Natural Variation in Adventitious Rooting in the Alpine Perennial Arabis alpina
Abstract
1. Introduction
2. Results
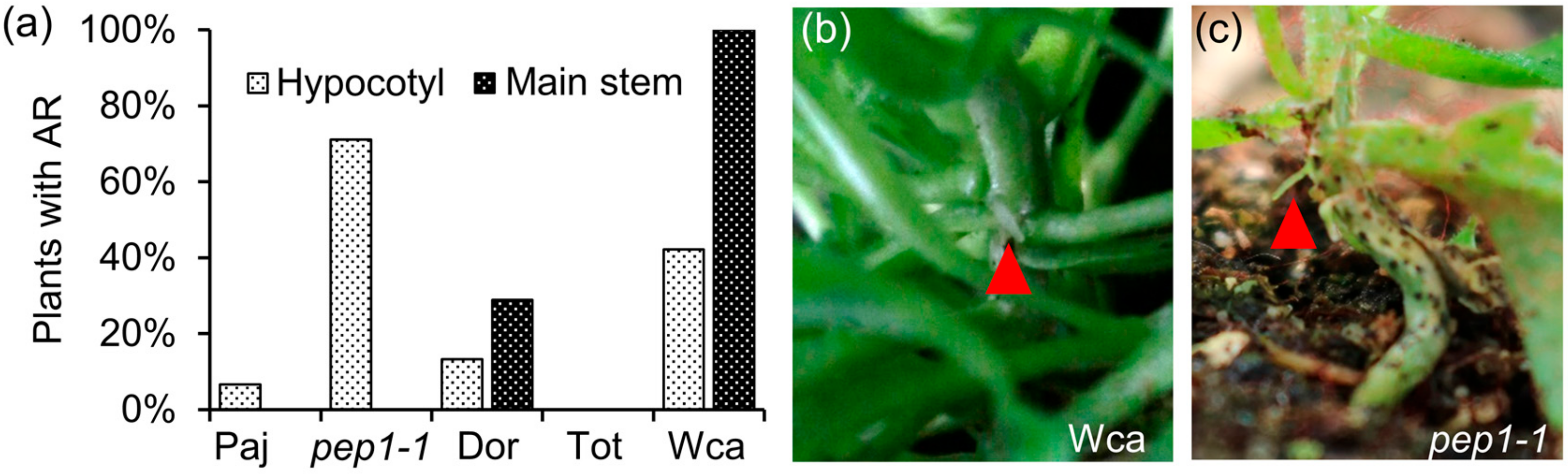
2.1. The Wca Accession Has Higher Propensity to Produce Adventitious Roots on the Main Stem
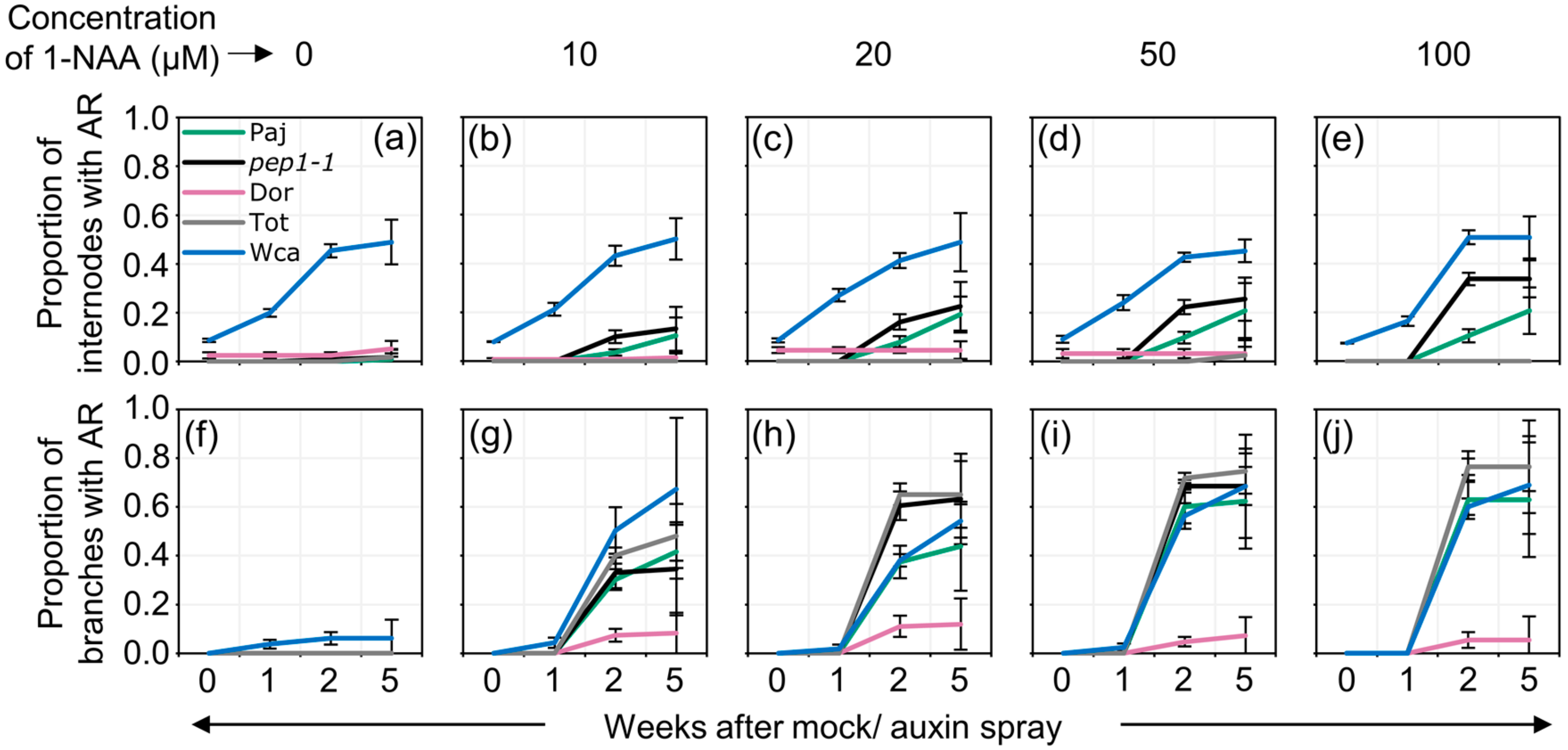
2.2. Auxin Spray Induces Adventitious Roots in A Dosage-, Age- and Genotype-Dependent Manner
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
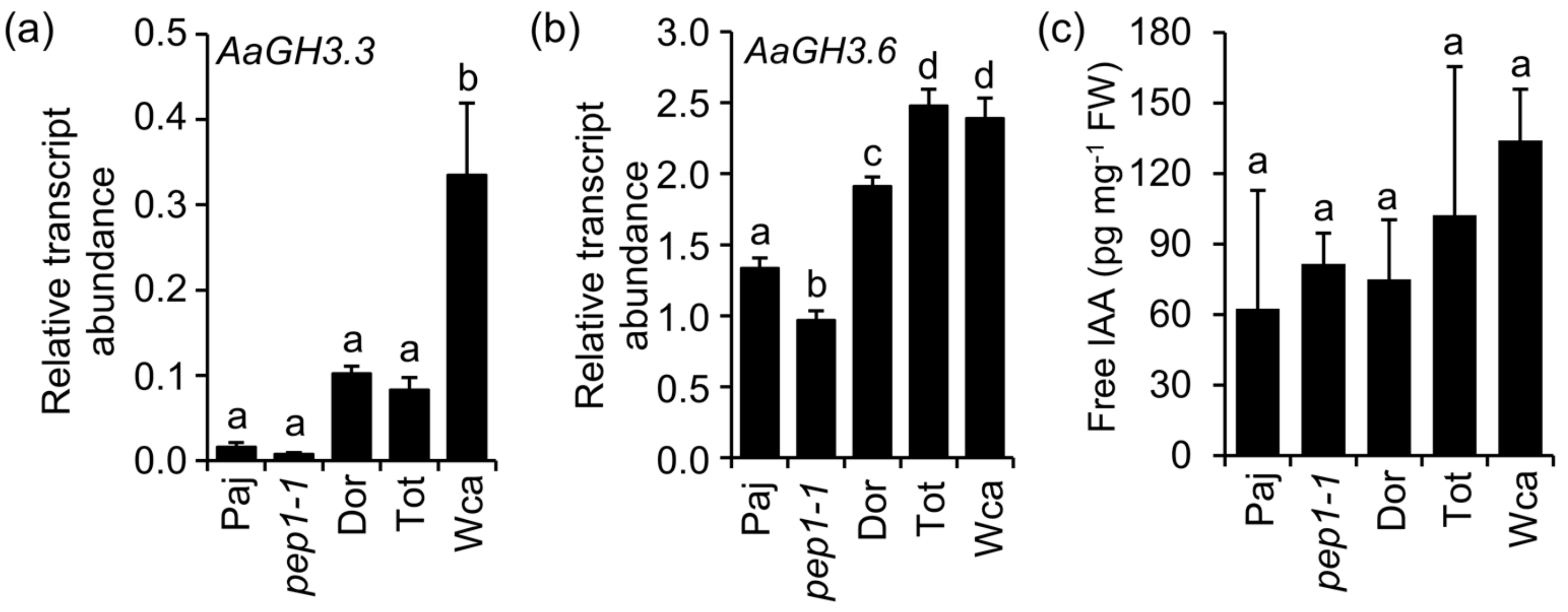
4.2. Gene Expression Analysis
4.3. Free IAA Quantification
4.4. Auxin Spray
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Billings, W.D.; Mooney, H.A. The ecology of arctic and alpine plants. Biol. Rev. 1968, 43, 481–529. [Google Scholar] [CrossRef]
- Bliss, L.C. Arctic and alpine plant life cycles. Annu. Rev. Ecol. Syst. 1971, 2, 405–438. [Google Scholar] [CrossRef]
- Jonsdottir, I.; Callaghan, T.V.; Headley, A.D. Resource dynamics within arctic clonal plants. Ecol. Bull. 1996, 45, 53–64. [Google Scholar]
- Reynolds, D.N. Alpine annual plants: Phenology, germination, photosynthesis, and growth of three Rocky Mountain species. Ecology 1984, 65, 759–766. [Google Scholar] [CrossRef]
- Klimeš, L.; Klimešová, J.; Hendriks, R.; van Groenendael, J. Clonal plant architecture: A comparative analysis of form and function. Ecol. Evol. Clonal Plants 1997, 8, 1–29. [Google Scholar]
- Czakó, M.; Wilson, J.; Yu, X.; Márton, L. Sustained root culture for generation and vegetative propagation of transgenic Arabidopsis thaliana. Plant Cell Rep. 1993, 12, 603–606. [Google Scholar] [CrossRef]
- Clauss, M.J.; Koch, M.A. Poorly known relatives of Arabidopsis thaliana. Trends Plant Sci. 2006, 11, 449–459. [Google Scholar] [CrossRef]
- Ansell, S.W.; Grundmann, M.; Russell, S.J.; Schneider, H.; Vogel, J.C. Genetic discontinuity, breeding-system change and population history of Arabis alpina in the Italian Peninsula and adjacent Alps. Mol. Ecol. 2008, 17, 2245–2257. [Google Scholar] [CrossRef]
- Tedder, A.; Ansell, S.W.; Lao, X.; Vogel, J.C.; Mable, B.K. Sporophytic self-incompatibility genes and mating system variation in Arabis alpina. Ann. Bot. 2011, 108, 699–713. [Google Scholar] [CrossRef]
- Tedder, A.; Helling, M.; Pannell, J.R.; Shimizu-Inatsugi, R.; Kawagoe, T.; Van Campen, J.; Sese, J.; Shimizu, K.K. Female sterility associated with increased clonal propagation suggests a unique combination of androdioecy and asexual reproduction in populations of Cardamine amara (Brassicaceae). Ann. Bot. 2015, 115, 763–776. [Google Scholar] [CrossRef]
- Laenen, B.; Tedder, A.; Nowak, M.D.; Toräng, P.; Wunder, J.; Wötzel, S.; Steige, K.A.; Kourmpetis, Y.; Odong, T.; Drouzas, A.D.; et al. Demography and mating system shape the genome-wide impact of purifying selection in Arabis alpina. Proc. Natl. Acad. Sci. USA 2018, 115, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Buehler, D.; Graf, R.; Holderegger, R.; Gugerli, F. Contemporary gene flow and mating system of Arabis alpina in a Central European alpine landscape. Ann. Bot. 2012, 109, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Thimann, K.V.; Went, F.W. On the Chemical Nature of the Rootforming Hormone. Proc. Kon. Acad. Wetensch. Amst. 1934, 37, 456–459. [Google Scholar]
- De Klerk, G.-J.; Van der Krieken, W.; De Jong, J.C. Review the formation of adventitious roots: New concepts, new possibilities. Vitr. Cell. Dev. Biol. Plant 1999, 35, 189–199. [Google Scholar] [CrossRef]
- Smith, D.L.; Fedoroff, N. V LRP1, a gene expressed in lateral and adventitious root primordia of arabidopsis. Plant Cell 1995, 7, 735–745. [Google Scholar]
- Gutierrez, L.; Bussell, J.D.; Pacurar, D.I.; Schwambach, J.; Pacurar, M.; Bellini, C. Phenotypic Plasticity of Adventitious Rooting in Arabidopsis Is Controlled by Complex Regulation of AUXIN RESPONSE FACTOR Transcripts and MicroRNA Abundance. Plant Cell 2009, 21, 3119–3132. [Google Scholar] [CrossRef]
- Boerjan, W.; Cervera, M.-T.; Delarue, M.; Beeckman, T.; Dewitte, W.; Bellini, C.; Caboche, M.; Onckelen, H.V.A.N.; Van Montagu, M.; Inzé, D. Superroot, a Recessive Mutation in Arabidopsis, Confers Auxin Overproduction. Plant Cell Online 1995, 7, 1405–1419. [Google Scholar]
- Wang, R.; Farrona, S.; Vincent, C.; Joecker, A.; Schoof, H.; Turck, F.; Alonso-Blanco, C.; Coupland, G.; Albani, M.C. PEP1 regulates perennial flowering in Arabis alpina. Nature 2009, 459, 423–427. [Google Scholar] [CrossRef]
- Gutierrez, L.; Mongelard, G.; Floková, K.; Păcurar, D.I.; Novák, O.; Staswick, P.; Kowalczyk, M.; Păcurar, M.; Demailly, H.; Geiss, G.; et al. Auxin Controls Arabidopsis Adventitious Root Initiation by Regulating Jasmonic Acid Homeostasis. Plant Cell 2012, 24, 2515–2527. [Google Scholar] [CrossRef]
- Staswick, P.E.; Serban, B.; Rowe, M.; Tiryaki, I.; Maldonado, M.T.; Maldonado, M.C.; Suza, W. Characterization of an arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 2005, 17, 616–627. [Google Scholar] [CrossRef]
- Albani, M.C.; Castaings, L.; Wötzel, S.; Mateos, J.L.; Wunder, J.; Wang, R.; Reymond, M.; Coupland, G. PEP1 of Arabis alpina Is Encoded by Two Overlapping Genes That Contribute to Natural Genetic Variation in Perennial Flowering. PLoS Genet. 2012, 8, e1003130. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, H.; Yang, Y.; Zhi, H.; Li, C.; Guo, J. Vegetative propagation capacity of invasive alligator weed through small stolon fragments under different treatments OPEN. Sci. Rep. 2017, 7, 43826. [Google Scholar] [CrossRef] [PubMed]
- Visser, E.; Cohen, J.D.; Barendse, G.W.M.; Blom, C.W.P.M.; Voesenek, L. An Ethylene-Mediated Increase in Sensitivity to Auxin Induces Adventitious Root Formation in Flooded Rumex palustris Sm. Plant Physiol. 1996, 112, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Vo, S.T.K.; Johnson, E.A. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems. Mt. Res. Dev. 2001, 21, 202. [Google Scholar] [CrossRef]
- Hughes, P.W.; Soppe, W.J.J.; Albani, M.C. Seed traits are pleiotropically regulated by the flowering time gene PERPETUAL FLOWERING 1 (PEP1) in the perennial Arabis alpina. Mol. Ecol. 2019, 28, 1183–1201. [Google Scholar] [CrossRef]
- Blythe, G.; Sibley, J.L. Novel Methods of Applying Rooting Hormones in Cutting Propagation ©. Comb. Proc. Int. Plant Propagators Soc. 2003, 53, 406–410. [Google Scholar]
- Little, C.H.A.; Macdonald, J.E. Effects of exogenous gibberellin and auxin on shoot elongation and vegetative bud development in seedlings of Pinus sylvestris and Picea glauca. Tree Physiol. 2003, 23, 73–83. [Google Scholar] [CrossRef]
- Drahn, S.R. Auxin Application via Foliar Sprays ©. Proc. Int. Plant Propagators Soc. 2007, 57, 44–48. [Google Scholar]
- Quan, J.; Meng, S.; Guo, E.; Zhang, S.; Zhao, Z.; Yang, X. De novo sequencing and comparative transcriptome analysis of adventitious root development induced by exogenous indole-3-butyric acid in cuttings of tetraploid black locust. BMC Genom. 2017, 18, 179. [Google Scholar] [CrossRef]
- Villacorta-Martín, C.; Sánchez-García, A.B.; Villanova, J.; Cano, A.; van de Rhee, M.; de Haan, J.; Acosta, M.; Passarinho, P.; Pérez-Pérez, J.M. Gene expression profiling during adventitious root formation in carnation stem cuttings. BMC Genom. 2015, 16, 789. [Google Scholar] [CrossRef]
- Busov, V.B.; Johannes, E.; Whetten, R.W.; Sederoff, R.R.; Spiker, S.L.; Lanz-Garcia, C.; Goldfarb, B. An auxin-inducible gene from loblolly pine (Pinus taeda L.) is differentially expressed in mature and juvenile-phase shoots and encodes a putative transmembrane protein. Planta 2004, 218, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Ahkami, A.H.; Lischewski, S.; Haensch, K.T.; Porfirova, S.; Hofmann, J.; Rolletschek, H.; Melzer, M.; Franken, P.; Hause, B.; Druege, U.; et al. Molecular physiology of adventitious root formation in Petunia hybrida cuttings: Involvement of wound response and primary metabolism. New Phytol. 2009, 181, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.; Hosseini, S.A.; Hajirezaei, M.R.; Druege, U.; Geelen, D. Adventitious rooting declines with the vegetative to reproductive switch and involves a changed auxin homeostasis. J. Exp. Bot. 2015, 66, 1437–1452. [Google Scholar] [CrossRef]
- Lakehal, A.; Bellini, C. Control of adventitious root formation: Insights into synergistic and antagonistic hormonal interactions. Physiol. Plant. 2018, 165, 90–100. [Google Scholar] [CrossRef]
- Rasmussen, A.; Mason, M.G.; De Cuyper, C.; Brewer, P.B.; Herold, S.; Agusti, J.; Geelen, D.; Greb, T.; Goormachtig, S.; Beeckman, T.; et al. Strigolactones Suppress Adventitious Rooting in Arabidopsis and Pea. Plant Physiol. 2012, 158, 1976–1987. [Google Scholar] [CrossRef]
- Brondani, G.E.; Baccarin, F.J.B.; de Wit Ondas, H.W.; Stape, J.L.; Gonçalves, A.N.; de Almeida, M. Low temperature, IBA concentrations and optimal time for adventitious rooting of Eucalyptus benthamii mini-cuttings. J. For. Res. 2012, 23, 583–592. [Google Scholar] [CrossRef]
- Kroin, J. Advances Using lndole-3-butyric Acid (IBA) Dissolved in Water for-Rooting Cuttings, Transplanting, and Grafting. Comb. Proc. Int. Plant Propagators Soc. 1992, 42, 345–346. [Google Scholar]
- Alvarez, R.; Nissen, S.J.; Sutter, E.G. Relationship between Indole-3-Acetic Acid Levels in Apple (Malus pumila Mill) Rootstocks Cultured in Vitro and Adventitious Root Formation in the Presence of Indole-3-Butyric Acid. Plant Physiol. 1989, 89, 439–443. [Google Scholar] [CrossRef]
- Grattapaglia, D.; Bertolucci, F.L.; Sederoff, R.R. Genetic mapping of QTLs controlling vegetative propagation in Eucalyptus grandis and E. urophylla using a pseudo-testcross strategy and RAPD markers. Theor. Appl. Genet. 1995, 90, 933–947. [Google Scholar] [CrossRef]
- Da Rocha Corrêa, L.; Fett-Neto, A.G. Effects of temperature on adventitious root development in microcuttings of Eucalyptus saligna Smith and Eucalyptus globulus Labill. J. Therm. Biol. 2004, 29, 315–324. [Google Scholar] [CrossRef]
- Saranga, J.; Cameron, R. Adventitious root formation in Anacardium occidentale L. in response to phytohormones and removal of roots. Sci. Hortic. Amst. 2007, 111, 164–172. [Google Scholar] [CrossRef]
- Negishi, N.; Oishi, M.; Kawaoka, A. Chemical screening for promotion of adventitious root formation in Eucalyptus globulus. BMC Proc. 2011, 5, P139. [Google Scholar] [CrossRef]
- Abarca, D.; Pizarro, A.; Hernandez, I.; Sanchez, C.; Solana, S.P.; del Amo, A.; Carneros, E.; Díaz-Sala, C. The GRAS gene family in pine: Transcript expression patterns associated with the maturation-related decline of competence to form adventitious roots. BMC Plant Biol. 2014, 14, 1583. [Google Scholar] [CrossRef] [PubMed]
- Fett-Neto, A.G.; Fett, J.P.; Vieira Goulart, L.W.; Pasquali, G.; Termignoni, R.R.; Ferreira, A.G. Distinct effects of auxin and light on adventitious root development in Eucalyptus saligna and Eucalyptus globulus. Tree Physiol. 2001, 21, 457–464. [Google Scholar] [CrossRef]
- Andersen, S.U.; Buechel, S.; Zhao, Z.; Ljung, K.; Novák, O.; Busch, W.; Schuster, C.; Lohmanna, J.U. Requirement of B2-type cyclin-dependent kinases for meristem integrity in Arabidopsis thaliana. Plant Cell 2008, 20, 88–100. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, P.; Roggen, A.; Ljung, K.; Albani, M.C. Natural Variation in Adventitious Rooting in the Alpine Perennial Arabis alpina. Plants 2020, 9, 184. https://doi.org/10.3390/plants9020184
Mishra P, Roggen A, Ljung K, Albani MC. Natural Variation in Adventitious Rooting in the Alpine Perennial Arabis alpina. Plants. 2020; 9(2):184. https://doi.org/10.3390/plants9020184
Chicago/Turabian StyleMishra, Priyanka, Adrian Roggen, Karin Ljung, and Maria C. Albani. 2020. "Natural Variation in Adventitious Rooting in the Alpine Perennial Arabis alpina" Plants 9, no. 2: 184. https://doi.org/10.3390/plants9020184
APA StyleMishra, P., Roggen, A., Ljung, K., & Albani, M. C. (2020). Natural Variation in Adventitious Rooting in the Alpine Perennial Arabis alpina. Plants, 9(2), 184. https://doi.org/10.3390/plants9020184




