Application of Deep Eutectic Solvents for the Extraction of Rutin and Rosmarinic Acid from Satureja montana L. and Evaluation of the Extracts Antiradical Activity
Abstract
1. Introduction
2. Results
2.1. Comparison of the Possibility of Extraction by DESs
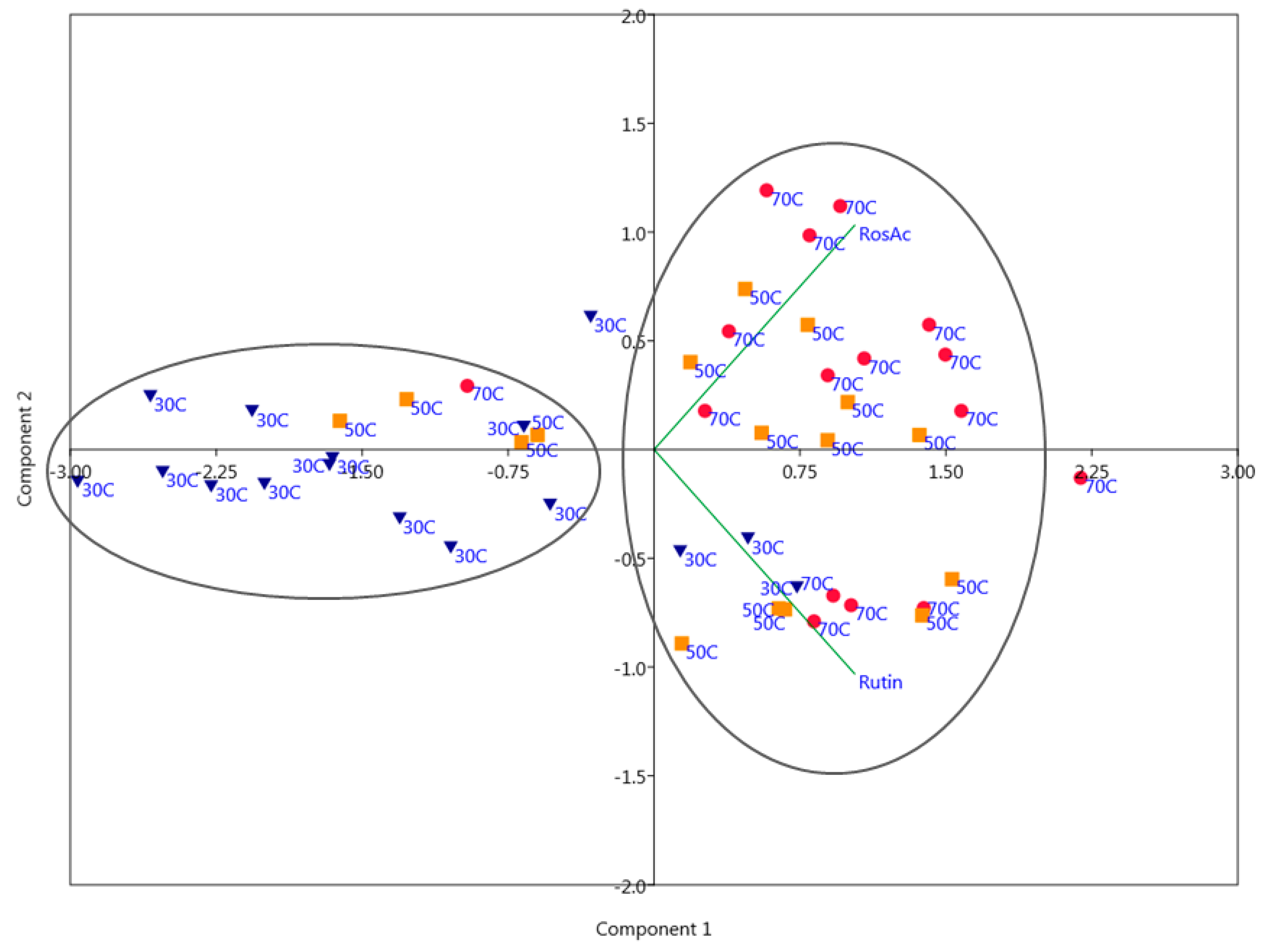
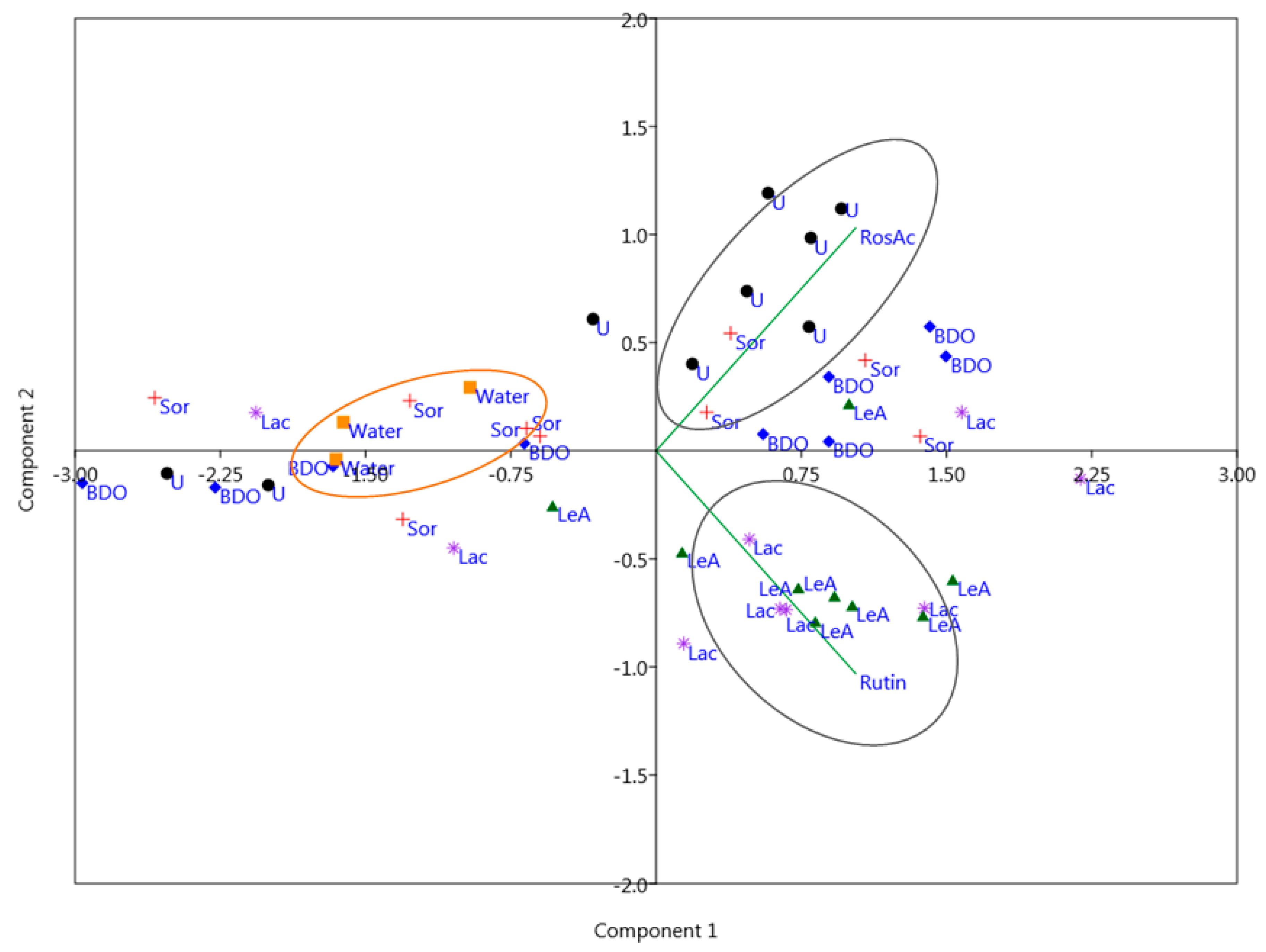
2.2. Principal Component Analysis
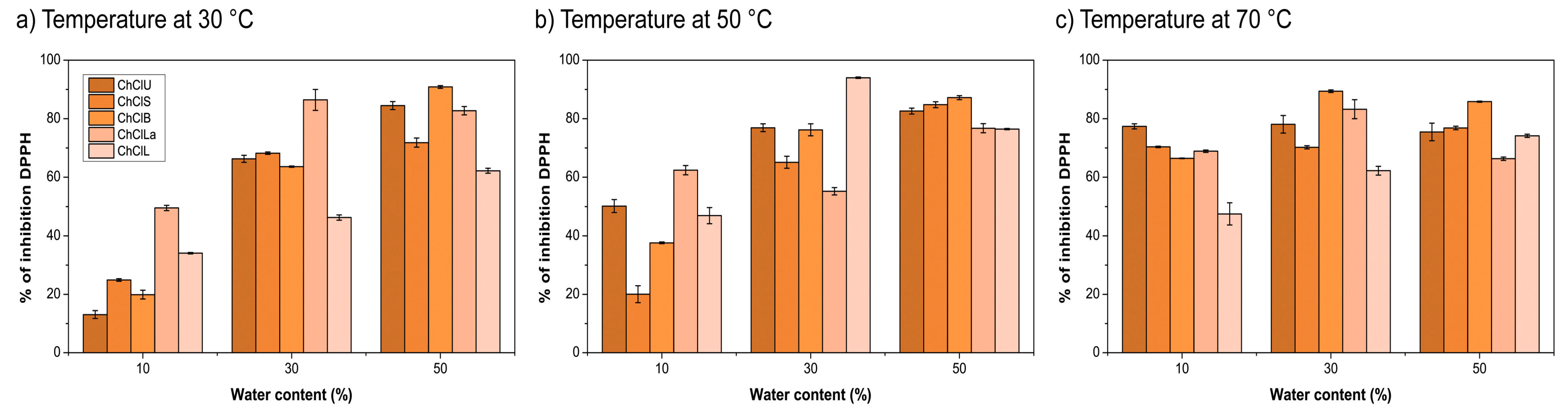
2.3. Antioxidant Activity
3. Discussion
3.1. Comparison of the Possibility of Extraction by DESs
3.2. Antiradical Activity
4. Materials and Methods
4.1. Preparation of DESs
4.2. DESs Extraction of Bioactive Components
4.3. Chemical Characterization of the Extracts
4.4. Antiradical Activity
4.5. Data Processing
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Ćurko, N.; Gaurina Srček, V.; Cvjetko Bubalo, M.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT- Food Sci. Technol. 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Yang, D.; Hou, M.; Ning, H.; Zhang, J.; Ma, J.; Yang, G.; Han, B. Efficient SO2 absorption by renewable choline chloride–glycerol deep eutectic solvents. Green Chem. 2013, 15, 2261–2265. [Google Scholar] [CrossRef]
- Jeong, K.M.; Lee, M.S.; Nam, M.W.; Zhao, J.; Jin, Y.; Lee, D.K.; Kwon, S.W.; Jeong, J.H.; Lee, J. Tailoring and recycling of deep eutectic solvents as sustainable and efficient extraction media. J. Chromatogr. A 2015, 1424, 10–17. [Google Scholar] [CrossRef]
- Min, W.N.; Jing, Z.; Min, S.L.; Ji, H.J.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J. Chromatogr. A 2013, 1285, 22–30. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Erwann, D. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef] [PubMed]
- Alañón, M.E.; IvanoviĆ, M.; Gómez-Caravaca, A.M.; Arráez-Román, D.; Segura-Carretero, A. Choline chloride derivative-based deep eutectic liquids as novel green alternative solvents for extraction of phenolic compounds from olive leaf. Arab. J. Chem. 2020, in press. [Google Scholar] [CrossRef]
- Stanic, G.; Samarzija, I. Diuretic activity of Satureja montana subsp. montana extracts and oil in rats. Phytother. Res. 1993, 7, 363–366. [Google Scholar] [CrossRef]
- Skočibušić, M.; Bezić, N. Chemical composition and antidiarrhoeal activities of winter savory (Satureja montana L.) essential oil. Pharm. Biol. 2003, 41, 622–626. [Google Scholar] [CrossRef]
- Cavar, S.; Maksimovic, M.; Solic, M.E.; Jerkovic-Mujkic, A.; Besta, R. Chemical composition and antioxidant and antimicrobial activity of two Satureja essential oils. Food Chem. 2008, 111, 648–653. [Google Scholar] [CrossRef]
- Serrano, C.; Matos, O.; Teixeira, B.; Ramos, C.; Neng, N.; Nogueira, J.; Nunes, M.L.; Marques, A. Antioxidant and antimicrobial activity of Satureja montana L. extracts. J. Sci. Food Agric. 2011, 91, 1554–1560. [Google Scholar] [CrossRef]
- Vidovic, S.S.; Vladic, J.Z.; Vastag, Z.G.; Zekovic, Z.P.; Popovic, L.M. Maltodextrin as a carrier of health benefit compounds in Satureja montana dry powder extract obtained by spray drying technique. Powder Technol. 2014, 258, 209–215. [Google Scholar] [CrossRef]
- Elgndi, M.A.; Filip, S.; Pavlic, B.; Vladic, J.; Stanojkovic, T.; Zizak, Z.; Zekovic, Z. Antioxidative and cytotoxic activity of essential oils and extracts of Satureja montana L., Coriandrum sativum L. and Ocimum basilicum L. obtained by supercritical fluid extraction. J. Supercrit. Fluid. 2017, 128, 128–137. [Google Scholar] [CrossRef]
- Vladić, J.; Vidović, S.; Aćimović, M.; Gavarić, A.; Jokić, S. Satureja montana: Cultivation, Production and Uses. In Medicinal Plants: Production, Cultivation and Uses; Matthias, A., Laisné, N., Eds.; Nova Science Publishers: New York, NY, USA, 2017; pp. 27–57. [Google Scholar]
- Vladić, J.; Canli, O.; Pavlić, B.; Zeković, Z.; Vidović, S.; Kaplan, M. Optimization of Satureja montana subcritical water extraction process and chemical characterization of volatile fraction of extracts. J. Supercrit. Fluid. 2017, 120, 86–94. [Google Scholar] [CrossRef]
- Zekovic, Z.; Gavaric, A.; Pavlic, B.; Vidovic, S.; Vladic, J. Optimization: Microwave irradiation effect on polyphenolic compounds extraction from winter savory (Satureja montana L.). Sep. Sci. Technol. 2017, 52, 1377–1386. [Google Scholar] [CrossRef]
- Zavatti, M.; Zanoli, P.; Benelli, A.; Rivasi, M.; Baraldi, C.; Baraldi, M. Experimental study on Satureja montana as a treatment for premature ejaculation. J. Ethnopharmacol. 2011, 133, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Mastelic, J.; Jerkovic, I. Gas chromatography–mass spectrometry analysis of free and glycoconjugated aroma compounds of seasonally collected Satureja montana L. Food Chem. 2003, 80, 135–140. [Google Scholar] [CrossRef]
- Prieto, J.M.; Iacopini, P.; Cioni, P.; Chericoni, S. In vitro activity of the essential oils of Origanum vulgare, Satureja montana and their main constituents in peroxynitrite-induced oxidative processes. Food Chem. 2007, 104, 889–895. [Google Scholar] [CrossRef]
- Grosso, C.; Figueiredo, A.C.; Burillo, J.; Mainar, A.M.; Urieta, J.S.; Barroso, J.G.; Coelho, J.; Palavra, A.M. Enrichment of the thymoquinone content in volatile oil from Satureja montana using supercritical fluid extraction. J. Sep. Sci. 2009, 32, 328–334. [Google Scholar] [CrossRef]
- Grosso, C.; Oliveira, A.C.; Mainar, A.M.; Urieta, J.S.; Barroso, J.G.; Palavra, A.M.F. Antioxidant activities of the supercritical and conventional Satureja montana extracts. J. Food Sci. 2009, 74, 713–717. [Google Scholar] [CrossRef]
- Silva, F.V.; Martins, A.; Salta, J.; Neng, N.R.; Nogueira, J.M.; Mira, D.; Gaspar, N.; Justino, J.; Grosso, C.; Urieta, J.S.; et al. Phytochemical profile and anticholinesterase and antimicrobial activities of supercritical versus conventional extracts of Satureja montana. J. Agric. Food Chem. 2009, 57, 11557–11563. [Google Scholar] [CrossRef]
- Miladi, H.; Ben Slama, R.; Mili, D.; Zouari, S.; Bakhrouf, A.; Ammar, E. Chemical composition and cytotoxic and antioxidant activities of Satureja montana L. essential oil and its antibacterial potential against Salmonella spp. strains. J. Chem. 2013, 2013, 275698. [Google Scholar] [CrossRef]
- Haloci, E.; Toska, V.; Baldisserotto, A.; Goci, E.; Vertuani, S.; Manfredini, S. Evaluation of antifungal activity of Satureja montana essential oil before and after inclusion in beta-cyclodextrine. Int. J. Pharm. Pharm. Sci. 2014, 6, 189–191. [Google Scholar]
- Vidovic, S.; Zekovic, Z.; Marosanovic, B.; Todorovic, M.P.; Vladic, J. Influence of pre-treatments on yield, chemical composition and antioxidant activity of Satureja montana extracts obtained by supercritical carbon dioxide. J. Supercrit. Fluid. 2014, 95, 468–473. [Google Scholar] [CrossRef]
- Vladic, J.; Zekovic, Z.; Jokic, S.; Svilovic, S.; Kovacevic, S.; Vidovic, S. Winter savory: Supercritical carbon dioxide extraction and mathematical modeling of extraction process. J. Supercrit. Fluid. 2016, 117, 89–97. [Google Scholar] [CrossRef]
- Rezvanpanah, S.; Rezaei, K.; Razavi, S.H.; Moini, S. Use of microwave-assisted hydrodistillation to extract the essential oils from Satureja hortensis and Satureja montana. Food Sci. Technol. Res. 2008, 14, 311–314. [Google Scholar] [CrossRef]
- El Tawab, A.M.A.; Shahin, N.N.; Abdel Mohsen, M.M. Protective effect of Satureja montana extract on cyclophosphamide-induced testicular injury in rats. Chem. Biol. Interact. 2014, 224, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Vladic, J.; Zekovic, Z.; Cvejin, A.; Adamovic, D.; Vidovic, S.S. Optimization of Satureja montana extraction process considering phenolic antioxidants and antioxidant activity. Sep. Sci. Technol. 2014, 49, 2066–2072. [Google Scholar] [CrossRef]
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef]
- Deng, J.; Xu, Z.; Xiang, C.; Liu, J.; Zhou, L.; Li, T.; Yang, Z.; Ding, C. Comparative evaluation of maceration and ultrasonic-assisted extraction of phenolic compounds from fresh olives. Ultrason. Sonochem. 2017, 37, 328–334. [Google Scholar] [CrossRef]
- Gu, H.; Chen, F.; Zhang, Q.; Zang, J. Application of ionic liquids in vacuum microwave-assisted extraction followed by macroporous resin isolation of three flavonoids rutin, hyperoside and hesperidin from Sorbus tianschanica leaves. J. Chromatogr. B 2016, 1014, 45–55. [Google Scholar] [CrossRef]
- Gan, Z.; Chen, Q.; Fu, Y.; Chen, G. Determination of bioactive constituents in Flos sophorae immaturus and Cortex fraxini by capillary electrophoresis in combination with far infrared-assisted solvent extraction. Food Chem. 2012, 130, 1122–1126. [Google Scholar] [CrossRef]
- Kraujalis, P.; Venskutonisa, P.R.; Ibañez, E.; Herrero, M. Optimization of rutin isolation from Amaranthus paniculatus leaves by high pressure extraction and fractionation techniques. J. Supercrit. Fluid. 2015, 104, 234–242. [Google Scholar] [CrossRef]
- Xie, J.; Shi, L.; Zhu, X.; Wang, P.; Zhao, Y.; Su, W. Mechanochemical-assisted efficient extraction of rutin from Hibiscus mutabilis L. Innov. Food Sci. Emerg. Technol. 2011, 12, 146–152. [Google Scholar] [CrossRef]
- Solana, L.M.; Boschiero, I.; Dall’Acqua, S.; Bertucco, A. A comparison between supercritical fluid and pressurized liquid extraction methods for obtaining phenolic compounds from Asparagus officinalis L. J. Supercrit. Fluid. 2015, 100, 201–208. [Google Scholar] [CrossRef]
- Molnar, M.; Jakovljević, M.; Jokić, S. Optimization of the process conditions for the extraction of rutin from Ruta graveolens L. by choline chloride based deep eutectic solvents. Solvent Extr. Res. Dev. 2018, 25, 109–116. [Google Scholar] [CrossRef]
- Jeliński, T.; Cysewski, P. Application of a computational model of natural deep eutectic solvents utilizing the COSMO-RS approach for screening of solvents with high solubility of rutin. J. Mol. Model. 2018, 24, 180. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, R.; Mishra, N.; Kumar Bansal, P. Phytochemical, pharmacological and pharmacokinetics effects of rosmarinic acid. J. Pharm. Sci. Innov. 2013, 2, 28–34. [Google Scholar] [CrossRef]
- Ngo, Y.L.; Lau, C.H.; Chua, L.S. Review on rosmarinic acid extraction, fractionation and its anti-diabetic potential. Food Chem. Toxicol. 2018, 121, 687–700. [Google Scholar] [CrossRef]
- Akar, Z.; Küçük, M.; Doğan, H. A new colorimetric DPPH• scavenging activity method with no need for a spectrophotometer applied on synthetic and natural antioxidants and medicinal herbs. J. Enzyme Inhib. Med. Chem. 2017, 32, 640–647. [Google Scholar] [CrossRef]
- Cozzolino, D.; Power, A.; Chapman, J. Interpreting and reporting principal component analysis in food science analysis and beyond. Food Anal. Methods. 2019, 12, 2469–2473. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef]
- Mitar, A.; Panić, M.; Prlić Kardum, J.; Halambek, J.; Sander, A.; Zagajski Kučan, K.; Radojčić Redovniković, I.; Radošević, K. Physicochemical Properties, Cytotoxicity, and Antioxidative Activity of Natural Deep Eutectic Solvents Containing Organic Acid. Chem. Biochem. Eng. Q. 2019, 33, 1–18. [Google Scholar] [CrossRef]
- Kremer, D.; Košir, I.J.; Končić, M.Z.; Čerenak, A.; Potočnik, T.; Srečec, S.; Randić, M.; Kosalec, I. Antimicrobial and antioxidant properties of Satureja montana L. and S. subspicata Vis. (Lamiaceae). Curr. Drug Targets 2015, 16, 1623–1633. [Google Scholar] [CrossRef]
- Xia, B.; Yan, D.; Bai, Y.; Xie, J.; Cao, Y.; Liao, D.; Lin, L. Determination of phenolic acids in Prunella vulgaris L.: a safe and green extraction method using alcohol-based deep eutectic solvents. Anal. Methods 2015, 7, 9354–9364. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.-L.; Guo, L.; Li, P.; Liu, E.-H. Comprehensive evaluation of deep eutectic solvents in extraction of bioactive natural products. ACS Sustainable Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Švarc-Gajic, J.; Segura-Carreteroa, A.; Fernández-Gutiérreza, A. Determination of phenolic compounds and antioxidant activity of a Mediterranean plant: The case of Satureja montana subsp. kitaibelii. J. Funct. Foods 2015, 18, 1167–1178. [Google Scholar] [CrossRef]
- Banožić, M.; Banjari, I.; Jakovljević, M.; Šubarić, D.; Tomas, S.; Babić, J.; Jokić, S. Optimization of Ultrasound-Assisted Extraction of Some Bioactive Compounds from Tobacco Waste. Molecules 2019, 24, 1611. [Google Scholar] [CrossRef]
- Antolovic, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. R. Soc. Chem. 2002, 127, 183–198. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods-UK 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Cordella, C.B. PCA: the basic building block of chemometrics. In Analytical Chemistry; Krull, S.I., Ed.; Intech Open Limited: London, UK, 2012; p. 154. [Google Scholar]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]

| Solvent | Parameters | EC50 (µg mL−1) |
|---|---|---|
| ChCl-U | 30 °C 50% H2O | 100.64 ± 15.74 |
| ChCl-Sor | 50 °C 50% H2O | 568.05 ± 21.51 |
| ChCl-BDO | 30 °C 50% H2O | 497.96 ± 2.82 |
| ChCL-Lac | 30 °C 30% H2O | 207.03 ± 6.76 |
| ChCL-LeA | 50 °C 30% H2O | 459.01 ± 8.83 |
| Components | Mole Ration (HBA:HBD) | Appearance | ||
|---|---|---|---|---|
| Hydrogen Bond Acceptor (HBA) | Hydrogen Bond Donors (HBDs) | |||
| ChCl-U | Choline chloride | Urea | 1:2 | Clear and in liquid state at 80 °C |
| ChCl-Sor | Sorbitol | 1:1 | Clear, viscous and in liquid state at 80 °C | |
| ChCl-BDO | Butane-1,4-diol | 1:2 | Clear and in liquid state at 80 °C | |
| ChCL-Lac | Lactic acid | 1:2 | Clear and in liquid state at 80 °C | |
| ChCL-LeA | Levulinic acid | 1:2 | Clear and in liquid state at 80 °C | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakovljević, M.; Vladić, J.; Vidović, S.; Pastor, K.; Jokić, S.; Molnar, M.; Jerković, I. Application of Deep Eutectic Solvents for the Extraction of Rutin and Rosmarinic Acid from Satureja montana L. and Evaluation of the Extracts Antiradical Activity. Plants 2020, 9, 153. https://doi.org/10.3390/plants9020153
Jakovljević M, Vladić J, Vidović S, Pastor K, Jokić S, Molnar M, Jerković I. Application of Deep Eutectic Solvents for the Extraction of Rutin and Rosmarinic Acid from Satureja montana L. and Evaluation of the Extracts Antiradical Activity. Plants. 2020; 9(2):153. https://doi.org/10.3390/plants9020153
Chicago/Turabian StyleJakovljević, Martina, Jelena Vladić, Senka Vidović, Kristian Pastor, Stela Jokić, Maja Molnar, and Igor Jerković. 2020. "Application of Deep Eutectic Solvents for the Extraction of Rutin and Rosmarinic Acid from Satureja montana L. and Evaluation of the Extracts Antiradical Activity" Plants 9, no. 2: 153. https://doi.org/10.3390/plants9020153
APA StyleJakovljević, M., Vladić, J., Vidović, S., Pastor, K., Jokić, S., Molnar, M., & Jerković, I. (2020). Application of Deep Eutectic Solvents for the Extraction of Rutin and Rosmarinic Acid from Satureja montana L. and Evaluation of the Extracts Antiradical Activity. Plants, 9(2), 153. https://doi.org/10.3390/plants9020153










