The Effect of Photoperiod Genes and Flowering Time on Yield and Yield Stability in Durum Wheat
Abstract
1. Introduction
2. Results
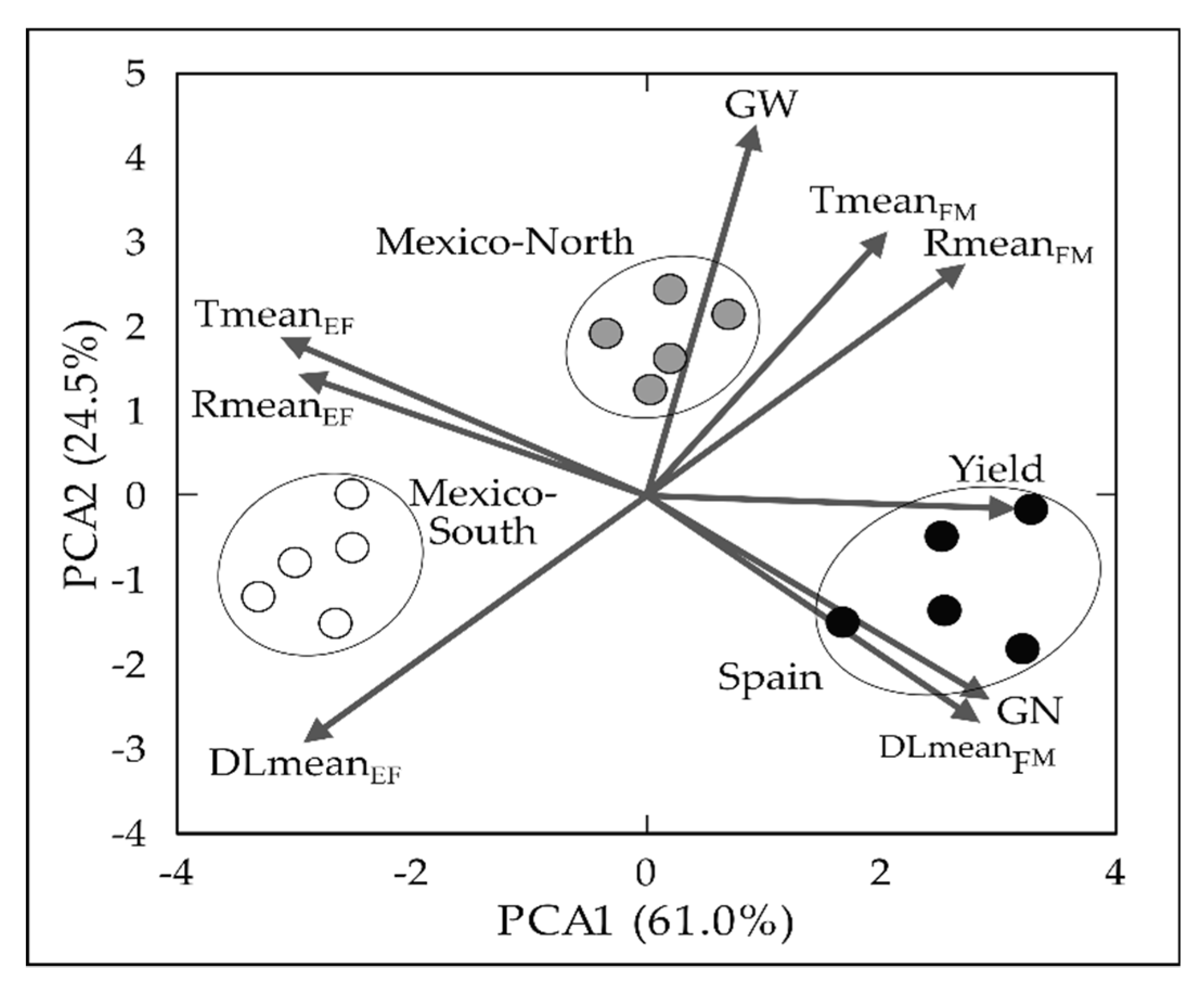
2.1. Environmental and Genetic Effects
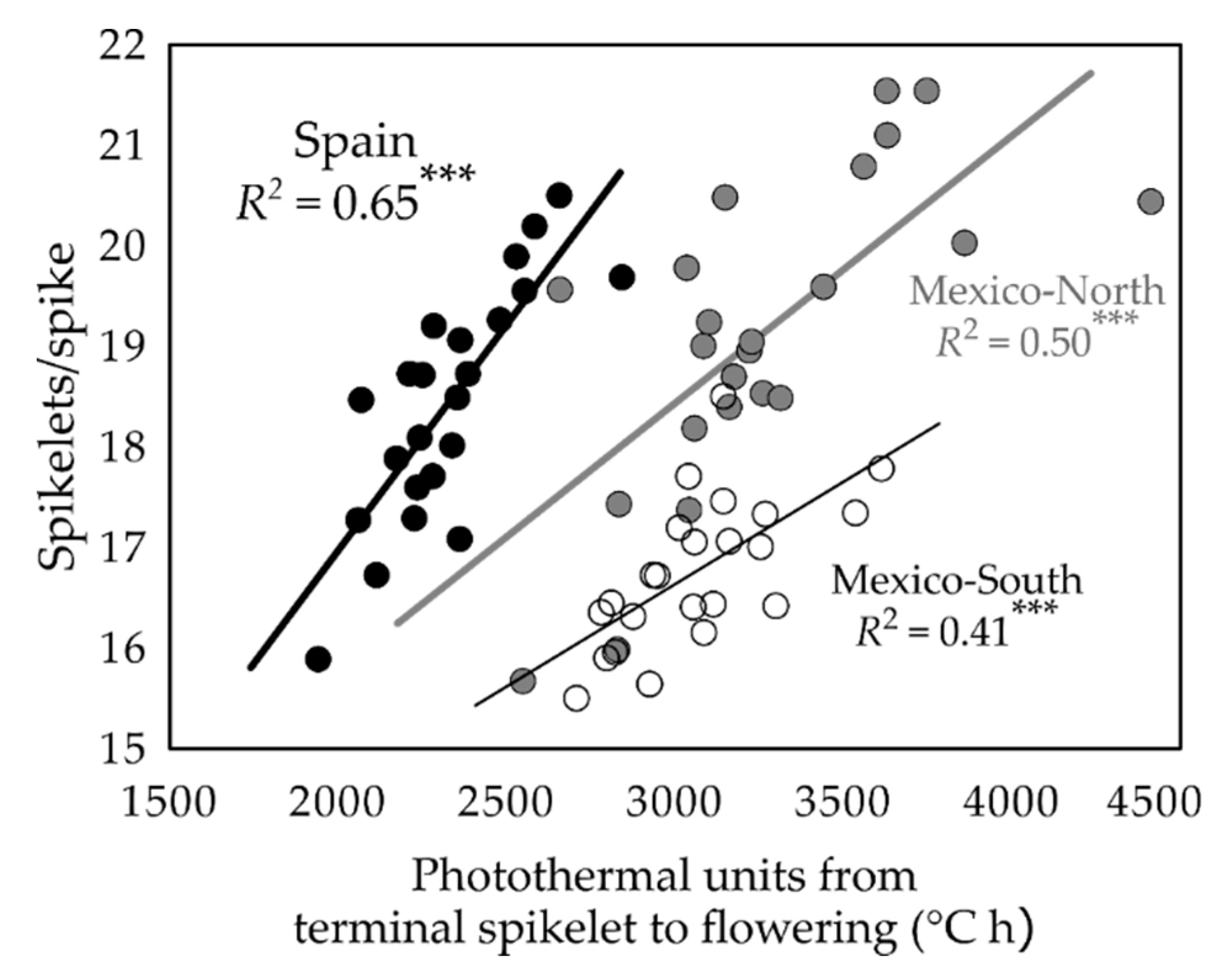
2.2. Relationships between Phenology Variation Due to Eps and Yield Formation
2.3. Ppd-1 Allele Combinations and Yield Stability
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Field Setup
4.3. Data Recording
4.4. Statistical Analysis
- Yij is the trait studied for genotype i in experiment j;
- ACi is the effect of allele combination for genotype i, considered as a fixed effect;
- G(AC)ij is the genotype effect within its allele combination for genotype i on experiment j, considered as a random effect;
- Rj is the effect of the replicate on experiment j, considered as a random effect; and
- Eij is the experimental error of genotype i in experiment j, considered as a random effect.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO Food Outlook. Biannual Report on Global Food Markets; Food and Agriculture Organization: Rome, Italy, 2017; p. 152. [Google Scholar]
- Trnka, M.; Rötter, R.P.; Ruiz-Ramos, M.; Kersebaum, K.C.; Olesen, J.E.; Žalud, Z.; Semenov, M.A. Adverse weather conditions for European wheat production will become more frequent with climate change. Nat. Clim. Chang. 2014, 4, 637–643. [Google Scholar] [CrossRef]
- Liu, B.; Asseng, S.; Liu, L.; Tang, L.; Cao, W.; Zhu, Y. Testing the responses of four wheat crop models to heat stress at anthesis and grain filling. Glob. Chang. Biol. 2016, 22, 1890–1903. [Google Scholar] [CrossRef] [PubMed]
- Curtis, T.; Halford, N.G. Food security: The challenge of increasing wheat yield and the importance of not compromising food safety. Ann. Appl. Biol. 2014, 164, 354–372. [Google Scholar] [CrossRef] [PubMed]
- Royo, C.; Martos, V.; Ramdani, A.; Villegas, D.; Rharrabti, Y.; García Del Moral, L.F. Changes in yield and carbon isotope discrimination of Italian and Spanish durum wheat during the 20th century. Agron. J. 2008, 100, 352–360. [Google Scholar] [CrossRef]
- De Vita, P.; Mastrangelo, A.M.; Matteu, L.; Mazzucotelli, E.; Virzì, N.; Palumbo, M.; Lo Storto, M.; Rizza, F.; Cattivelli, L.; De Vita, P.; et al. Genetic improvement effects on yield stability in durum wheat genotypes grown in Italy. F. Crop. Res. 2010, 119, 68–77. [Google Scholar] [CrossRef]
- Cubero, J.I.; Flores, F. Métodos Estadísticos para el Estudio de la Estabilidad Varietal en Ensayos Agrícolas; Junta de Andalucía, Consejeriía de Agricultura y Pesca: Sevilla, Spain, 1994; ISBN 8487564100. [Google Scholar]
- Becker, H.C.; Léon, J. Stability Analysis in Plant Breeding. Plant Breed. 1988, 101, 1–23. [Google Scholar] [CrossRef]
- Finlay, K.; Wilkinson, G. The analysis of adaptation in a plant-breeding programme. Aust. J. Agric. Res. 1963, 14, 742. [Google Scholar] [CrossRef]
- Lin, C.S.; Binns, M.R. A superiority measure of cultivar performance for cultivar × location data. Can. J. Plant Sci. 1988, 68, 193–198. [Google Scholar] [CrossRef]
- Shukla, G.K. Some statistical aspects of partitioning genotype-environmental components of variability. Heredity 1972, 29, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Wricke, G. Über eine methode zur erfassung der ökologischen streubreite in feldversuchen. Zeitschr. F. Pflanzenz. 1962, 47, 92–96. [Google Scholar]
- Kang, M.S. Simultaneous selection for yield and stability in crop performance trials: Consequences for growers. Agron. J. 1993, 85, 754–757. [Google Scholar] [CrossRef]
- Kang, M.S. A rank-sum method for selecting high-yielding, stable corn genotypes. Cereal Res. Commun. 1988, 16, 113–115. [Google Scholar]
- Annicchiarico, P. Genotype x environment interactions: Challenges and opportunities for plant breeding and cultivar recommendations. FAO Plant Prod. Prot. 2002, 174, 115. [Google Scholar]
- Worland, A.J.; Bo, A.; Börner, A.; Korzun, V.; Li, W.M.; Petrovíc, S.; Sayers, E.J. The influence of photoperiod genes on the adaptability of European winter wheats. Euphytica 1998, 100, 385–394. [Google Scholar] [CrossRef]
- Snape, J.W.W.; Butterworth, K.; Whitechurch, E.; Worland, A.J.J. Waiting for fine times: Genetics of flowering time in wheat. Euphytica 2001, 119, 185–190. [Google Scholar] [CrossRef]
- Casadebaig, P.; Zheng, B.; Chapman, S.; Huth, N.; Faivre, R.; Chenu, K. Assessment of the potential impacts of wheat plant traits across environments by combining crop modeling and global sensitivity analysis. PLoS ONE 2016, 11, 1–27. [Google Scholar] [CrossRef]
- Rezaei, E.E.; Webber, H.; Gaiser, T.; Naab, J.; Ewert, F. Heat stress in cereals: Mechanisms and modelling. Eur. J. Agron. 2015, 64, 98–113. [Google Scholar] [CrossRef]
- Frederiks, T.M.; Christopher, J.T.; Sutherland, M.W.; Borrell, A.K. Post-head-emergence frost in wheat and barley: Defining the problem, assessing the damage, and identifying resistance. J. Exp. Bot. 2015, 66, 3487–3498. [Google Scholar] [CrossRef]
- Atlin, G.N.; Cairns, J.E.; Das, B. Rapid breeding and varietal replacement are critical to adaptation of cropping systems in the developing world to climate change. Glob. Food Sec. 2017, 12, 31–37. [Google Scholar] [CrossRef]
- Distelfeld, A.; Li, C.; Dubcovsky, J. Regulation of flowering in temperate cereals. Curr. Opin. Plant Biol. 2009, 12, 178–184. [Google Scholar] [CrossRef]
- Yan, L.; Helguera, M.; Kato, K.; Fukuyama, S.; Sherman, J.; Dubcovsky, J. Allelic variation at the VRN-1 promoter region in polyploid wheat. Theor. Appl. Genet. 2004, 109, 1677–1686. [Google Scholar] [CrossRef]
- Fu, D.; Szűcs, P.; Yan, L.; Helguera, M.; Skinner, J.S.; von Zitzewitz, J.; Hayes, P.M.; Dubcovsky, J.; Szucs, P.; Yan, L.; et al. Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol. Genet. Genom. 2005, 273, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, E.P.; Turner, A.S.; Laurie, D.A. Photoperiod insensitive Ppd-A1a mutations in tetraploid wheat (Triticum durum Desf.). Theor. Appl. Genet. 2009, 118, 285–294. [Google Scholar] [CrossRef]
- Maccaferri, M.; Sanguineti, M.C.; Corneti, S.; Araus, J.L.; Salem, M.B.; Bort, J.; De Ambrogio, E.; Garcia Del Moral, L.F.; Demontis, A.; El-Ahmed, A.; et al. Quantitative trait loci for grain yield and adaptation of durum wheat (Triticum durum Desf.) across a wide range of water availability. Genetics 2008, 178, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Royo, C.; Dreisigacker, S.; Alfaro, C.; Ammar, K.; Villegas, D. Effect of Ppd-1 genes on durum wheat flowering time and grain filling duration in a wide range of latitudes. J. Agric. Sci. 2016, 154, 612–631. [Google Scholar] [CrossRef]
- Braun, H.J.; Rajaram, S.; Van Ginkel, M. CIMMYT’s approach to breeding for wide adaptation. Euphytica 1996, 92, 175–183. [Google Scholar] [CrossRef]
- Basualdo, J.; Díaz, M.L.; Cuppari, S.; Cardone, S.; Soresi, D.; Camargo, G.P.; Carrera, A. Allelic variation and differential expression of VRN-A1 in durum wheat genotypes varying in the vernalization response. Plant Breed. 2015, 134, 520–528. [Google Scholar] [CrossRef]
- Muterko, A.; Kalendar, R.; Salina, E. Allelic variation at the VERNALIZATION-A1, VRN-B1, VRN-B3, and PHOTOPERIOD-A1 genes in cultivars of Triticum durum Desf. Planta 2016, 244, 1253–1263. [Google Scholar] [CrossRef]
- Tanio, M.; Kato, K. Development of near-isogenic lines for photoperiod-insensitive genes, Ppd-B1 and Ppd-D1, carried by the Japanese wheat cultivars and their effect on apical development. Breed. Sci. 2007, 57, 65–72. [Google Scholar] [CrossRef]
- Royo, C.; Dreisigacker, S.; Soriano, J.M.; Lopes, M.S.; Ammar, K.; Villegas, D. Allelic variation at the vernalization response (Vrn-1) and photoperiod sensitivity (Ppd-1) genes and their association with the development of durum wheat landraces and modern cultivars. Front. Plant Sci. 2020, 11, 838. [Google Scholar] [CrossRef]
- Würschum, T.; Rapp, M.; Miedaner, T.; Longin, C.F.H.; Leiser, W.L. Copy number variation of Ppd-B1 is the major determinant of heading time in durum wheat. BMC Genet. 2019, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bentley, A.R.; Horsnell, R.; Werner, C.P.; Turner, A.S.; Rose, G.A.; Bedard, C.; Howell, P.; Wilhelm, E.P.; Mackay, I.J.; Howells, R.M.; et al. Short, natural, and extended photoperiod response in BC2F4 lines of bread wheat with different Photoperiod-1 (Ppd-1) alleles. J. Exp. Bot. 2013, 64, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Worland, A.J. The influence of flowering time genes on environmental adaptability in European wheats. Euphytica 1996, 89, 49–57. [Google Scholar] [CrossRef]
- Villegas, D.; Alfaro, C.; Ammar, K.; Cátedra, M.M.; Crossa, J.; García del Moral, L.F.; Royo, C. Daylength, temperature and solar radiation effects on the phenology and yield formation of spring durum wheat. J. Agron. Crop Sci. 2016, 202, 203–216. [Google Scholar] [CrossRef]
- Royo, C.; Ammar, K.; Alfaro, C.; Dreisigacker, S.; García del Moral, L.F.; Villegas, D. Effect of Ppd-1 photoperiod sensitivity genes on dry matter production and allocation in durum wheat. F. Crop. Res. 2018, 221, 358–367. [Google Scholar] [CrossRef]
- Arjona, J.M.; Royo, C.; Dreisigacker, S.; Ammar, K.; Villegas, D. Effect of allele combinations at Ppd-1 loci on durum wheat grain filling at contrasting latitudes. J. Agron. Crop Sci. 2020, 206, 64–75. [Google Scholar] [CrossRef]
- Arjona, J.M.J.; Royo, C.; Dreisigacker, S.; Ammar, K.; Villegas, D. Effect of Ppd-A1 and Ppd-B1 allelic variants on grain number and thousand kernel weight of durum wheat and their impact on final grain yield. Front. Plant Sci. 2018, 9, 888. [Google Scholar] [CrossRef]
- Ortiz-Monasterio, J.I.; Dhillon, S.S.; Fischer, R.A. Date of sowing effects on grain yield and yield components of irrigated spring wheat cultivars and relationships with radiation and temperature in Ludhiana, India. F. Crop. Res. 1994, 37, 169–184. [Google Scholar] [CrossRef]
- Royo, C.; Dreisigacker, S.; Ammar, K.; Villegas, D. Agronomic performance of durum wheat landraces and modern cultivars and its association with genotypic variation in vernalization response (Vrn-1) and photoperiod sensitivity (Ppd-1) genes. Eur. J. Agron. 2020, 120, 126129. [Google Scholar] [CrossRef]
- Trethowan, R.M.; van Ginkel, M.; Ammar, K.; Crossa, J.; Payne, T.S.; Cukadar, B.; Rajaram, S.; Hernandez, E. Associations among twenty years of international bread wheat yield evaluation environments. Crop Sci. 2003, 43, 1698–1711. [Google Scholar] [CrossRef]
- Roncallo, P.F.; Akkiraju, P.C.; Cervigni, G.L.; Echenique, V.C. QTL mapping and analysis of epistatic interactions for grain yield and yield-related traits in Triticum turgidum L. var. durum. Euphytica 2017, 213, 277. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Wang, H.; Li, L.; Wu, J.; Yang, X.; Li, X.; Gao, A. QTL mapping of yield-related traits in the wheat germplasm 3228. Euphytica 2011, 177, 277–292. [Google Scholar] [CrossRef]
- Soriano, J.M.; Malosetti, M.; Roselló, M.; Sorrells, M.E.; Royo, C. Dissecting the old Mediterranean durum wheat genetic architecture for phenology, biomass and yield formation by association mapping and QTL meta-analysis. PLoS ONE 2017, 12, e0178290. [Google Scholar] [CrossRef] [PubMed]
- Terrile, I.I.; Miralles, D.J.; González, F.G.; Gonzalez, F.G.; González, F.G. Fruiting efficiency in wheat (Triticum aestivum L.): Trait response to different growing conditions and its relation to spike dry weight at anthesis and grain weight at harvest. F. Crop. Res. 2017, 201, 86–96. [Google Scholar] [CrossRef]
- Draeger, T.; Moore, G. Short periods of high temperature during meiosis prevent normal meiotic progression and reduce grain number in hexaploid wheat (Triticum aestivum L.). Theor. Appl. Genet. 2017, 130, 1785–1800. [Google Scholar] [CrossRef]
- Fischer, R.A. The effect of duration of the vegetative phase in irrigated semi-dwarf spring wheat on phenology, growth and potential yield across sowing dates at low latitude. F. Crop. Res. 2016, 198, 188–199. [Google Scholar] [CrossRef]
- Stone, P.; Nicolas, M. Wheat cultivars vary widely in responses of grain yield and quality to short periods of post-anthesis heat stress. Aust. J. Plant Physiol. 1994, 21, 887. [Google Scholar] [CrossRef]
- Bergkamp, B.; Impa, S.M.; Asebedo, A.R.; Fritz, A.K.; Jagadish, S.V.K. Prominent winter wheat varieties response to post-flowering heat stress under controlled chambers and field based heat tents. F. Crop. Res. 2018, 222, 143–152. [Google Scholar] [CrossRef]
- García, G.A.; Serrago, R.A.; Dreccer, M.F.; Miralles, D.J. Post-anthesis warm nights reduce grain weight in field-grown wheat and barley. F. Crop. Res. 2016, 195, 50–59. [Google Scholar] [CrossRef]
- Milner, S.G.; Maccaferri, M.; Huang, B.E.; Mantovani, P.; Massi, A.; Frascaroli, E.; Tuberosa, R.; Salvi, S. A multiparental cross population for mapping QTL for agronomic traits in durum wheat (Triticum turgidum ssp. durum). Plant Biotechnol. J. 2016, 14, 735–748. [Google Scholar] [CrossRef]
- Mehrabi, Z.; Ramankutty, N. Synchronized failure of global crop production. Nat. Ecol. Evol. 2019, 3, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Mühleisen, J.; Peter, H.; Peter, H.; Yusheng, M. Exploitation of yield stability in barley. Theor. Appl. Genet. 2014, 1949–1962. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Cabrera, A.; Hoffstetter, A.; Griffey, C.; Van Sanford, D.; Costa, J.; McKendry, A.; Chao, S.; Sneller, C. Genomic selection for wheat traits and trait stability. Theor. Appl. Genet. 2016, 129, 1697–1710. [Google Scholar] [CrossRef] [PubMed]
- Bentley, A.R.; Turner, A.S.; Gosman, N.; Leigh, F.J.; Maccaferri, M.; Dreisigacker, S.; Greenland, A.; Laurie, D.A. Frequency of photoperiod-insensitive Ppd-A1a alleles in tetraploid, hexaploid and synthetic hexaploid wheat germplasm. Plant Breed. 2011, 130, 10–15. [Google Scholar] [CrossRef]
- Forsythe, W.C.; Rykiel, E.J.; Stahl, R.S.; Wu, H.-I.; Schoolfield, R.M. A model comparison for daylength as a function of latitude and day of year. Ecol. Modell. 1995, 80, 87–95. [Google Scholar] [CrossRef]
- Dalezios, N.R.; Loukas, A.; Bampzelis, D. The role of agrometeorological and agrohydrological indices in the phenology of wheat in central Greece. Phys. Chem. Earth 2002, 27, 1019–1023. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Kirby, E.J.M.; Appleyard, M. Cereal Development Guide, 2nd ed.; Arable Unit, National Agricultural Centre: Kenilworth, UK, 1986. [Google Scholar]
- SAS institute Inc RRID:SCR_014242 JMP 13 Pro 2016. SAS Institute Inc., Cary, NC, USA. Available online: http://www.jmp.com (accessed on 5 December 2020).
- SAS RRID:SCR_008567 Statistical Analysis System Software 2009. SAS Institute Inc., Cary, NC, USA. Available online: https://www.sas.com/es_es/home.html (accessed on 5 December 2020).
- Dia, M.; Wehner, T.C.; Arellano, C. RGxE: An R program for genotype x environment interaction analysis. Am. J. Plant Sci. 2017, 8, 1672–1698. [Google Scholar] [CrossRef]
- Mendiburu, F. de Agricolae: Statistical Procedures for Agricultural Research. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 5 December 2020).
- R Core Team RRID:SCR_001905. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2018. [Google Scholar]


| Ppd-A1 | Ppd-B1 | Allele Combination Acronym | Number of Genotypes | ||
|---|---|---|---|---|---|
| Allele (1) | Photoperiod Response | Allele | Photoperiod Response | ||
| Ppd-A1b | Sensitive | Ppd-B1b | Sensitive | SS | 5 |
| Ppd-A1b | Sensitive | Ppd-B1a | Insensitive | SI | 5 |
| GS105-Ppd-A1a | Insensitive | Ppd-B1b | Sensitive | I5S | 4 |
| GS105-Ppd-A1a | Insensitive | Ppd-B1a | Insensitive | I5I | 6 |
| GS100-Ppd-A1a | Insensitive | Ppd-B1b | Sensitive | I0S (2) | 1 |
| GS100-Ppd-A1a | Insensitive | Ppd-B1a | Insensitive | I0I | 3 |
| Source of Variation | Df | Days Emergence-Flowering | Days Flowering-Maturity | Grains/m2 (GN) (1) | Grain Weight (GW, mg) (2) | Grain Yield (GY, g/m2) |
|---|---|---|---|---|---|---|
| Site | 2 | 86.4 *** | 43.6 * | 42.4 *** | 14.1 * | 50.5 *** |
| Year | 4 | 2.8 ns | 7.1 ns | 5.0 ns | 5.1 ns | 10.5 * |
| Site × year | 8 | 6.1 *** | 27.0 *** | 2.9 *** | 8.9 *** | 3.8 *** |
| Genotype | 22 | 3.1 *** | 4.4 * | 27.5 *** | 55.8 *** | 11.0 *** |
| Between allele combinations | 4 | 1.5 * | 0.3 ns | 11.5 * | 20.5 ns | 2.7 ns |
| Within allele combinations | 18 | 1.6 *** | 4.1 *** | 16.0 *** | 35.3 *** | 8.3 *** |
| Genotype × Site | 44 | 1.0 *** | 3.6 *** | 2.3 ns | 3.4 *** | 3.4 ** |
| Between allele combinations × Site | 8 | 0.2 ns | 2.2 *** | 1.2 * | 1.2 * | |
| Within allele combinations × Site | 36 | 0.8 *** | 1.4 ns | 2.2 *** | 2.2 * | |
| Genotype × Year | 88 | 0.2 *** | 3.9 * | 3.5 ns | 2.9 * | 3.4 ns |
| Genotype × Site × Year | 176 | 0.3 *** | 5.5 *** | 6.8 *** | 3.9 *** | 7.4 *** |
| Between allele combinations × Site × Year | 32 | 0.1 ns | 1.3 ns | 1.6 ns | 0.9 ns | 1.9 * |
| Within allele combinations × Site × Year | 144 | 0.2 *** | 4.2 *** | 5.2 *** | 3.0 *** | 5.5 *** |
| Block | 30 | 0.01 *** | 0.5 *** | 1.8 *** | 0.8 *** | 1.8 *** |
| Residual | 651 | 0.1 | 4.3 | 7.9 | 5.1 | 8.1 |
| Allele Combination | Grains/Spike | Spikelets/Spike | Grains/Spikelet | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spain | Mexico-North | Mexico-South | Mean | Spain | Mexico-North | Mexico-South | Mean | Spain | Mexico-North | Mexico-South | Mean | |
| I0I | 36.8 b | 36.3 b | 32.9 a | 35.4 b | 16.8 c | 17.3 a | 15.4 c | 16.5 c | 2.2 a | 2.1 a | 2.1 a | 2.1 ab |
| I5I | 36.2 b | 34.4 b | 29.9 a | 33.5 b | 17.4 bc | 17.4 a | 16.0 bc | 16.9 bc | 2.1 a | 2.0 a | 1.9 a | 2.0 b |
| I5S | 47.4 a | 47.7 a | 38.9 a | 44.6 a | 19.8 a | 20.5 a | 17.1 ab | 19.1 a | 2.4 a | 2.3 a | 2.3 a | 2.3 a |
| SS | 38.9 b | 40.1 b | 33.4 a | 37.4 b | 19.1 ab | 20.3 a | 17.7 a | 19.0 a | 2.1 a | 2.0 a | 1.9 a | 2.0 b |
| SI | 36.6 b | 40.4 b | 32.8 a | 36.6 b | 18.9 ab | 19.9 a | 17.3 ab | 18.7 ab | 2.0 a | 2.0 a | 1.9 a | 2.0 b |
| Year | Days from Emergence to Flowering | Days from Flowering to Maturity | ||||
|---|---|---|---|---|---|---|
| GN | GW | GY | GN | GW | GY | |
| Spain | ||||||
| 2007 | 0.23 ns | −0.61 ** | −0.71 *** | −0.19 ns | 0.45 * | 0.46 * |
| 2008 | 0.27 ns | −0.58 ** | −0.57 ** | −0.22 ns | 0.45 * | 0.45 * |
| 2010 | 0.33 ns | −0.67 *** | −0.58 ** | −0.15 ns | 0.31 ns | 0.28 ns |
| 2011 | 0.55 ** | −0.62 ** | 0.01 ns | 0.36 ns | −0.01 ns | 0.38 ns |
| 2012 | −0.03 ns | −0.50 * | −0.57 ** | 0.28 ns | 0.03 ns | 0.37 ns |
| Mexico-North | ||||||
| 2007 | 0.16 ns | −0.54 ** | −0.37 ns | 0.16 ns | −0.14 ns | 0.06 ns |
| 2008 | 0.09 ns | −0.57 ** | −0.70 *** | −0.14 ns | 0.13 ns | 0.13 ns |
| 2010 | −0.09 ns | −0.41 ns | −0.55 ** | −0.03 ns | −0.06 ns | −0.14 ns |
| 2011 | −0.04 ns | −0.59 ** | −0.70 *** | −0.14 ns | 0.68 *** | 0.56 ** |
| 2012 | −0.34 ns | −0.74 *** | −0.95 *** | 0.34 ns | 0.57 ** | 0.81 *** |
| Mexico-South | ||||||
| 2007 | −0.17 ns | −0.62 ** | −0.81 *** | 0.24 ns | 0.06 ns | 0.36 ns |
| 2008 | −0.08 ns | −0.47 * | −0.76 *** | 0.15 ns | 0.06 ns | 0.32 ns |
| 2010 | 0.74 ** | −0.51 * | 0.67 *** | −0.17 ns | 0.03 ns | −0.18 ns |
| 2011 | 0.39 ns | −0.78 *** | −0.49 * | −0.04 ns | 0.26 ns | 0.40 ns |
| 2012 | 0.29 ns | −0.62 ** | −0.23 ns | 0.02 ns | −0.09 ns | 0.02 ns |
| Year | Days from Emergence to Flowering | Grains/m2 (GN) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Spikes/m2 | Grains/Spike | Spikelets/Spike | Grains/Spikelet | Spikes/m2 | Grains/Spike1 | Spikelets/Spike | Grains/Spikelet | ||
| Spain | |||||||||
| 2010 | 0.14 ns | −0.04 ns | 0.83 *** | −0.29 ns | −0.12 ns | 0.59 *** | 0.22 ns | 0.59 ** | |
| 2011 | 0.46 * | 0.10 ns | 0.74 *** | −0.37 ns | 0.38 ns | 0.67 *** | 0.66 *** | 0.21 ns | |
| 2012 | −0.41 ns | 0.26 ns | 0.66 *** | −0.12 ns | 0.32 ns | 0.72 *** | 0.53 ** | 0.57 ** | |
| Mexico-North | |||||||||
| 2010 | 0.31 ns | −0.22 ns | 0.70 *** | −0.74 *** | 0.44 * | 0.58 ** | 0.45 * | 0.28 ns | |
| 2011 | −0.30 ns | 0.21 ns | 0.70 *** | −0.41 * | 0.36 ns | 0.75 *** | 0.44 * | 0.63 ** | |
| 2012 | −0.50 * | 0.02 ns | 0.75 *** | −0.54 *** | 0.32 ns | 0.65 *** | 0.10 ns | 0.72 *** | |
| Mexico-South | |||||||||
| 2010 | 0.07 ns | 0.63 ** | 0.12 ns | 0.68 *** | 0.15 ns | 0.69 *** | 0.48 * | 0.55 ** | |
| 2011 | −0.41 ns | 0.56 ** | 0.80 *** | 0.34 ns | 0.06 ns | 0.83 *** | 0.55 ** | 0.74 *** | |
| 2012 | −0.14 ns | 0.45 * | 0.41 * | 0.32 ns | 0.54 *** | 0.78 *** | 0.41 * | 0.74 *** | |
| Allele Combination | Grain Yield (GY, g/m2) | Regression Slope (b) | Superiority Measure (Pi) | Shukla’s Stability (σ2) | Wricke’s Ecovalence (Wi2) | Kang’s Yield-Stability (YSi) |
|---|---|---|---|---|---|---|
| I0I | 600 a | 1.12 a | 2542 e | 755 ns | 1563 | +8 |
| SS | 554 a | 0.96 b | 63966 d | −365 ns | 219 | +5 |
| I5I | 547 a | 0.90 b | 80228 c | −80 ns | 561 | +2 |
| I5S | 542 a | 0.87 b | 103535 b | 4143 ** | 5628 | −7 |
| SI | 516 a | 1.19 a,(1) | 139795 a | 3754 * | 5162 | −5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arjona, J.M.; Villegas, D.; Ammar, K.; Dreisigacker, S.; Alfaro, C.; Royo, C. The Effect of Photoperiod Genes and Flowering Time on Yield and Yield Stability in Durum Wheat. Plants 2020, 9, 1723. https://doi.org/10.3390/plants9121723
Arjona JM, Villegas D, Ammar K, Dreisigacker S, Alfaro C, Royo C. The Effect of Photoperiod Genes and Flowering Time on Yield and Yield Stability in Durum Wheat. Plants. 2020; 9(12):1723. https://doi.org/10.3390/plants9121723
Chicago/Turabian StyleArjona, Jose M., Dolors Villegas, Karim Ammar, Susanne Dreisigacker, Christian Alfaro, and Conxita Royo. 2020. "The Effect of Photoperiod Genes and Flowering Time on Yield and Yield Stability in Durum Wheat" Plants 9, no. 12: 1723. https://doi.org/10.3390/plants9121723
APA StyleArjona, J. M., Villegas, D., Ammar, K., Dreisigacker, S., Alfaro, C., & Royo, C. (2020). The Effect of Photoperiod Genes and Flowering Time on Yield and Yield Stability in Durum Wheat. Plants, 9(12), 1723. https://doi.org/10.3390/plants9121723






