Physiological and Biochemical Mechanisms Mediated by Allelochemical Isoliquiritigenin on the Growth of Lettuce Seedlings
Abstract
1. Introduction
2. Results
2.1. Synthesis of Isoliquiritigenin
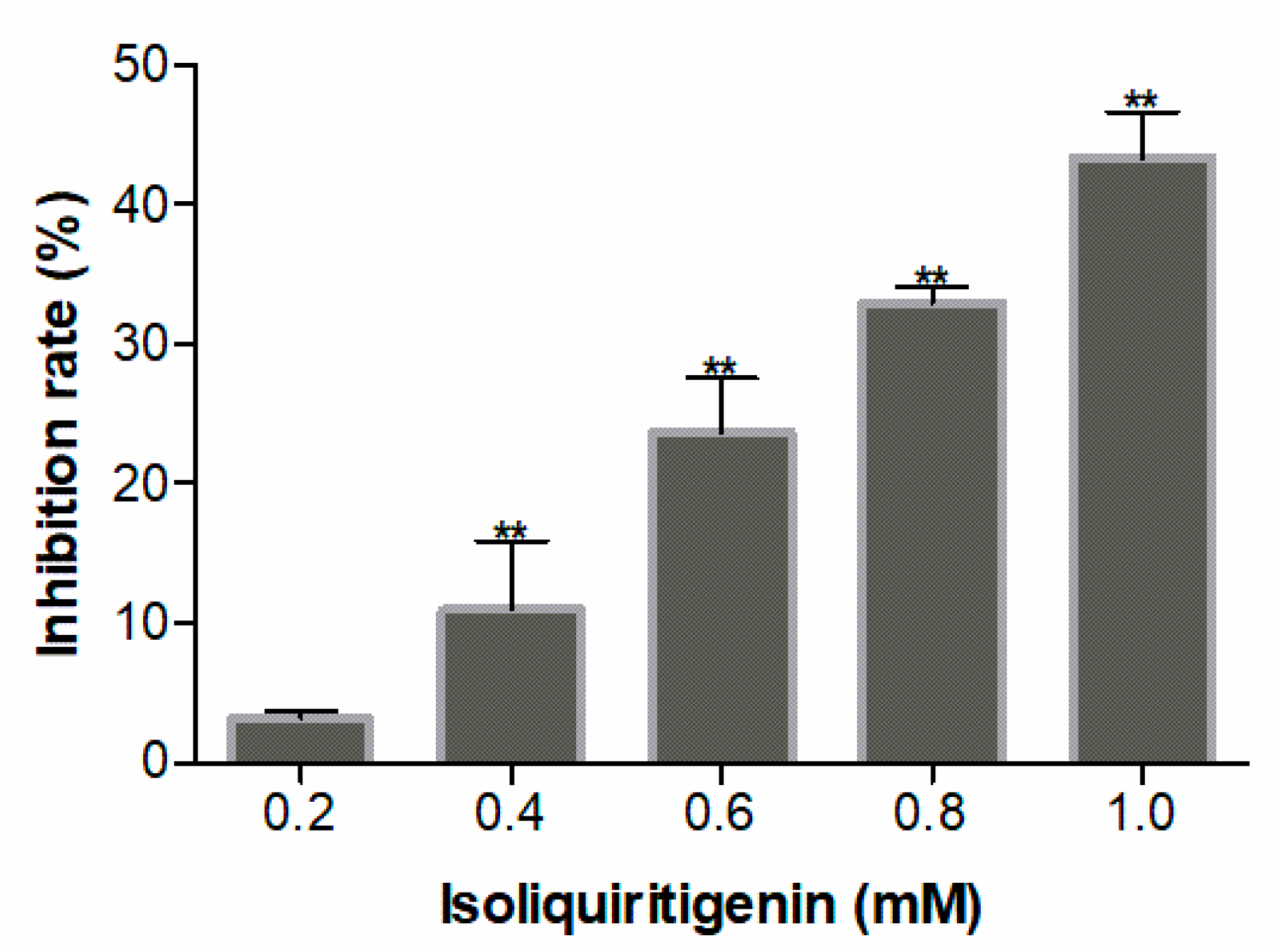
2.2. Allelopathic Effects of Isoliquiritigenin on Lettuce Germination and Growth
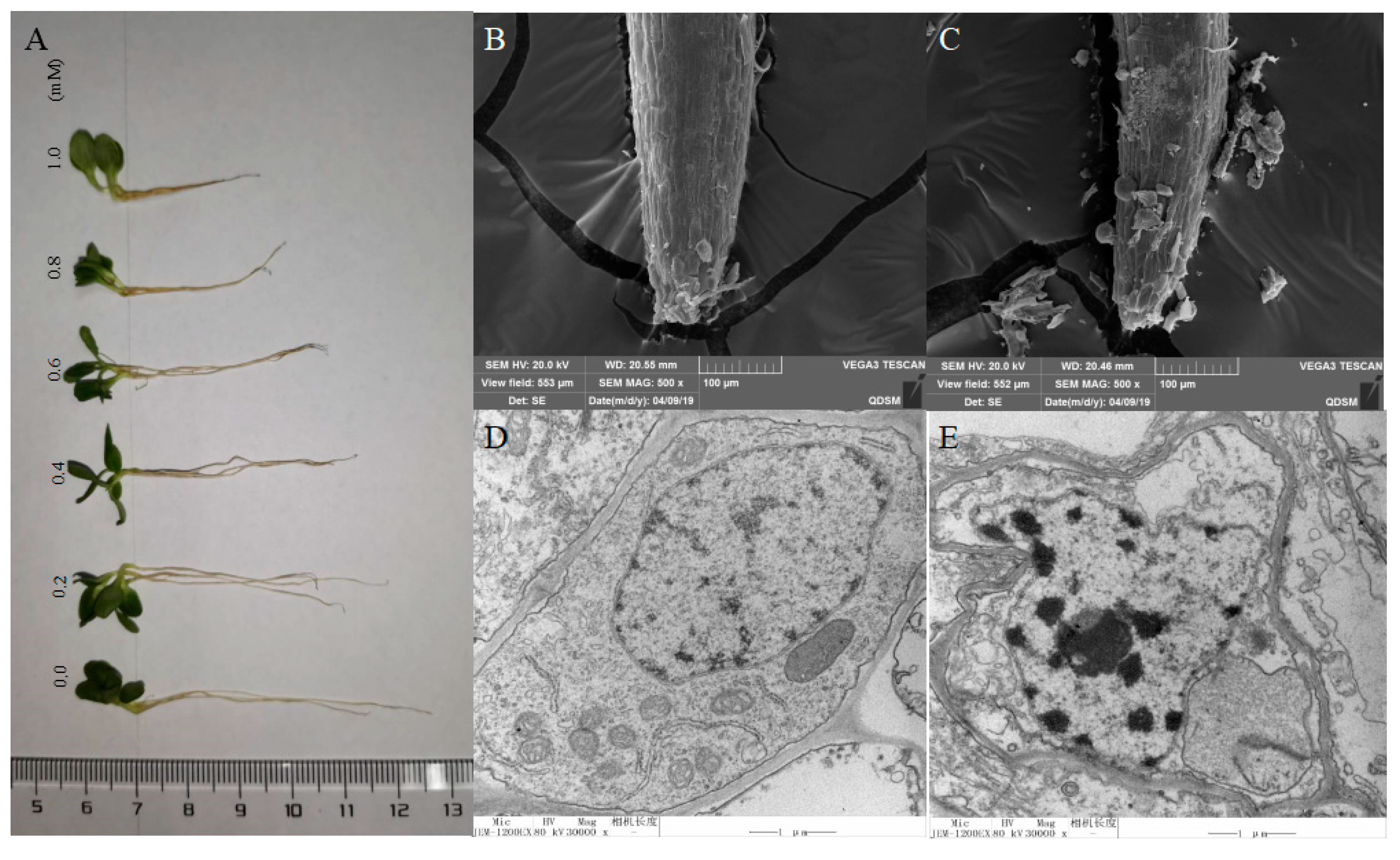
2.3. Morphology Analysis
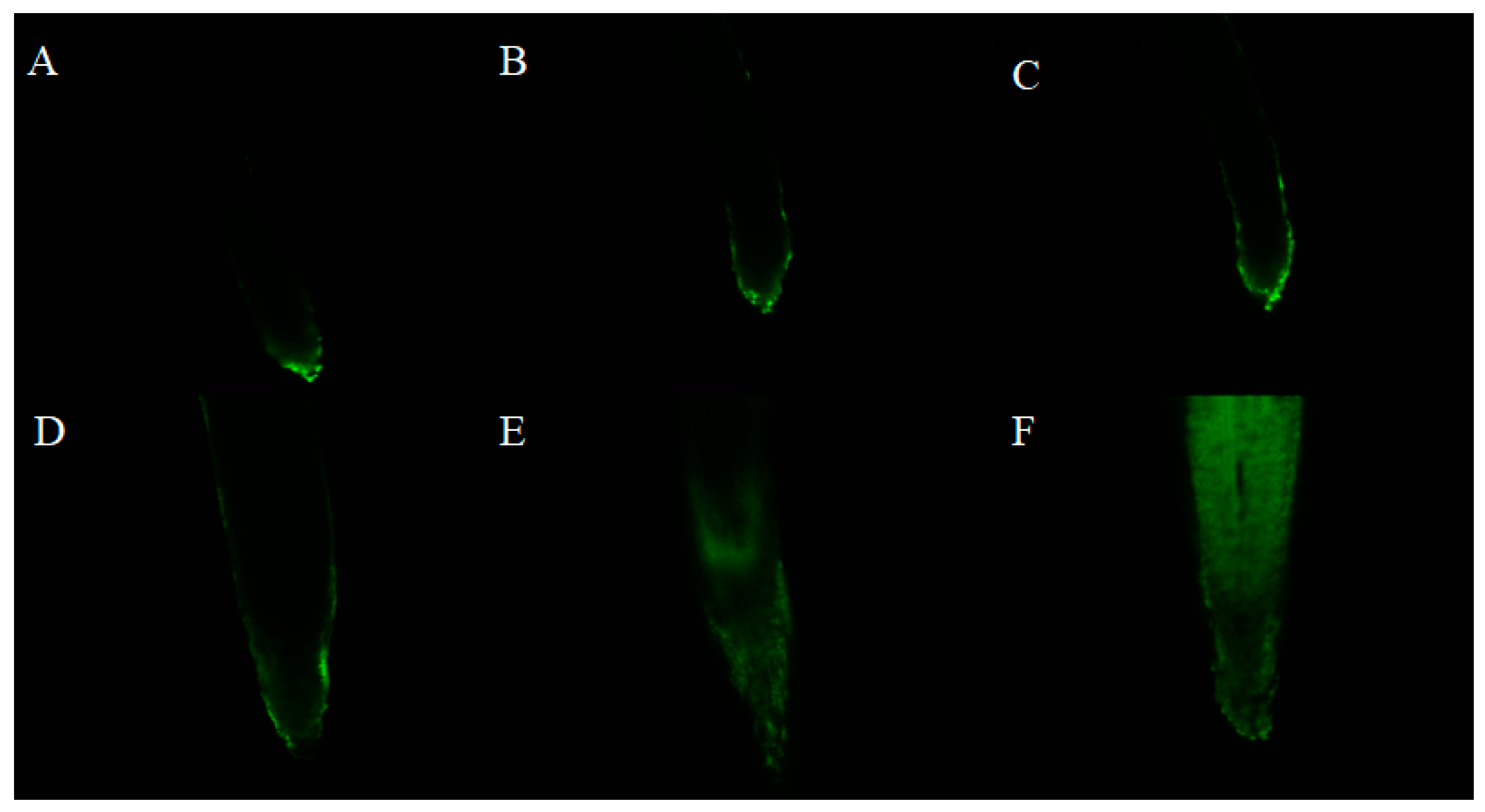
2.4. Effect of Isoliquiritigenin on ROS Production
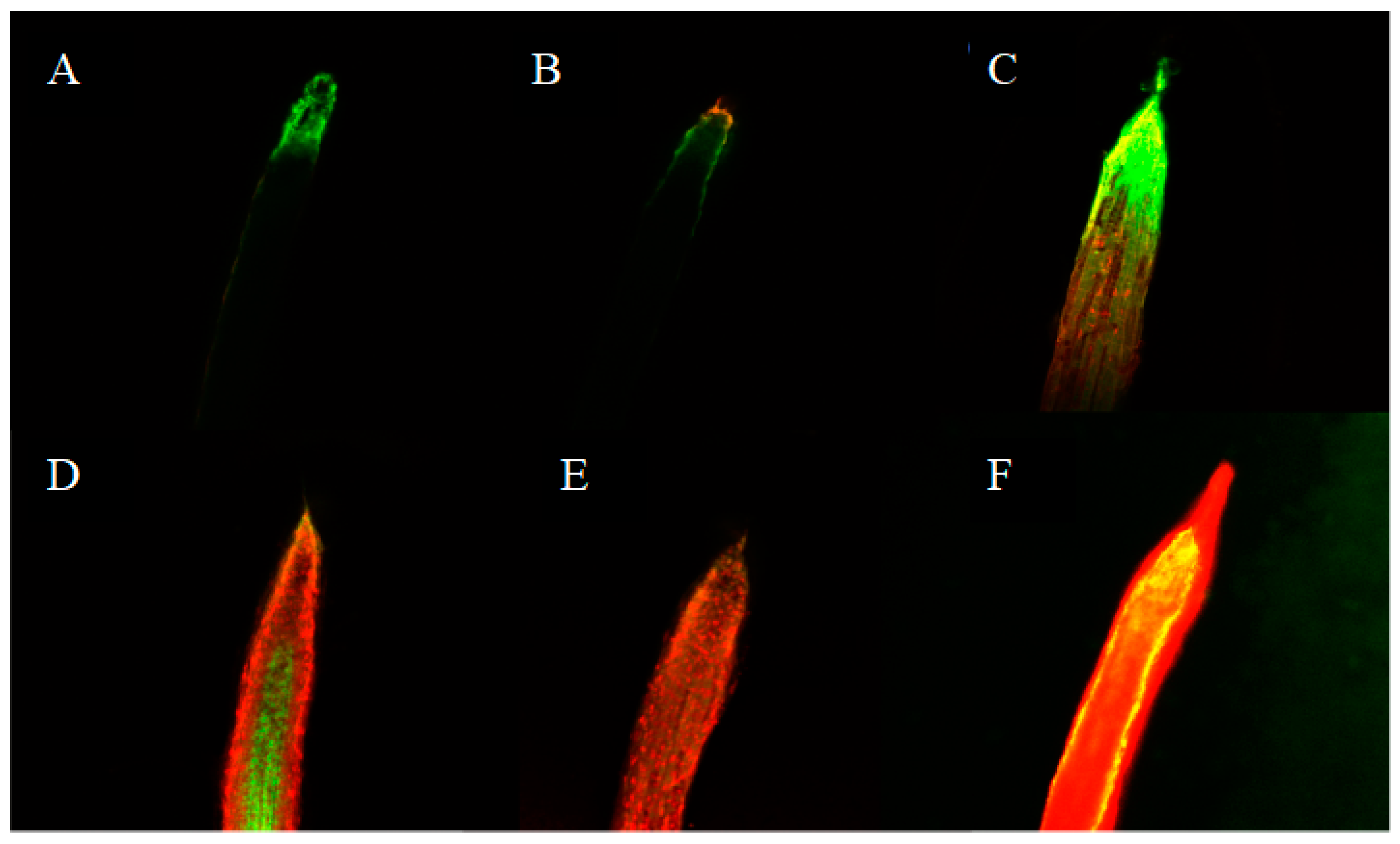
2.5. Cell Viability in Radicle Tips of Lettuce Treated with Isoliquiritigenin
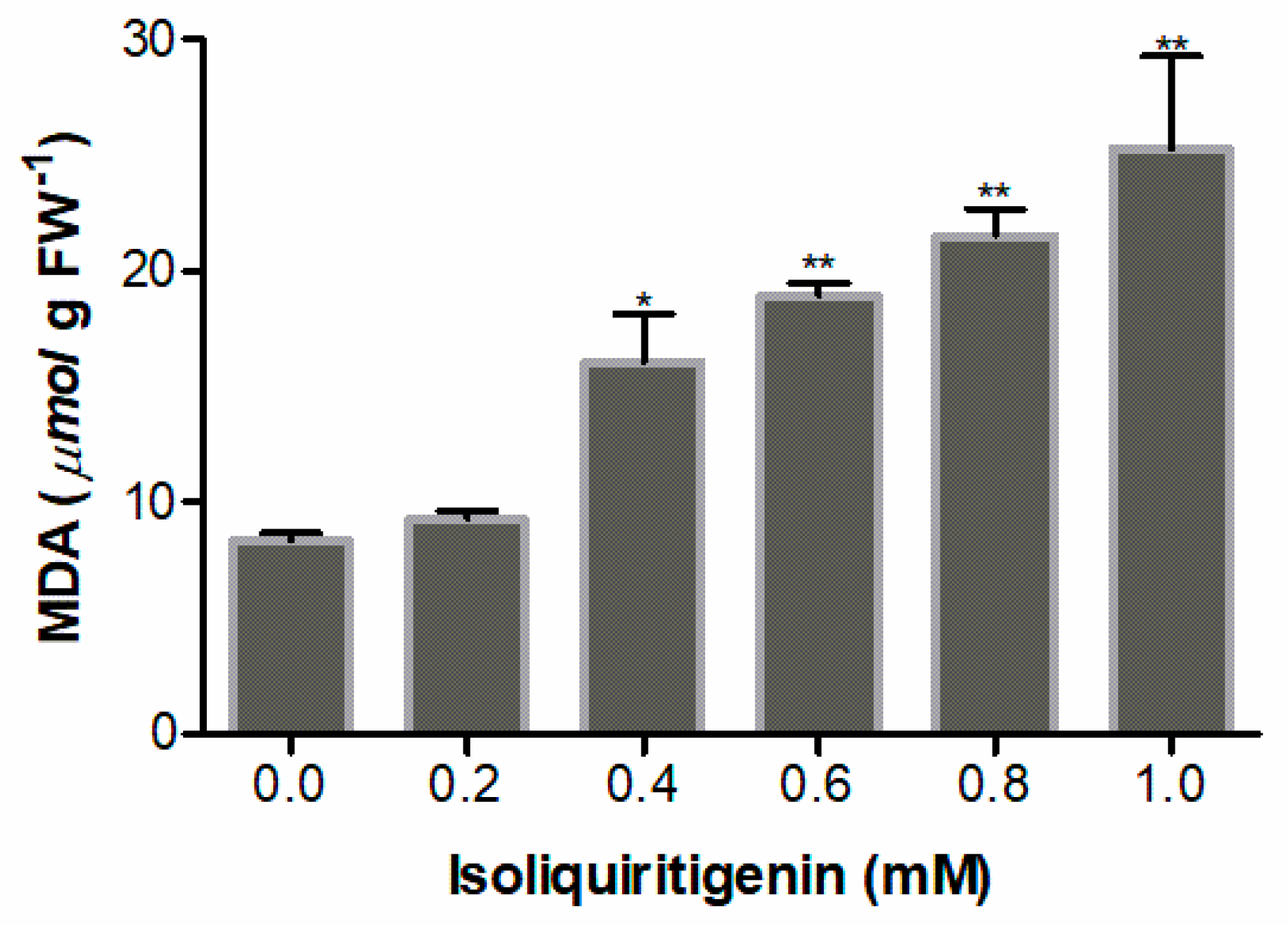
2.6. Effect of Isoliquiritigenin on Lipid Peroxidation
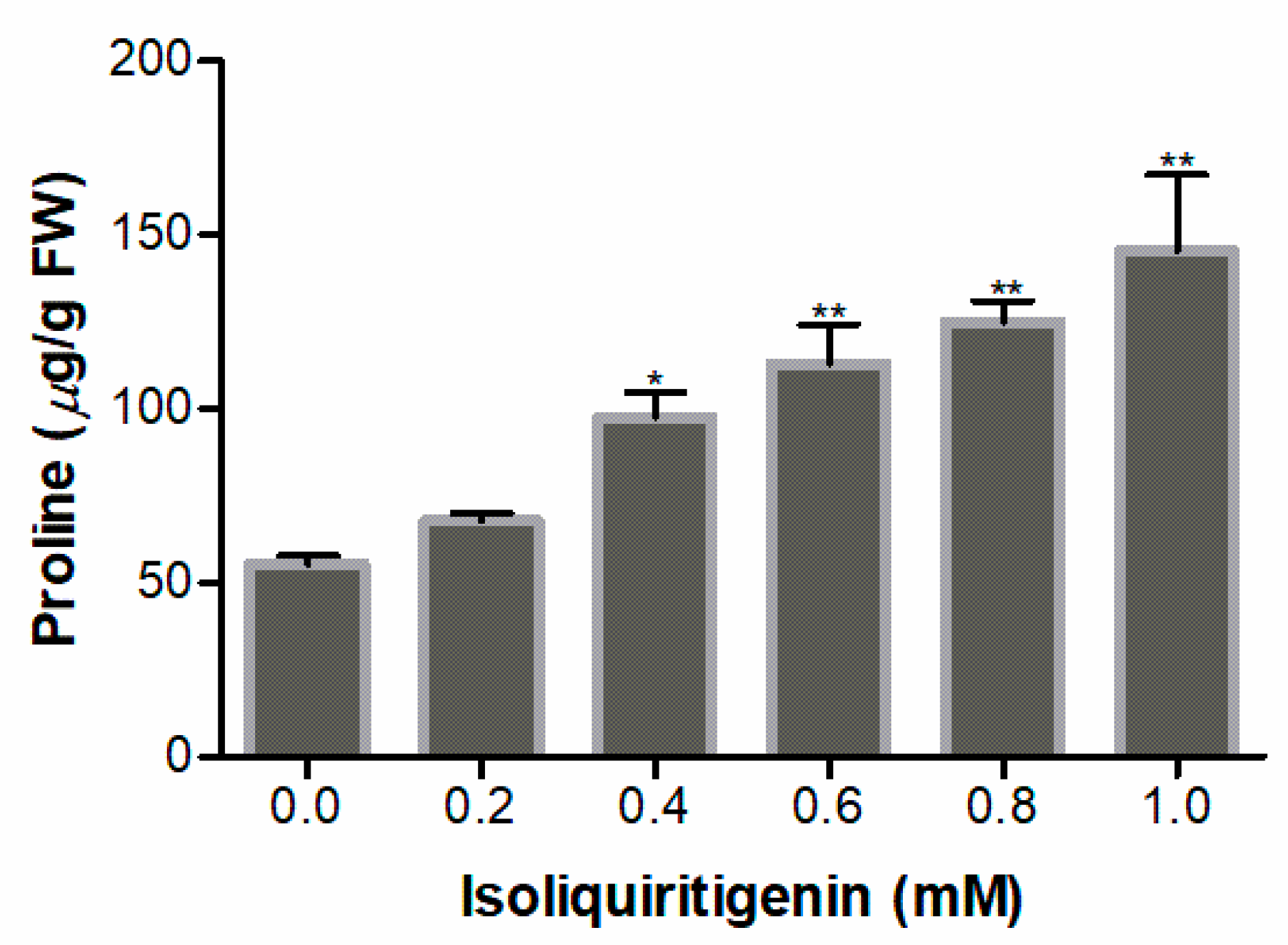
2.7. Effect of Isoliquiritigenin on Free Proline
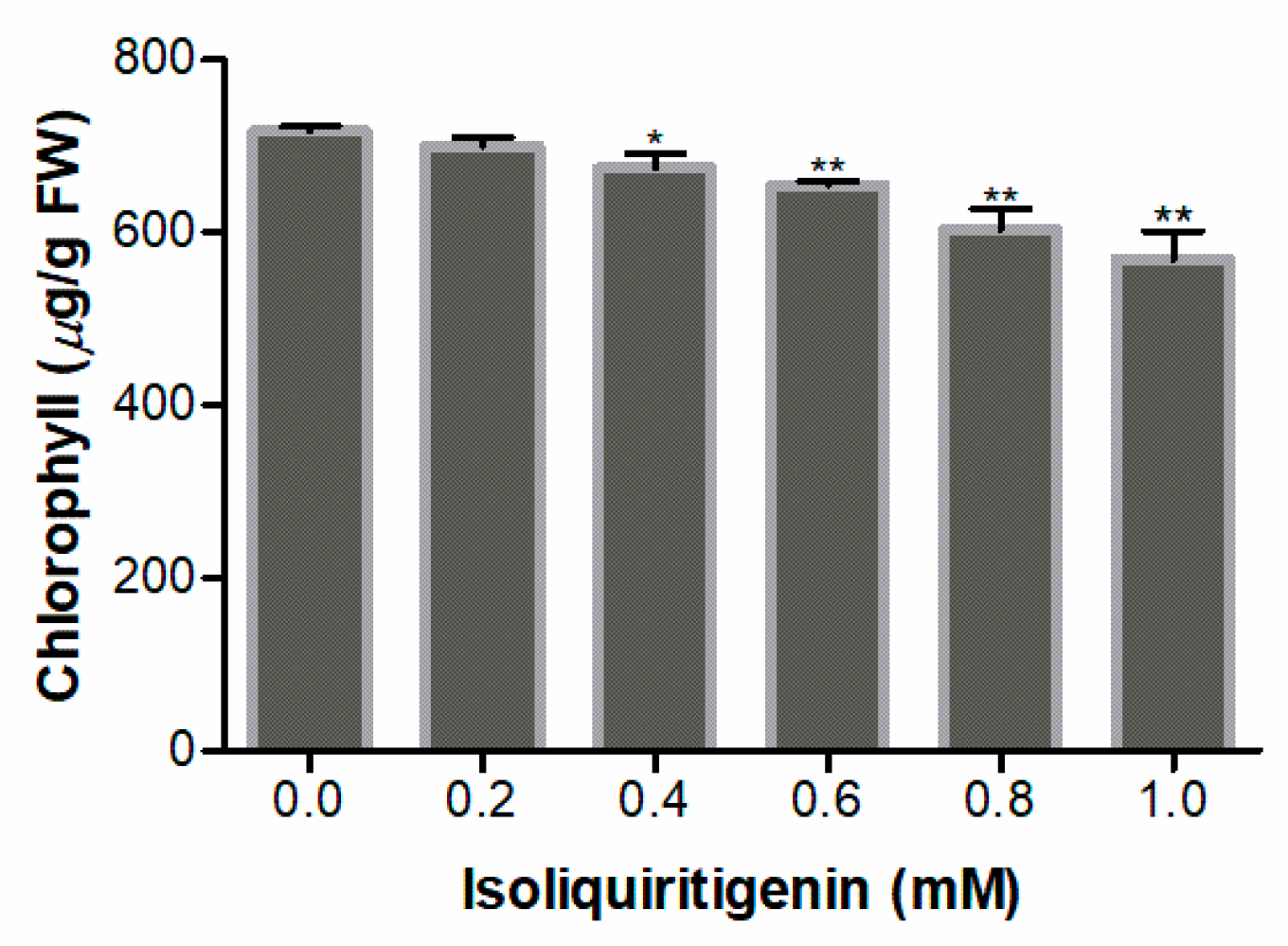
2.8. Effect of Isoliquiritigenin on Chlorophyll
3. Discussion
4. Materials and Methods
4.1. General
4.2. Synthesis of Isoliquiritigenin
4.3. Evaluation of the Allelopathic Effects of Isoliquiritigenin on Lettuce Growth
4.4. Determination of Cell Viability
4.5. Determination of O2-
4.6. Determination of Lipid Peroxidation
4.7. Determination of Free Proline
4.8. Determination of H2O2
4.9. Determination of Chlorophyll
4.10. Morphological Examination
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, V.G.; Krutovsky, K.V.; Shestibratov, K.A. Fell upas sits, the hydra-tree of death (dagger), or the phytotoxicity of trees. Molecules 2019, 24, 1636. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, B.K.; Ghimire, B.; Yu, C.Y.; Chung, I.M. Allelopathic and autotoxic effects of Medicago sativa-derived allelochemicals. Plants 2019, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.H.; Xuan, T.D.; Khanh, T.D.; Tran, H.D.; Trung, N.T. Allelochemicals and signaling chemicals in plants. Molecules 2019, 24, 2737. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhao, S.; Yang, S.; Lin, X.; He, X.; Wei, X.; Song, Q.; Li, R.; Fu, C.; Zhang, J.; et al. An “essential herbal medicine”-licorice: A review of phytochemicals and its effects in combination preparations. J. Ethnopharmacol. 2019, 19, 112439. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.; Dawid, C.; Peters, V.; Hofmann, T. Saponins from european licorice roots (Glycyrrhiza glabra). J. Nat. Prod. 2018, 81, 1734–1744. [Google Scholar] [CrossRef]
- Ren, X.; Yan, Z.Q.; He, X.F.; Li, X.Z.; Qin, B. Allelochemicals from rhizosphere soils of Glycyrrhiza uralensis Fisch: Discovery of the autotoxic compounds of a traditional herbal medicine. Ind. Crop. Prod. 2017, 97, 302–307. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Kim, J.H.; Park, S.J.; Chang, J.S.; Rho, M.C.; Bae, K.H.; Park, K.H.; Lee, W.S. Inhibition of neuraminidase activity by polyphenol compounds isolated from the roots of Glycyrrhiza uralensis. Bioorg. Med. Chem. Lett. 2010, 3, 971–974. [Google Scholar] [CrossRef]
- Baker, C.J.; Mock, N.M. An improved method for monitoring cell death in cell suspension and leaf disc assays using Evans blue. Plant Cell Tiss. Org. 1994, 39, 7–12. [Google Scholar] [CrossRef]
- Peng, F.; Du, Q.H.; Peng, C.; Wang, N.; Tang, H.L.; Xie, X.M.; Shen, J.G.; Chen, J.P. A review: The pharmacology of isoliquiritigenin. Phytother. Res. 2015, 29, 969–977. [Google Scholar] [CrossRef]
- Sánchez-Moreiras, A.M.; de la Peña, T.C.; Reigosa, M.J. The natural compound benzoxazolin-2(3H)-one selectively retards cell cycle in lettuce root meristems. Phytochemistry 2008, 69, 2172–2179. [Google Scholar] [CrossRef] [PubMed]
- Soltys, D.; Rudzińska-Langwald, A.; Gniazdowska, A.; Wiśniewska, A.; Bogatek, R. Inhibition of tomato (Solanum lycopersicum L.) root growth by cyanamide is due to altered cell division, phytohormone balance and expansin gene expression. Planta 2012, 236, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Huang, T.C.; Shieh, T.M.; Wu, C.H.; Lin, L.C.; Hsia, S.M. Isoliquiritigenin induces autophagy and inhibits ovarian cancer cell growth. Int. J. Mol. Sci. 2017, 18, 2025. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, J.E.; Chae, I.G.; Park, G.; Lee, S.; Chun, K.S. Isoliquiritigenin inhibits the proliferation of human renal carcinoma Caki cells through the ROS-mediated regulation of the Jak2/STAT3 pathway. Oncol. Rep. 2017, 38, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, L.; Deng, X.; Sun, Y. Effects of isoliquiritigenin on ovarian cancer cells. Onco Targets Ther. 2018, 11, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Escobar, S.J.M.; Fong, G.M.; Winnischofer, S.M.B.; Simone, M.; Munoz, L.; Dennis, J.M.; Rocha, M.E.M.; Witting, P.K. Anti-proliferative and cytotoxic activities of the flavonoid isoliquiritigenin in the human neuroblastoma cell line SH-SY5Y. Chem.-Biol. Interact. 2019, 299, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Reape, T.J.; Molony, E.M.; McCabe, P.F. Programmed cell death in plants: Distinguishing between different modes. J. Exp. Bot. 2008, 59, 435–444. [Google Scholar] [CrossRef]
- Scott, I.; Logan, D.C. Mitochondria and cell death pathways in plants. Plant Signal. Behav. 2008, 3, 475–477. [Google Scholar] [CrossRef]
- Araniti, F.; Costas-Gil, A.; Cabeiras-Freijanes, L.; Lupini, A.; Sunseri, F.; Reigosa, M.J.; Abenavoli, M.R.; Sánchez-Moreiras, A.M. Rosmarinic acid induces programmed cell death in Arabidopsis seedlings through reactive oxygen species and mitochondrial dysfunction. PLoS ONE 2018, 13, e0208802. [Google Scholar] [CrossRef]
- Babula, P.; Vaverkova, V.; Poborilova, Z.; Ballova, L.; Masarik, M.; Provaznik, I. Phytotoxic action of naphthoquinone juglone demonstrated on lettuce seedling roots. Plant Physiol. Biochem. 2014, 84, 78–86. [Google Scholar] [CrossRef]
- Koodkaew, I.; Sunohara, Y.; Matsuyama, S.; Matsumoto, H. Phytotoxic action mechanism of hapalocyclamide in lettuce seedlings. Plant Physiol. Biochem. 2012, 58, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Sun, Y.; Xiao, C.L.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Physiological basis of different allelopathic reactions of cucumber and figleaf gourd plants to cinnamic acid. J. Exp. Bot. 2007, 58, 3765–3773. [Google Scholar] [CrossRef] [PubMed]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Zemskov, E.A.; Lu, Q.; Ornatowski, W.; Klinger, C.N.; Desai, A.A.; Maltepe, E.; Yuan, J.X.; Wang, J.X.; Fineman, J.R.; Black, S.M. Biomechanical forces and oxidative stress: Implications for pulmonary vascular disease. Antioxid. Redox Signal. 2019, 31, 819–842. [Google Scholar] [CrossRef]
- Burbridge, E.; Diamond, M.; Dix, P.J.; McCabe, P.F. Use of cell morphology to evaluate the effect of a peroxidase gene on cell death induction thresholds in tobacco. Plant Sci. 2007, 172, 853–860. [Google Scholar] [CrossRef]
- Dat, J.F.; Pellinen, R.; Beeckman, T.; Van De Cotte, B.; Langebartels, C.; Kangasjärvi, J.; Inzé, D.; Van Breusegem, F. Changes in hydrogen peroxide homeostasis trigger an active cell death process in tobacco. Plant J. 2003, 33, 621–632. [Google Scholar] [CrossRef]
- Xin, A.Y.; Li, X.Z.; Jin, H.; Yang, X.Y.; Zhao, R.M.; Liu, J.K.; Qin, B. The accumulation of reactive oxygen species in root tips caused by autotoxic allelochemicals-a significant factor for replant problem of Angelica sinensis (Oliv.) Diels. Ind. Crop. Prod. 2019, 138, 111432. [Google Scholar] [CrossRef]
- Chen, X.Y.; Ren, H.H.; Wang, D.; Chen, Y.; Qu, C.J.; Pan, Z.H.; Liu, X.N.; Hao, W.J.; Xu, W.J.; Wang, K.J.; et al. Isoliquiritigenin induces mitochondrial dysfunction and apoptosis by inhibiting mitoNEET in a reactive oxygen species-dependent manner in A375 human melanoma cells. Oxid. Med. Cell. Longev. 2019, 2019, 9817576. [Google Scholar] [CrossRef]
- Wang, J.R.; Luo, Y.H.; Piao, X.J.; Zhang, Y.; Feng, Y.C.; Li, J.Q.; Xu, W.T.; Zhang, Y.; Zhang, T.; Wang, S.N.; et al. Mechanisms underlying isoliquiritigenin-induced apoptosis and cell cycle arrest via ROS-mediated MAPK/STAT3/NF-kappaB pathways in human hepatocellular carcinoma cells. Drug Dev. Res. 2019, 80, 461–470. [Google Scholar] [CrossRef]
- Yang, W.H.; Zheng, L.P.; Yuan, H.Y.; Wang, J.W. Glaucocalyxin A and B regulate growth and induce oxidative stress in lettuce (Lactuca sativa L.) roots. J. Plant Growth Regul. 2014, 33, 384–396. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.; Nali, C.; Ciompi, S.; Lorenzini, G.; Soldatini, G.F. The use of chlorophyll fluorescence and leaf gas exchange as methods for studying the different responses to ozone of two bean cultivars. J. Exp. Bot. 1997, 48, 173–179. [Google Scholar] [CrossRef]
- Yan, Z.Q.; Wang, D.D.; Ding, L.; Cui, H.Y.; Jin, H.; Yang, X.Y.; Yang, J.S.; Qin, B. Mechanism of artemisinin phytotoxicity action: Induction of reactive oxygen species and cell death in lettuce seedlings. Plant Physiol. Biochem. 2015, 88, 53–59. [Google Scholar] [CrossRef]
- Hou, Y.X.; Sun, S.W.; Liu, Y.; Li, Y.; Liu, X.H.; Wang, W.; Zhang, S.; Wang, W. An improved method for the synthesis of butein using SOCl2/EtOH as catalyst and deciphering its inhibition mechanism on xanthine oxidase. Molecules 2019, 24, 1948. [Google Scholar] [CrossRef]
- Dhaouadi, K.; Meliti, W.; Dallali, S.; Belkhir, M.; Ouerghemmi, S.; Sebei, H.; Fattouch, S. Commercial Lawsonia inermis L. dried leaves and processed powder: Phytochemical composition, antioxidant, antibacterial, and allelopathic activities. Ind. Crop. Prod. 2015, 77, 544–552. [Google Scholar] [CrossRef]
- Jones, K.H.; Senft, J.A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J. Histochem. Cytochem. 1985, 33, 77–79. [Google Scholar] [CrossRef]
- Sunohara, Y.; Matsumoto, H. Quinclorac-induced cell death is accompanied by generation of reactive oxygen species in maize root tissue. Phytochemistry 2008, 69, 2312–2319. [Google Scholar] [CrossRef]
- Health, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free prolin for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Yan, Z.Q.; Wang, D.D.; Cui, H.Y.; Zhang, D.H.; Sun, Y.H.; Jin, H.; Li, X.; Yang, X.; Guo, H.; He, X.; et al. Phytotoxicity mechanisms of two coumarin allelochemicals from Stellera chamaejasme in lettuce seedlings. Acta Physiol. Plant. 2016, 38, 248–258. [Google Scholar] [CrossRef]
- He, Y.L.; Liu, Y.L.; Cao, W.X.; Huai, M.F.; Xu, B.G.; Huang, B.G. Effects of salicylic acid on heat tolerance associated with antioxidant metabolism in kentucky bluegrass. Crop Sci. 2005, 45, 988–995. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophyll-a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Savage, W.; Berry, W.L.; Reed, C.A. Effects of trace element stress on the morphology of developing seedlings of lettuce (Lactuca sativa L. grand rapids) as shown by scanning electron microscopy. J. Plant Nutr. 1981, 3, 129–138. [Google Scholar] [CrossRef]
- Zhang, X.H.; Wu, J.; Huang, J.G.; Zhou, L.J. Cytotoxicity of the natural herbicidal chemical, berberine, on Nicotiana tabacum Bright yellow-2 cells. Pestic. Biochem. Physiol. 2018, 152, 131–137. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Sun, S.-W.; Shi, H.-L.; Zhao, K.; Wang, J.; Liu, Y.; Liu, X.-H.; Wang, W. Physiological and Biochemical Mechanisms Mediated by Allelochemical Isoliquiritigenin on the Growth of Lettuce Seedlings. Plants 2020, 9, 245. https://doi.org/10.3390/plants9020245
Zhang S, Sun S-W, Shi H-L, Zhao K, Wang J, Liu Y, Liu X-H, Wang W. Physiological and Biochemical Mechanisms Mediated by Allelochemical Isoliquiritigenin on the Growth of Lettuce Seedlings. Plants. 2020; 9(2):245. https://doi.org/10.3390/plants9020245
Chicago/Turabian StyleZhang, Shuang, Shi-Wei Sun, Hai-Lin Shi, Ke Zhao, Jin Wang, Yang Liu, Xiao-Hong Liu, and Wei Wang. 2020. "Physiological and Biochemical Mechanisms Mediated by Allelochemical Isoliquiritigenin on the Growth of Lettuce Seedlings" Plants 9, no. 2: 245. https://doi.org/10.3390/plants9020245
APA StyleZhang, S., Sun, S.-W., Shi, H.-L., Zhao, K., Wang, J., Liu, Y., Liu, X.-H., & Wang, W. (2020). Physiological and Biochemical Mechanisms Mediated by Allelochemical Isoliquiritigenin on the Growth of Lettuce Seedlings. Plants, 9(2), 245. https://doi.org/10.3390/plants9020245




