Accumulation of Anthocyanins and Other Phytochemicals in American Elderberry Cultivars during Fruit Ripening and its Impact on Color Expression
Abstract
1. Introduction
2. Results
2.1. Phytochemical Attributes of Different Cultivars
2.2. Color Development and Phytochemicals Accumulation during Ripening
2.3. Major Anthocyanins in American Elderberry
2.4. Anthocyanin Profile of Different Cultivars
2.5. Spectral Properties of American Elderberry Extract under Various pH
2.6. Colorimetric Properties of American Elderberry Extract under Various pH
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Collection of Plant Materials
4.3. Determination of Maturity Stage
4.4. Fruit Extracts and Their Quality Attributes
4.5. Quantification of Anthocyanin and Phenolic Content
4.6. Sample Purification
4.7. Anthocyanin Identification
4.8. Buffer and Sample Preparation
4.9. Spectrophotometric and Colorimetric Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACN | Anthocyanin |
| Cou | Coumaroyl |
| Cy | Cyanidin |
| FRFE | Extract from fully-ripe fruits |
| Glu | Glucoside |
| HPLC | High-performance liquid chromatography |
| HRFE | Extract from half-ripe fruits |
| Mono | Monomeric |
| MS | Mass spectroscopy |
| ND | Not detected |
| Poly | Polymeric |
| Sam | Sambubioside |
| TP | Total phenolics |
References
- Lee, J.; Finn, C.E. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (S. nigra) cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Charlebois, D.; Byers, P.L.; Finn, C.E.; Thomas, A.L. Elderberry: Botany, Horticulture, Potential. Hortic. Rev. 2010, 37, 213–280. [Google Scholar]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Todorovic, B.; Veberic, R.; Stampar, F.; Ivancic, A. Investigation of anthocyanin profile of four elderberry species and interspecific hybrids. J. Agric. Food Chem. 2014, 6, 5573–5580. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, M.; Scheerens, J.; Reese, R.; Miller, R. Total phenolic, anthocyanin contents and antioxidant capacity of selected elderberry (Sambucus canadensis L.) accessions. Pharmacogn. Mag. 2010, 6, 198–203. [Google Scholar] [CrossRef]
- Byers, P.L.; Thomas, A.L.; Millican, M. “Wyldewood” elderberry. HortScience 2010, 45, 312–313. [Google Scholar] [CrossRef]
- Finn, C.E.; Thomas, A.L.; Byers, P.L.; Serçe, S. Evaluation of American (Sambucus canadensis) and European (S. nigra) elderberry genotypes grown in diverse environments and implications for cultivar development. HortScience 2008, 43, 1385–1391. [Google Scholar] [CrossRef]
- Iannone, R.; Miranda, S.; Riemma, S.; De Marco, I. Life cycle assessment of red and white wines production in southern Italy. Chem. Eng. Trans. 2014, 39, 595–600. [Google Scholar]
- Wilson, R.J.; Business, R.J.W.; Nickerson, G.; Fried, D.; Tree ER, F.; Nursery, B.; Moonshine, U. Growing Elderberries: A Production Manual and Enterprise Viability Guide for Vermont and the Northeast; UVM Center for Sustainable Agriculture, University of Vermont Extension: Burlington, VT, USA, 2016. [Google Scholar]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food—A review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Torabian, G.; Valtchev, P.; Adil, Q.; Dehghani, F. Anti-influenza activity of elderberry (Sambucus nigra). J. Funct. Foods 2019, 54, 353–360. [Google Scholar] [CrossRef]
- Future Market Insight. Natural Antioxidants Market—Towards a Higher Quality Product Consumption. 2020. Available online: https://www.futuremarketinsights.com/reports/natural-antioxidants-market (accessed on 30 November 2020).
- Tang, P.; Giusti, M.M. Black goji as a potential source of natural color in a wide pH range. Food Chem. 2018, 269, 419–426. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Petkovsek, M.M.; Ivancic, A.; Todorovic, B.; Veberic, R.; Stampar, F. Fruit Phenolic Composition of Different Elderberry Species and Hybrids. J. Food Sci. 2015, 80, 2180–2190. [Google Scholar] [CrossRef] [PubMed]
- Dyrby, M.; Westergaard, N.; Stapelfeldt, H. Light and heat sensitivity of red cabbage extract in soft drink model systems. Food Chem. 2001, 72, 431–437. [Google Scholar] [CrossRef]
- Inami, O.; Tamura, I.; Kikuzaki, H.; Nakatani, N. Stability of Anthocyanins of Sambucus canadensis and Sambucus nigra. J. Agric. Food Chem. 1996, 44, 3090–3096. [Google Scholar] [CrossRef]
- Szalóki-Dorkó, L.; Stéger-Máté, M.; Abrankó, L. Effects of fruit juice concentrate production on individual anthocyanin species in elderberry. Int. J. Food Sci. Technol. 2016, 51, 641–648. [Google Scholar] [CrossRef]
- Szalóki-Dorkó, L.; Stéger-Máté, M.; Abrankó, L. Evaluation of colouring ability of main European elderberry (Sambucus nigra L.) varieties as potential resources of natural food colourants. Int. J. Food Sci. Technol. 2015, 50, 1317–1323. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Thomas, A.L.; Byers, P.L.; Finn, C.E. Fruit composition of elderberry (Sambucus spp.) genotypes grown in Oregon and Missouri, USA. Acta Hortic. 2015, 1061, 219–224. [Google Scholar] [CrossRef]
- Mathieu, F.; Charlebois, D.; Charles, M.T.; Chevrier, N. Biochemical changes in American Elder (Sambucus canadensis) fruits during development. Acta Hortic. 2015, 1061, 61–72. [Google Scholar] [CrossRef]
- Guedes, M.N.S.; de Abreu, C.M.P.; Maro, L.A.C.; Pio, R.; de Abreu, J.R.; de Oliveira, J.O. Caracterização química e teores de minerais em frutos de cultivares de amoreira-preta cultivadas em clima tropical de altitude. Acta Sci. Agron. 2013, 35, 191–196. [Google Scholar]
- Matiacevich, S.; Celis Cofré, D.; Silva, P.; Enrione, J.; Osorio, F. Quality parameters of six cultivars of blueberry using computer vision. Int. J. Food. Sci. 2013, 2013, 419535. [Google Scholar] [CrossRef]
- Urgar, D.D.; Duralija, B.; Voća, S.; Vokurka, A.; Ercisli, S. A comparison of fruit chemical characteristics of two wild grown Rubus species from different locations of Croatia. Molecules 2012, 17, 10390–10398. [Google Scholar]
- Charlebois, D. Elderberry as a Medicinal Plant. In Issues in New Crops and New Uses; ASHS Press: Alexandria, VA, USA, 2007; pp. 284–292. [Google Scholar]
- Elez-Garofulić, I.; Kovačević-Ganić, K.; Galić, I.; Dragovi-Uzelac, V.; Savić, Z. The influence of processing on physico-chemical parameters, phenolics, antioxidant activity and sensory attributes of elderberry (Sambucus nigra L.) fruit wine. Hrvat. Casopis Za prehrambenu Tehnol. Biotehnol. I Nutr. 2012, 7, 9–13. [Google Scholar]
- Sigurdson, G.T.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Impact of location, type, and number of glycosidic substitutions on the color expression of o-dihydroxylated anthocyanidins. Food Chem. 2018, 268, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Macheix, J.J.; Fleuriet, A.; Billot, J. Fruit Phenolics; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Ali, K.; Maltese, F.; Fortes, A.M.; Pais, M.S.; Choi, Y.H.; Verpoorte, R. Monitoring biochemical changes during grape berry development in Portuguese cultivars by NMR spectroscopy. Food Chem. 2011, 124, 1760–1769. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.T.; Wang, C.Y. The influence of light and maturity on fruit quality and flavonoid content of red raspberries. Food Chem. 2009, 112, 676–684. [Google Scholar] [CrossRef]
- He, J.J.; Liu, Y.X.; Pan, Q.H.; Cui, X.Y.; Duan, C.Q. Different anthocyanin profiles of the skin and the pulp of Yan73 (muscat hamburg × alicante bouschet) grape berries. Molecules 2010, 15, 1141–1153. [Google Scholar] [CrossRef]
- Zhou, Y.; Giusti, M.M.; Parker, J.; Salamanca, J.; Rodriguez-Saona, C. Frugivory by Brown Marmorated Stink Bug (Hemiptera: Pentatomidae) Alters Blueberry Fruit Chemistry and Preference by Conspecifics. Environ. Entomol. 2016, 45, 1227–1234. [Google Scholar] [CrossRef][Green Version]
- Harakotr, B.; Suriharn, B.; Tangwongchai, R.; Scott, M.P.; Lertrat, K. Anthocyanins and antioxidant activity in coloured waxy corn at different maturation stages. J. Funct. Foods 2014, 9, 109–118. [Google Scholar] [CrossRef]
- Ahmadiani, N.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Anthocyanins contents, profiles, and color characteristics of red cabbage extracts from different cultivars and maturity stages. J. Agric. Food Chem. 2014, 62, 7524–7531. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Cis-trans configuration of coumaric acid acylation affects the spectral and colorimetric properties of anthocyanins. Molecules 2018, 23, 598. [Google Scholar] [CrossRef]
- Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C.; García-Estévez, I. Wine Color Evolution and Stability. Red Wine Technol. 2018, 195–205. [Google Scholar]
- Schand, J. (Ed.) Colorimetry: Understanding the CIE System; John Wiley & Sons, Inc.: New York, NY, USA, 2007. [Google Scholar]
- Rodriguez-Saona, L.E.; Wrolstad, R.E. Unit F1.1.1-F1.1.11: Extraction, isolation, and purification of anthocyanins. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Unit F1.2.1-F1.2.13: Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Waterhouse, A.L. Unit I1.1: Determination of total phenolics. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Sigurdson, G.T.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Molar absorptivities (ε) and spectral and colorimetric characteristics of purple sweet potato anthocyanins. Food Chem. 2019, 271, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Farr, J.E.; Giusti, M.M. ColorBySpectra-Academic License. Available online: https://buckeyevault.com/ products/absorbance-to-color-al (accessed on 4 December 2020).
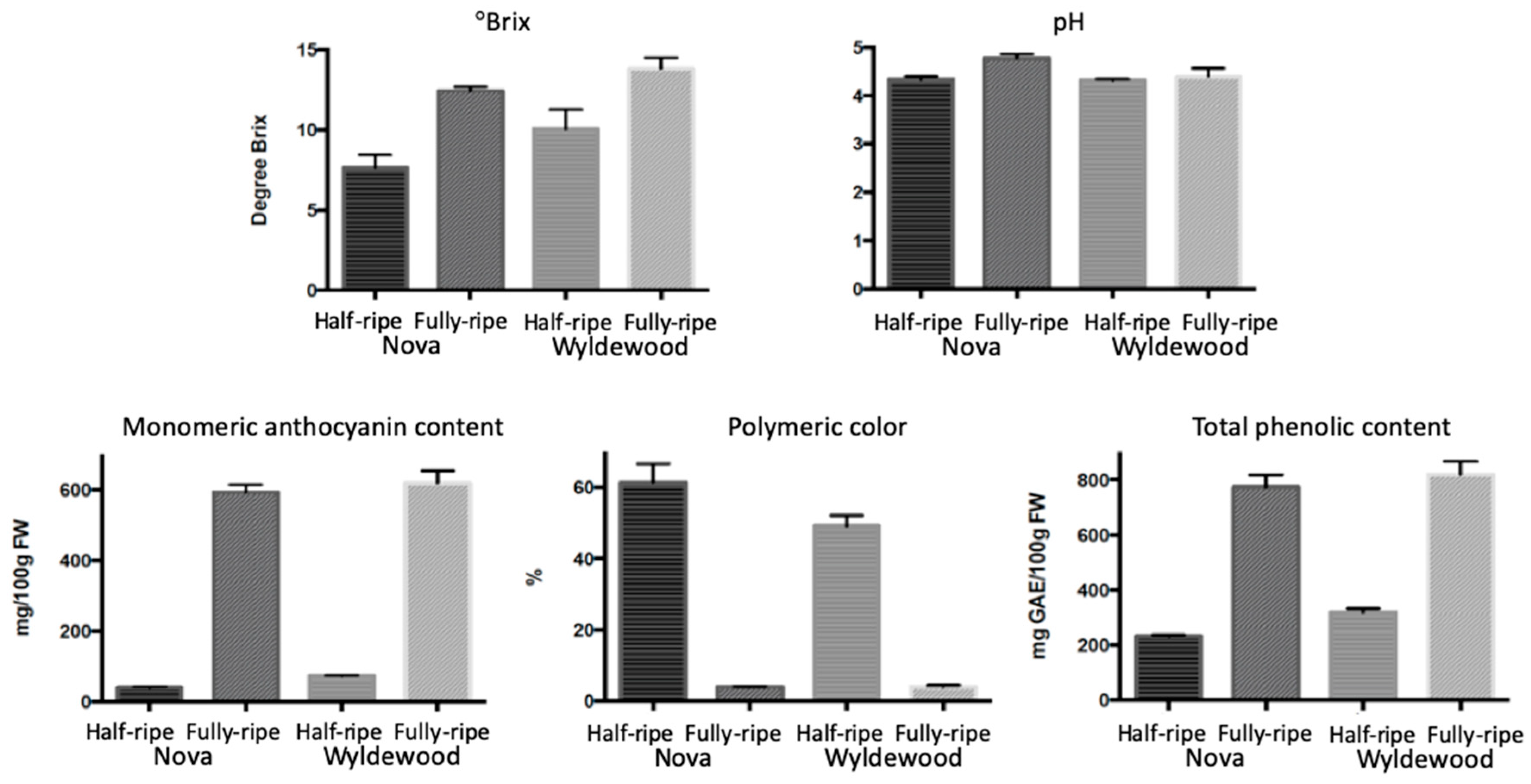
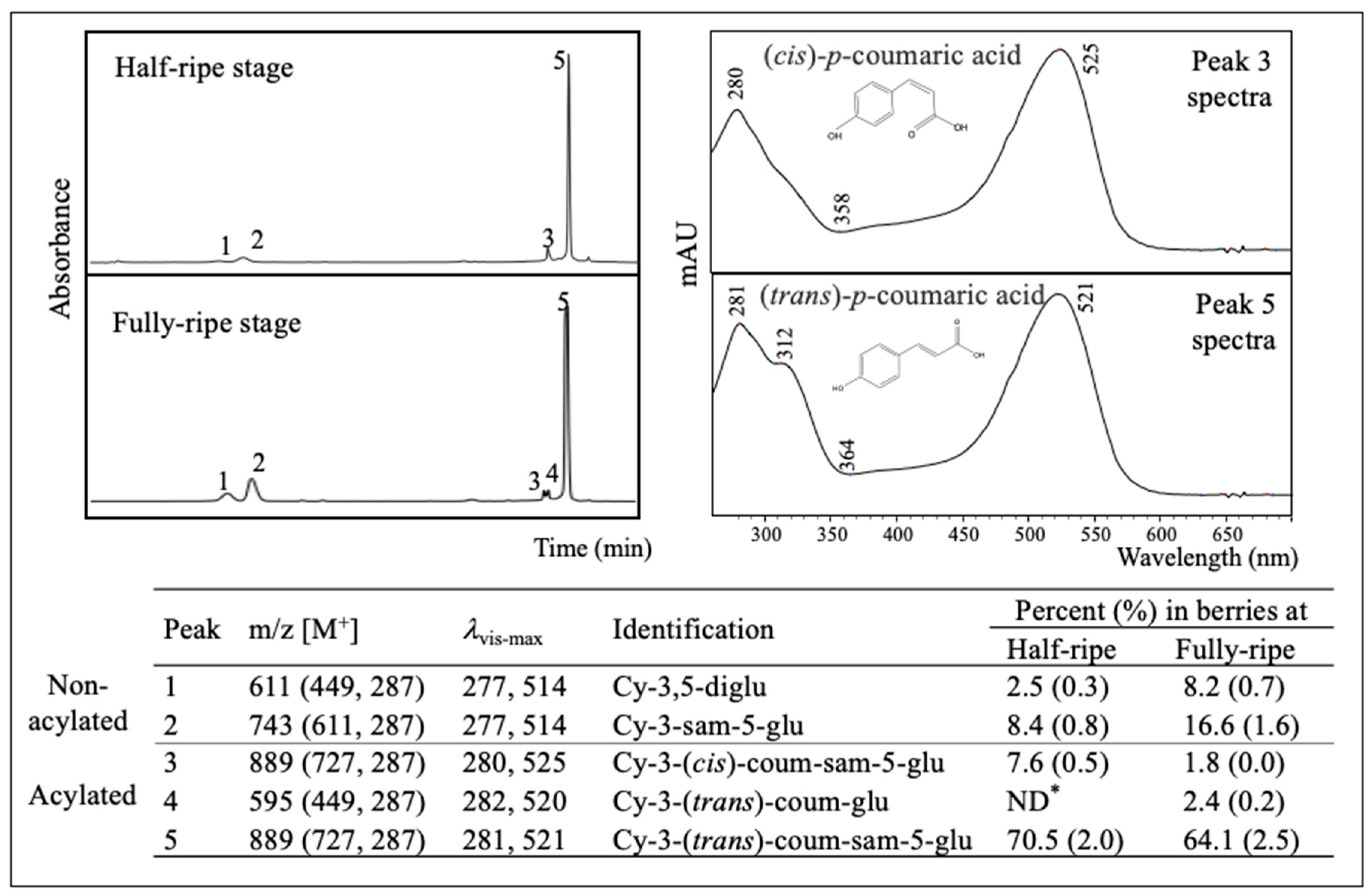


| Cultivar | °Brix | pH | Mono ACN 1 | Poly Color 2 | TP 3 | ACN/TP 4 |
|---|---|---|---|---|---|---|
| Adams | 12.9 ± 1.1 a | 4.7 ± 0.11 a,b | 499 ± 91 a,b | 7.5 ± 0.8 a,b | 684 ± 88 a | 73 ± 4 a |
| Johns | 12.0 ± 1.1 a | 4.8 ± 0.05 b | 595 ± 26 b | 5.0 ± 0.5 a | 706 ± 22 a | 84 ± 4 b |
| Nova | 12.4 ± 0.3 a | 4.8 ± 0.06 b | 456 ± 73 a,b | 9.1 ± 1.5 a,b | 700 ± 77 a | 65 ± 4 a |
| Wyldewood | 12.0 ± 1.1 a | 4.5 ± 0.08 a | 471 ± 36 a,b | 7.3 ± 0.4 a,b | 637 ± 51 a | 74 ± 2 a,b |
| York | 13.1 ± 0.4 a | 4.9 ± 0.12 b | 354 ± 59 a | 10.8 ± 1.1 b | 582 ± 52 a | 61 ± 3 a |
| Peak | Adam | Johns | Nova | Wyldewood | York | Overall | |
|---|---|---|---|---|---|---|---|
| Non-acylated | 1 | 7.2 ± 0.7 b | 7.9 ± 0.7 b | 8.2 ± 0.7 b | 8.5 ± 0.5 b | 5.2 ± 0.2 a | 7.4 ± 1.3 |
| 2 | 16.2 ± 2.3 a | 15.4 ± 1.1 a | 16.6 ± 1.6 a | 18.6 ± 0.5 a | 16.1 ± 0.7 a | 16.6 ± 1.7 | |
| Sum | 23.3 ± 3 a | 23.3 ± 1.8 a | 24.8 ± 2.2 a | 27.2 ± 1.0 b | 21.3 ± 0.9 a | 24.0 ± 2.6 | |
| Acylated | 3 | 2.3 ± 0.2 b | 1.7 ± 0.1 a | 1.8 ± 0.0 a | 1.9 ± 0.1 a | 2.3 ± 0.2 b | 2.0 ± 0.3 |
| 4 | 2.4 ± 0.2 b | 2.1 ± 0.1 b | 2.4 ± 0.2 b | 1.5 ± 0.1 a | 2.1 ± 0.3 b | 2.1 ± 0.4 | |
| 5 | 67.1 ± 2.7 b | 67.0 ± 2.3 b | 64.1 ± 2.5 a,b | 59.8 ± 0.6 a | 67.0 ± 1.8 b | 65.0 ± 3.5 | |
| Sum | 71.9 ± 2.4 b | 70.8 ± 2.2 b | 68.3 ± 2.6 a,b | 63.2 ± 0.6 a | 71.4 ± 1.6 b | 69.1 ± 3.7 | |
| Extracts | pH2 | pH3 | pH4 | pH5 | pH6 | pH7 | pH8 | pH9 | |
|---|---|---|---|---|---|---|---|---|---|
| Lambda Max (lmax, nm) | |||||||||
| Half-ripe | CE 1 | 520 ± 1 | 515 ± 1 | ND 2 | ND | ND | ND | 593 ± 2 | 580 ± 0 |
| C18 | 519 ± 3 | 522 ± 1 | 516 ± 3 | ND | ND | 540 ± 3 | 596 ± 1 | 577 ± 5 | |
| Fully-ripe | CE | 519 ± 2 | 524 ± 3 | 523 ± 1 | 529 ± 9 | 528 ± 2 | 549 ± 1 | 600 ± 0 | 600 ± 0 |
| C18 | 520 ± 0 | 522 ± 0 | 524 ± 3 | 527 ± 1 | 523 ± 6 | 551 ± 2 | 597 ± 1 | 597 ± 1 | |
| L* (Lightness) | |||||||||
| Half-ripe | CE | 54.8 ± 0.3 | 59.8 ± 0.1 | 64.2 ± 1.5 | 66.9 ± 0.1 | 68.0 ± 0.1 | 66.0 ± 0.3 | 54.6 ± 0.6 | 54.6 ± 0.4 |
| C18 | 55.5 ± 0.1 | 60.0 ± 0.5 | 70.5 ± 0.4 | 73.1 ± 0.3 | 74.1 ± 2.3 | 56.3 ± 0.4 | 35.5 ± 0.4 | 54.9 ± 0.5 | |
| Fully-ripe | CE | 64.0 ± 0.7 | 75.6 ± 0.4 | 85.4 ± 0.3 | 88.1 ± 0.3 | 88.3 ± 0.2 | 83.0 ± 0.3 | 73.8 ± 0.3 | 73.4 ± 0.7 |
| C18 | 63.7 ± 0.1 | 73.0 ± 0.2 | 85.1 ± 0.1 | 87.3 ± 0.5 | 87.8 ± 0.7 | 58.3 ± 0.9 | 39.4 ± 0.1 | 45.3 ± 0.4 | |
| C* (Chroma) | |||||||||
| Half-ripe | CE | 50.8 ± 0.5 | 40.5 ± 0.2 | 33.7 ± 0.3 | 32.5 ± 0.1 | 31.4 ± 0.3 | 30.3 ± 0.3 | 27.0 ± 0.5 | 48.9 ± 0.4 |
| C18 | 67.5 ± 0.1 | 52.6 ± 0.2 | 30.7 ± 0.1 | 26.3 ± 0.2 | 25.5 ± 2.1 | 22.4 ± 0.1 | 7.4 ± 0.4 | 46.5 ± 0.5 | |
| Fully-ripe | CE | 52.6 ± 0.3 | 28.6 ± 0.3 | 8.1 ± 0.2 | 3.2 ± 0.2 | 2.5 ± 0.2 | 2.7 ± 0.3 | 17.5 ± 0.2 | 14.3 ± 0.6 |
| C18 | 66.0 ± 0.1 | 45.7 ± 0.5 | 15.4 ± 0.2 | 7.7 ± 0.2 | 7.1 ± 0.2 | 34.4 ± 0.7 | 41.5 ± 0.4 | 30.9 ± 0.2 | |
| h* (Hue Angle) | |||||||||
| Half-ripe | CE | 31.9 ± 0 | 42.5 ± 0.1 | 58.0 ± 0.3 | 67.4 ± 0.2 | 70.8 ± 0.2 | 72.2 ± 0.2 | 103.1 ± 0.1 | 88.3 ± 0.4 |
| C18 | 23.9 ± 0.1 | 23.2 ± 0.3 | 44.6 ± 0.3 | 60.5 ± 0.3 | 64.7 ± 1.2 | 32.4 ± 0.4 | 168.0 ± 4.6 | 85.5 ± 0.5 | |
| Fully-ripe | CE | 1.1 ± 0.6 | 356.9 ± 0.2 | 7.5 ± 0.7 | 36.2 ± 1.4 | 51.6 ± 0.8 | 341.8 ± 4.0 | 249.2 ± 0.3 | 153.4 ± 1.0 |
| C18 | 9.9 ± 0.1 | 1.4 ± 0.1 | 10.9 ± 0.6 | 28.9 ± 0.6 | 36.7 ± 1.6 | 316.2 ± 0.5 | 270.4 ± 0.4 | 238.1 ± 0.2 | |
| Johns | Nova | Wyldewood | York | |
|---|---|---|---|---|
| Adam | ||||
| pH2 | 1.25 | 4.61 | 0.91 | 3.31 |
| pH3 | 0.81 | 4.34 | 1.60 | 8.05 |
| pH4 | 1.09 | 0.86 | 0.17 | 3.52 |
| pH7 | 1.18 | 2.18 | 1.61 | 4.38 |
| pH8 | 6.06 | 6.11 | 8.78 | 14.44 |
| Johns | ||||
| pH2 | 5.34 | 1.81 | 4.21 | |
| pH3 | 4.79 | 2.16 | 8.21 | |
| pH4 | 1.75 | 1.05 | 3.60 | |
| pH7 | 2.71 | 12.13 | 4.62 | |
| pH8 | 1.49 | 3.00 | 9.21 | |
| Nova | ||||
| pH2 | 3.96 | 3.69 | ||
| pH3 | 2.92 | 8.25 | ||
| pH4 | 0.78 | 3.30 | ||
| pH7 | 2.48 | 2.40 | ||
| pH8 | 2.75 | 8.59 | ||
| Wyldewood | ||||
| pH2 | 2.50 | |||
| pH3 | 7.28 | |||
| pH4 | 3.41 | |||
| pH7 | 3.97 | |||
| pH8 | 6.73 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Gao, Y.G.; Giusti, M.M. Accumulation of Anthocyanins and Other Phytochemicals in American Elderberry Cultivars during Fruit Ripening and its Impact on Color Expression. Plants 2020, 9, 1721. https://doi.org/10.3390/plants9121721
Zhou Y, Gao YG, Giusti MM. Accumulation of Anthocyanins and Other Phytochemicals in American Elderberry Cultivars during Fruit Ripening and its Impact on Color Expression. Plants. 2020; 9(12):1721. https://doi.org/10.3390/plants9121721
Chicago/Turabian StyleZhou, Yucheng, Yu Gary Gao, and M. Monica Giusti. 2020. "Accumulation of Anthocyanins and Other Phytochemicals in American Elderberry Cultivars during Fruit Ripening and its Impact on Color Expression" Plants 9, no. 12: 1721. https://doi.org/10.3390/plants9121721
APA StyleZhou, Y., Gao, Y. G., & Giusti, M. M. (2020). Accumulation of Anthocyanins and Other Phytochemicals in American Elderberry Cultivars during Fruit Ripening and its Impact on Color Expression. Plants, 9(12), 1721. https://doi.org/10.3390/plants9121721








