif, A., Okla, M. K., Soufan, W., Al-Ghamdi, A. A., & Ahmad, A. (2020). Development of an In Vitro Propagation Protocol and a Sequence Characterized Amplified Region (SCAR) Marker of Viola serpens Wall. ex Ging. Plants, 9(2), 246. https://doi.org/10.3390/plants9020246





