Integrated Physiological and Transcriptomic Analyses Reveal a Regulatory Network of Anthocyanin Metabolism Contributing to the Ornamental Value in a Novel Hybrid Cultivar of Camellia japonica
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Condition
2.2. Measurement of Leaf Color Indices and Contents of Pigments
2.3. Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometer (UPLC-MS/MS)
2.4. Statistics for Physiological Indices
2.5. RNA-Seq and Data Processing
2.6. Quantitative Real-Time PCR (qPCR)
3. Results and Discussion
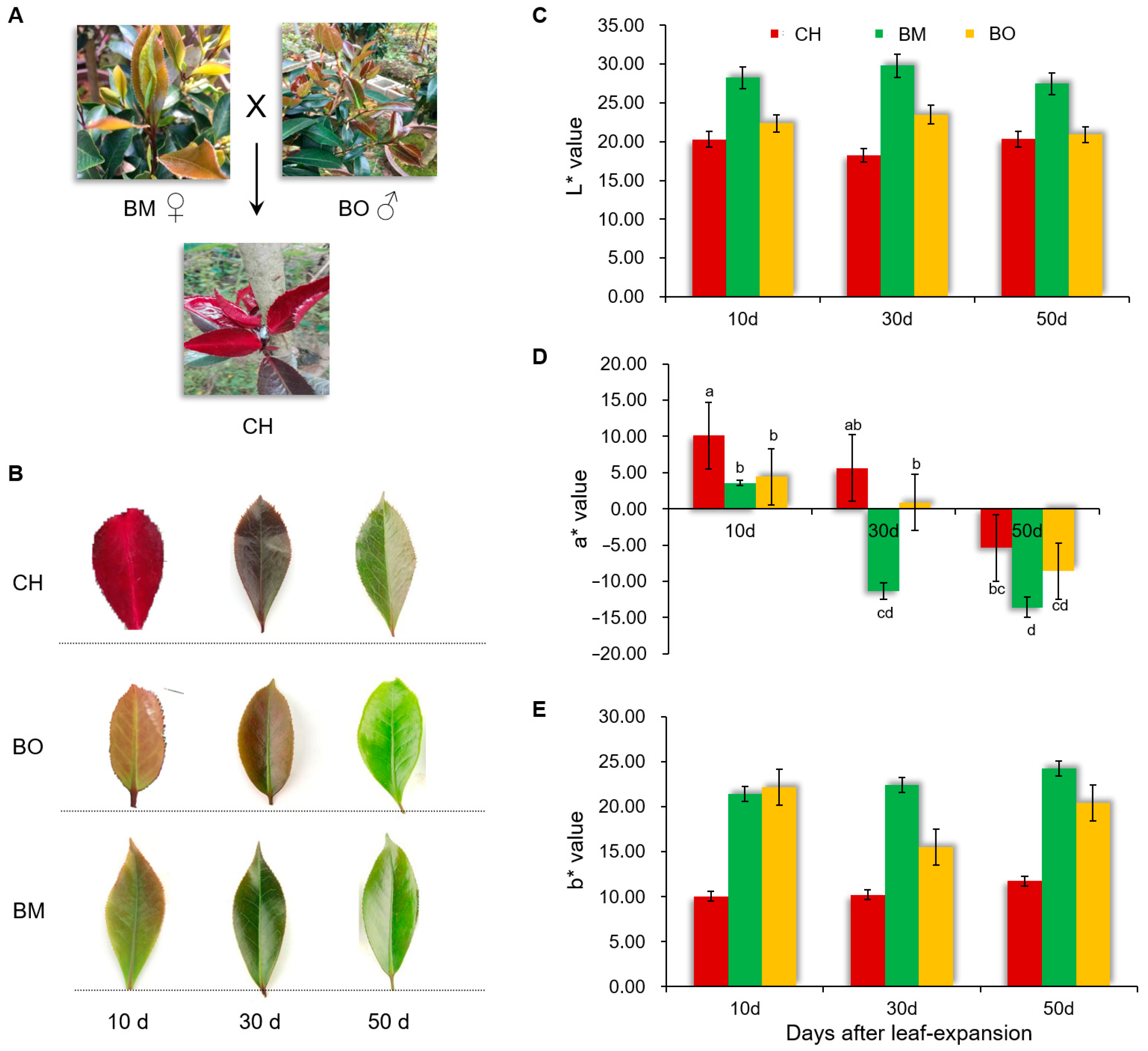
3.1. Characterization of the Red-Leaf Phenotype in Camellia japonica Cv. Chunjiang Hongxia
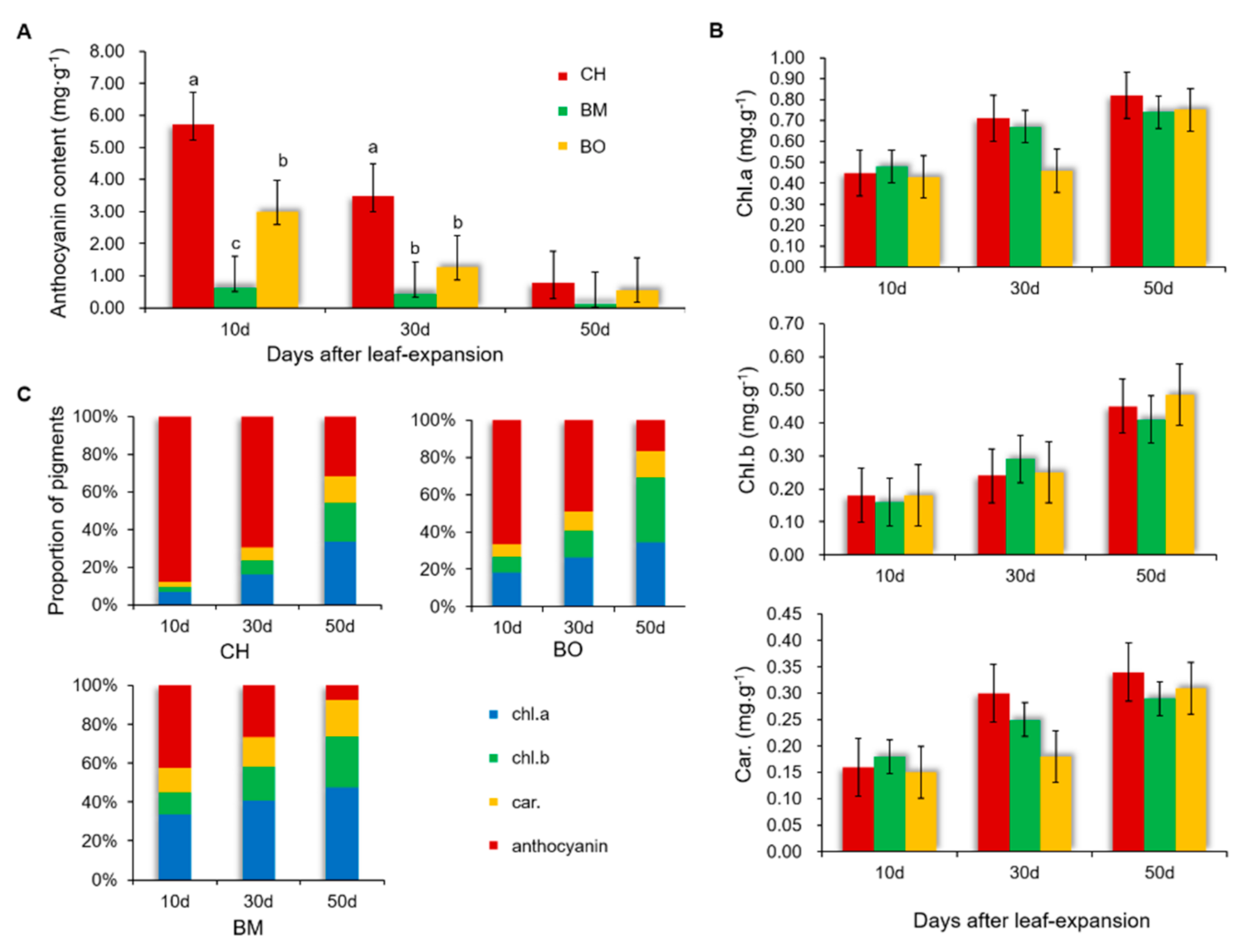
3.2. Contents of Pigments in CH Leaves
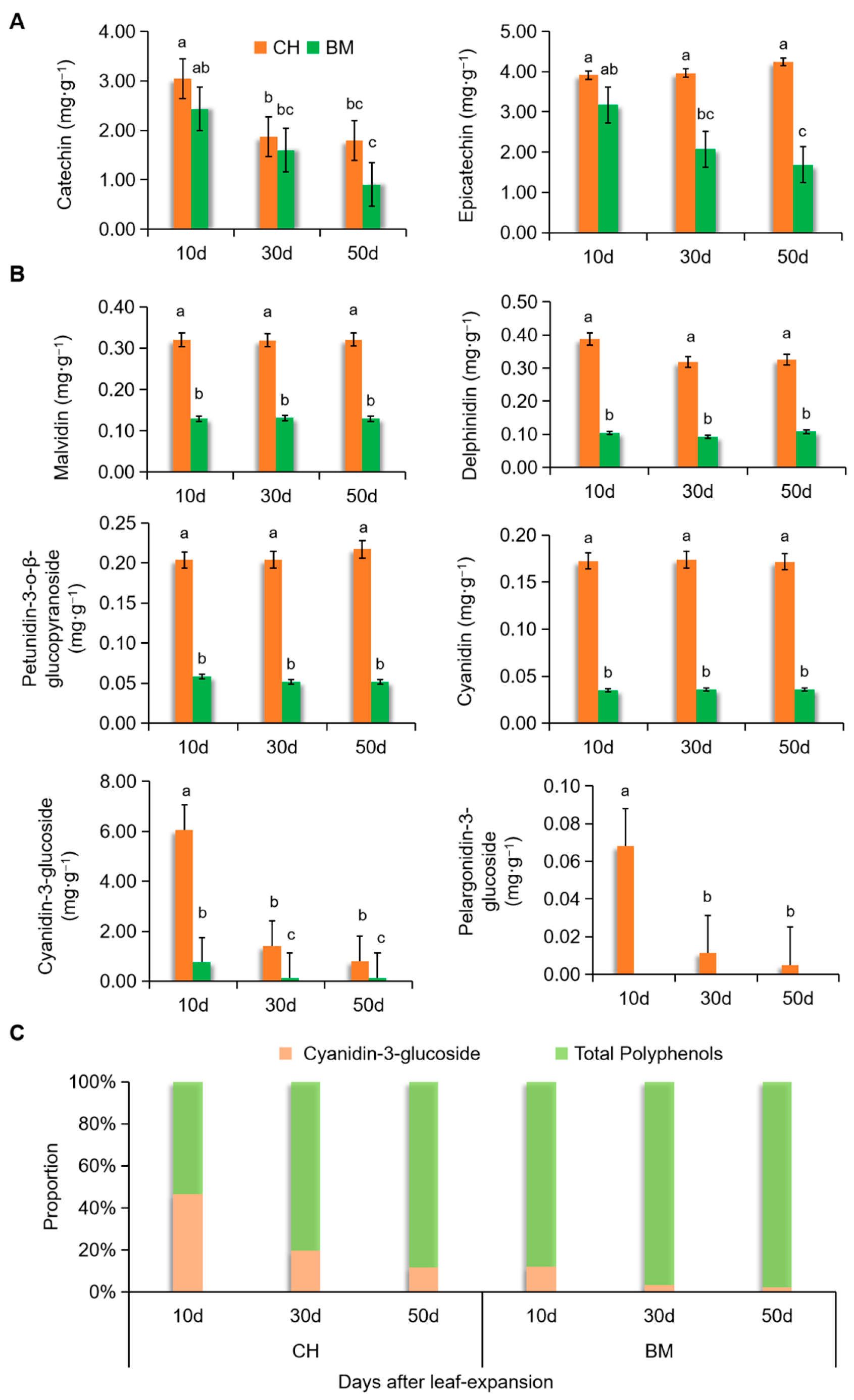
3.3. Dynamic Changes of Polyphenols and Anthocyanins in CH Leaves
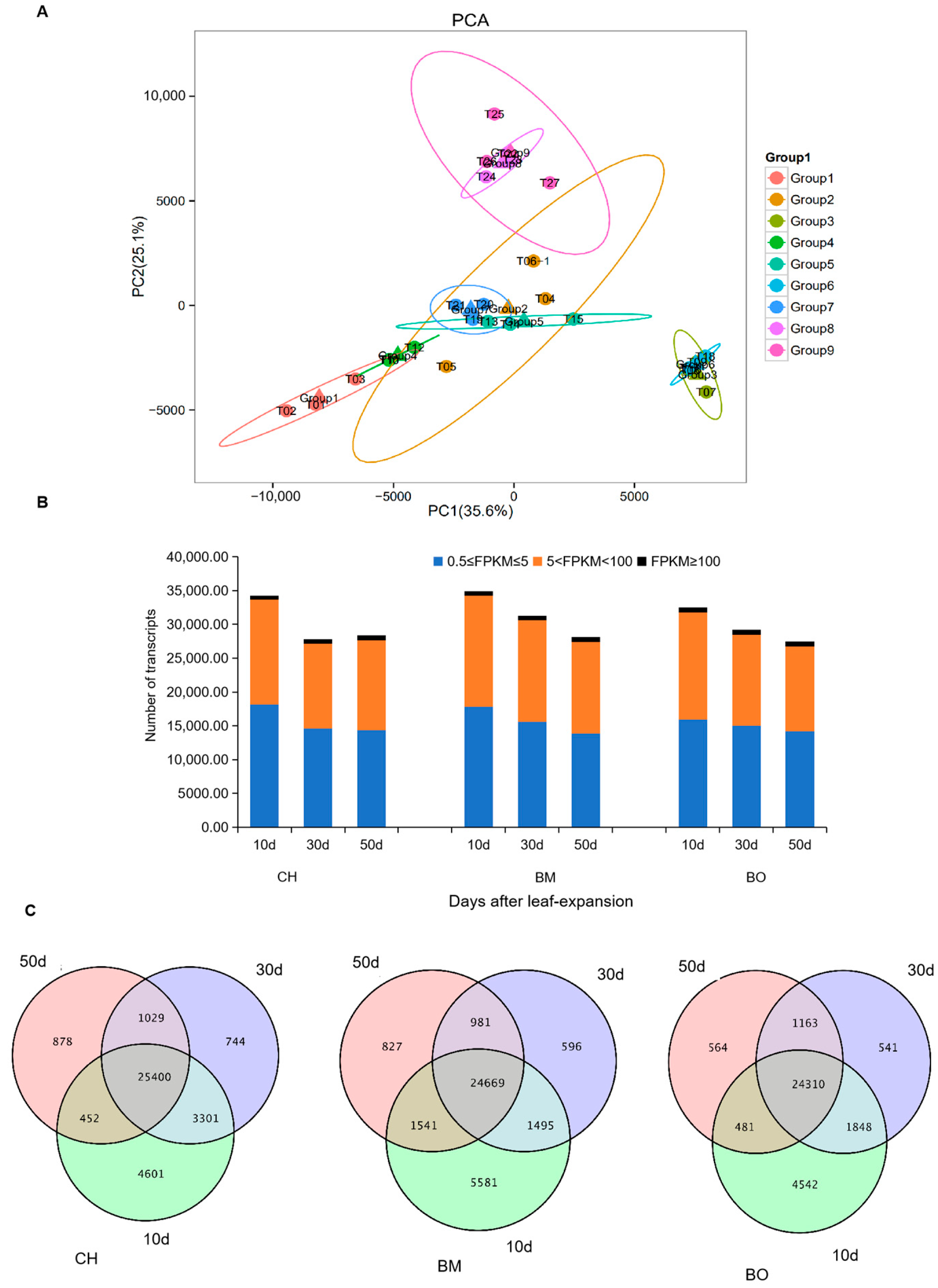
3.4. Transcriptome Assembling and Annotation
3.5. Co-Expression Module Analysis of Transcribed Unigenes
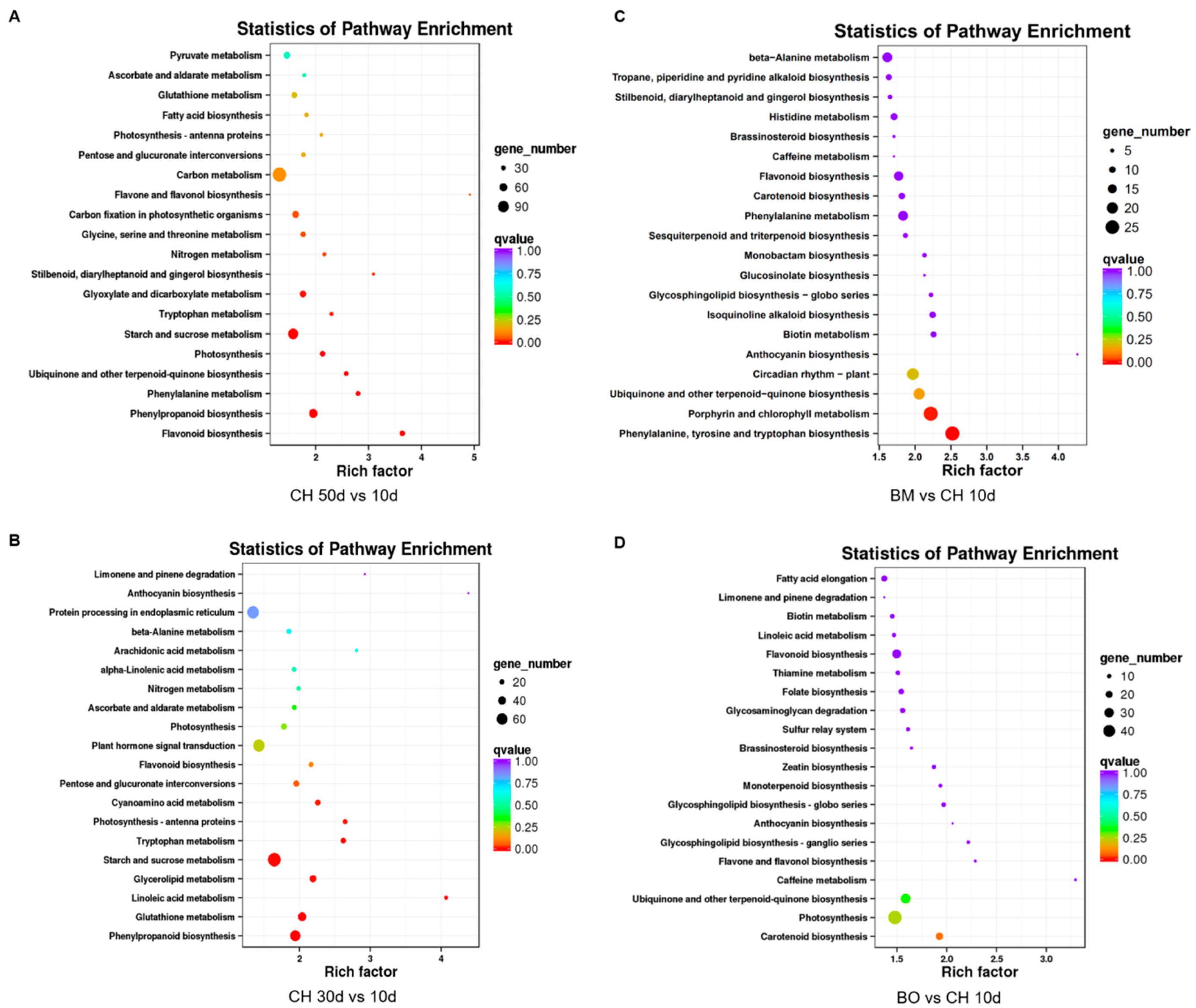
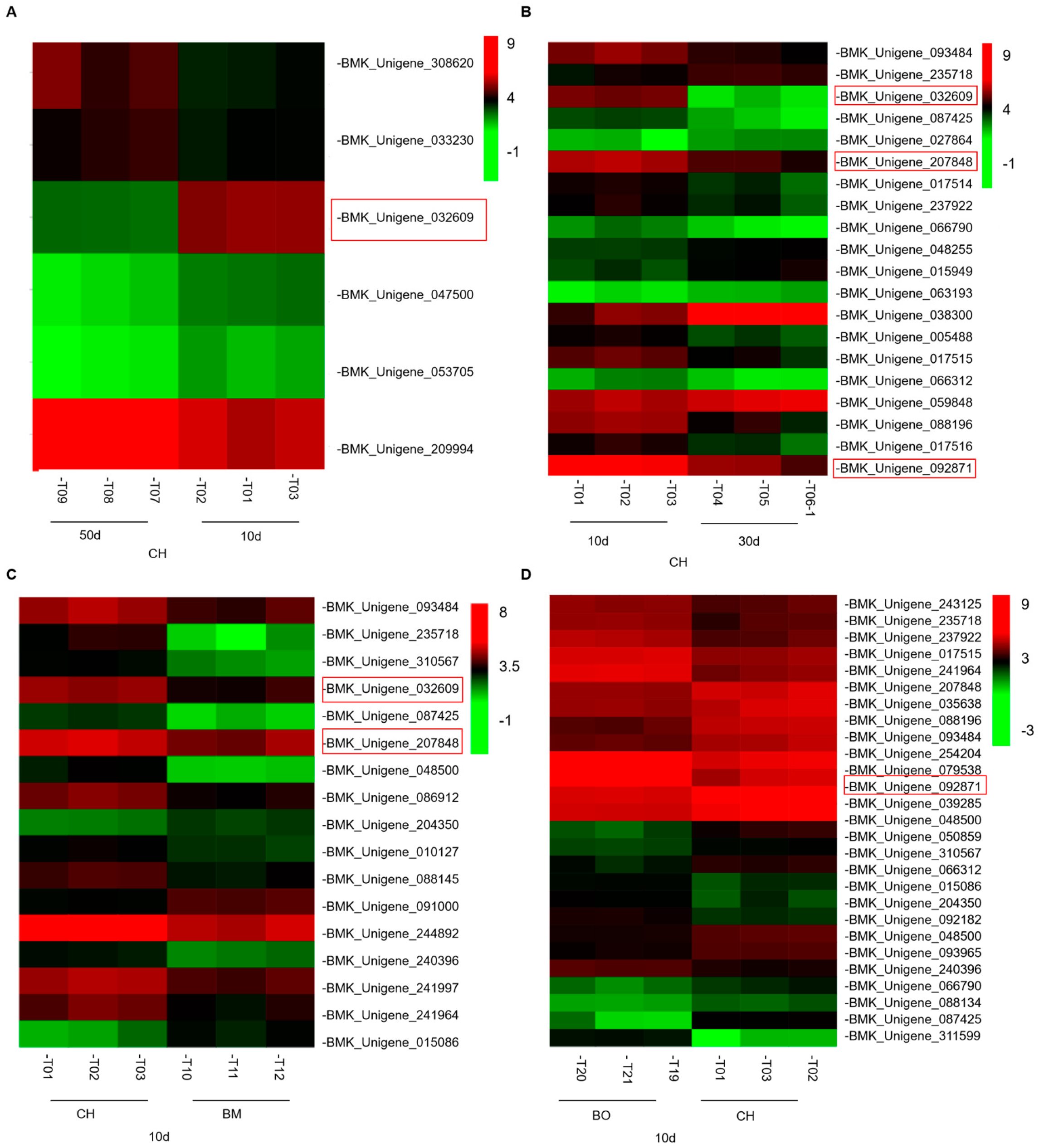
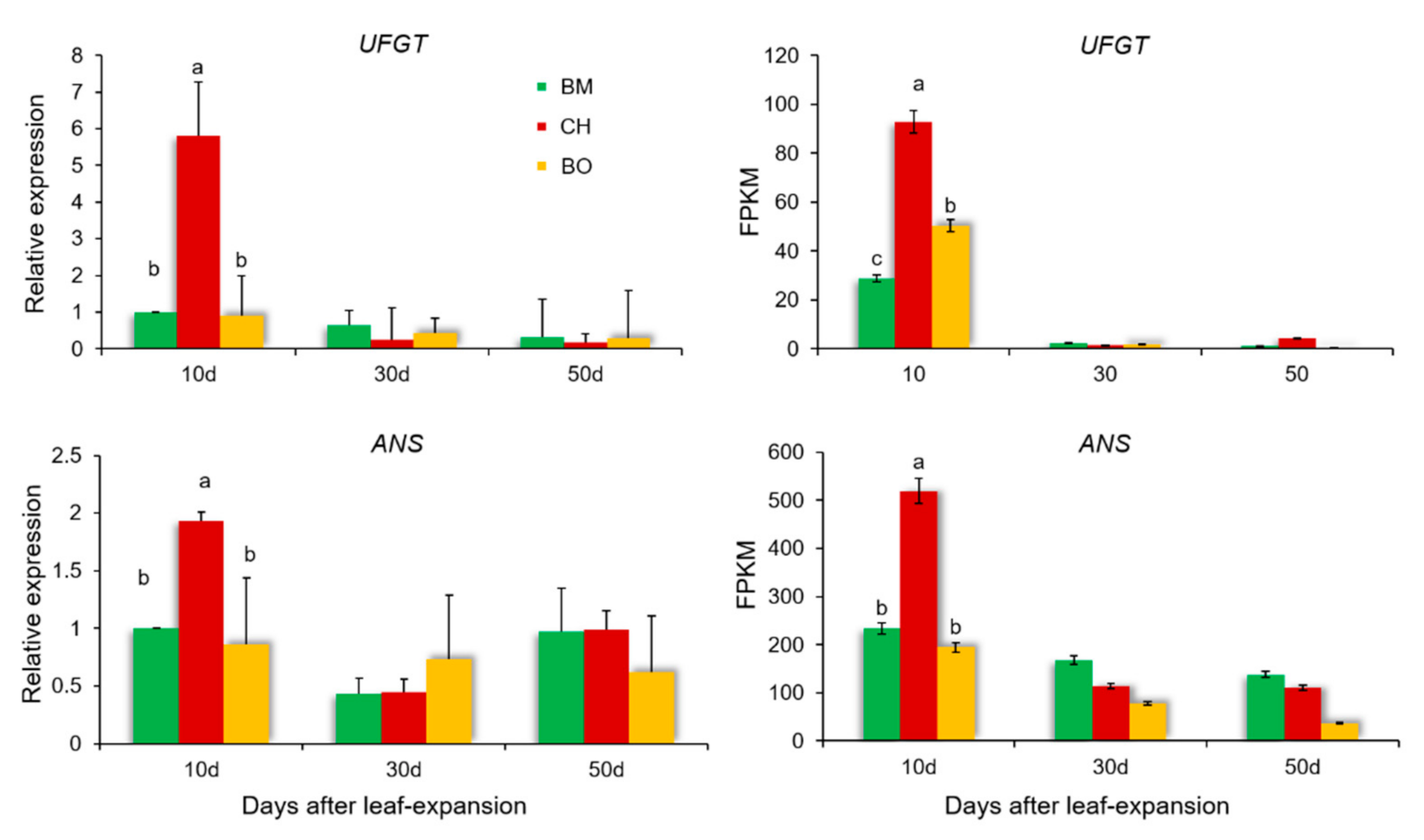
3.6. Differential Expression Analysis Reveals a Regulatory Network of Anthocyanin Metabolism Related to Red-Leaf Phenotype
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Savige, T.J. The International Camellia Register; The International Camellia Society: Wirlinga, Australia, 1993. [Google Scholar]
- Vela, P.; Salinero, C.; Sainz, M. Phenological growth stages of Camellia japonica. Ann. Appl. Biol. 2013, 162, 182–190. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Y.; Short, P.; Yamaguchi, S.; Bergamini, D.; Salinero, C.; Beard, D.; Powell, J.; Corneo, A. The progress of international camellia register and development of camellia cultivar dictionary. In Proceedings of the International Camellia Society Congress, Nantes, France, 24–29 March 2018; pp. 24–33. [Google Scholar]
- Ying, Z.; Zhang, J.; Ying, H.; Fan, Z.; Ni, S.; Wu, B.; Li, J. Content change of main chemical component relating to the red leaf of bud mutation Camellia variety ‘Jinhua Meinü’. Acta Hortic. Sin. 2017, 44, 723–732. [Google Scholar]
- Li, Y.; Zhang, Z.; Wang, P.; Wang, S.; Ma, L.; Li, L.; Yang, R.; Ma, Y.; Wang, Q. Comprehensive transcriptome analysis discovers novel candidate genes related to leaf color in a Lagerstroemia indica yellow leaf mutant. Genes Genomics 2015, 37, 851–863. [Google Scholar] [CrossRef]
- Li, C.-F.; Xu, Y.-X.; Ma, J.-Q.; Jin, J.-Q.; Huang, D.-J.; Yao, M.-Z.; Ma, C.-L.; Chen, L. Biochemical and transcriptomic analyses reveal different metabolite biosynthesis profiles among three color and developmental stages in ‘Anji Baicha’ (Camellia sinensis). BMC Plant Biol. 2016, 16, 195. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Xu, B.; Li, Y.; Ma, Y.; Wang, G. Phenotype and transcriptome analysis reveals chloroplast development and pigment biosynthesis together influenced the leaf color formation in mutants of Anthurium andraeanum ‘Sonate’. Front. Plant Sci. 2015, 6, 139. [Google Scholar] [CrossRef]
- Karageorgou, P.; Manetas, Y. The importance of being red when young: Anthocyanins and the protection of young leaves of Quercus coccifera from insect herbivory and excess light. Tree Physiol. 2006, 26, 613–621. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, M.; Meng, X.; Wan, H.; Zhang, J.; Tian, J.; Hao, S.; Jin, K.; Yao, Y. Photoperiod and shading regulate coloration and anthocyanin accumulation in the leaves of malus crabapples. Plant Cell Tissue Organ Cult. 2015, 121, 619–632. [Google Scholar] [CrossRef]
- Nesumi, A.; Ogino, A.; Yoshida, K.; Taniguchi, F.; Yamamoto, M.; Tanaka, J.; Murakami, A. ‘Sunrouge’, a New Tea Cultivar with High Anthocyanin. Jpn. Agric. Res. Q. 2012, 46, 321–328. [Google Scholar] [CrossRef]
- Zhang, L.-Q.; Wei, K.; Cheng, H.; Wang, L.-Y.; Zhang, C.-C. Accumulation of catechins and expression of catechin synthetic genes in Camellia sinensis at different developmental stages. Bot. Stud. 2016, 57. [Google Scholar] [CrossRef]
- Zhang, L.; Tai, Y.; Wang, Y.; Meng, Q.; Yang, Y.; Zhang, S.; Yang, H.; Zhang, Z.; Li, D.; Wan, X. The proposed biosynthesis of procyanidins by the comparative chemical analysis of five Camellia species using LC-MS. Sci. Rep. 2017, 7, 46131. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Fan, Z.; Liu, Z.; Tanaka, T.; Yin, H. Global gene expression defines faded whorl specification of double flower domestication in Camellia. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y.; She, G.; Zhang, X.; Jordan, B.; Chen, Q.; Zhao, J.; Wan, X. Metabolite profiling and transcriptomic analyses reveal an essential role of UVR8-mediated signal transduction pathway in regulating flavonoid biosynthesis in tea plants (Camellia sinensis) in response to shading. BMC Plant Biol. 2018, 18, 233. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, Z.; Lee, J.; Li, X.; Sun, W. Proteomic analysis of tea plants (Camellia sinensis) with purple young shoots during leaf development. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, S.; Xu, B. The World ’s Best Camellia Cultivars; Zhejiang Science and Technology Publishing House: Hangzhou, China, 1998. [Google Scholar]
- Gao, J.; Su, Y.; Hu, X. The Identification and Appreciation of the World’s Outstanding Camellias; Zhejiang Science and Technology Publishing House: Hangzhou, China, 2007. [Google Scholar]
- Wang, L.-S.; Shiraishi, A.; Hashimoto, F.; Aoki, N.; Shimizu, K.; Sakata, Y. Analysis of petal anthocyanins to investigate flower coloration of Zhongyuan (Chinese) and Daikon Island (Japanese) tree peony cultivars. J. Plant Res. 2001, 114, 33–43. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophyll-a and chlorophhyll-b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Kerio, L.; Wachira, F.; Wanyoko, J.; Rotich, M. Characterization of anthocyanins in Kenyan teas: Extraction and identification. Food Chem. 2012, 131, 31–38. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, L.; Liao, Y.; Zhou, Y.; Xu, X.; Dong, F.; Yang, Z. An alternative pathway for the formation of aromatic aroma compounds derived from L-phenylalanine via phenylpyruvic acid in tea (Camellia sinensis (L.) O. Kuntze) leaves. Food Chem. 2019, 270, 17–24. [Google Scholar] [CrossRef]
- Cock, P.J.A.; Fields, C.J.; Goto, N.; Heuer, M.L.; Rice, P.M. The Sanger FASTQ file format for sequences with quality scores, and the Solexa/Illumina FASTQ variants. Nucleic Acids Res. 2010, 38, 1767–1771. [Google Scholar] [CrossRef]
- Cox, M.P.; Peterson, D.A.; Biggs, P.J. SolexaQA: At-a-glance quality assessment of Illumina second-generation sequencing data. BMC Bioinform. 2010, 11. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Apweiler, R.; Bairoch, A.; Wu, C.; Barker, W.; Boeckmann, B.; Ferro, S.; Gasteiger, E.; Huang, H.; Lopez, R.; Magrane, M.; et al. UniProt: The Universal Protein knowledgebase. Nucleic Acids Res. 2004, 32, D115–D119. [Google Scholar] [CrossRef] [PubMed]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, J.; Wu, S.; Zhu, Y.; Chen, Y.; He, F. Integrated NR database in protein annotation system and its localization. Comput. Eng. 2006, 32, 71–72. [Google Scholar]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef]
- Ye, J.; Fang, L.; Zheng, H.; Zhang, Y.; Chen, J.; Zhang, Z.; Wang, J.; Li, S.; Li, R.; Bolund, L. WEGO: A web tool for plotting GO annotations. Nucleic Acids Res. 2006, 34, W293–W297. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Rismani-Yazdi, H.; Haznedaroglu, B.Z.; Bibby, K.; Peccia, J. Transcriptome sequencing and annotation of the microalgae Dunaliella tertiolecta: Pathway description and gene discovery for production of next-generation biofuels. BMC Genomics 2011, 12, 148. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Shi, Q.; Niu, L.; Zhang, Y. Transcriptomic Analysis of Leaf in Tree Peony Reveals Differentially Expressed Pigments Genes. Molecules 2017, 22, 324. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008, 53, 814–827. [Google Scholar] [CrossRef]
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Li, J.-B.; Hashimoto, F.; Shimizu, K.; Sakata, Y. Anthocyanins from red flowers of Camellia cultivar ‘Dalicha’. Phytochemistry 2008, 69, 3166–3171. [Google Scholar] [CrossRef]
- Li, J.-B.; Hashimoto, F.; Shimizu, K.; Sakata, Y. Chemical taxonomy of red-flowered wild Camellia species based on floral anthocyanins. Phytochemistry 2013, 85, 99–106. [Google Scholar] [CrossRef]
- Holton, T.A.; Cornish, E.C. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 1995, 7, 1071–1083. [Google Scholar] [CrossRef]
- Shimada, N.; Sasaki, R.; Sato, S.; Kaneko, T.; Tabata, S.; Aoki, T.; Ayabe, S.-I. A comprehensive analysis of six dihydroflavonol 4-reductases encoded by a gene cluster of the Lotus japonicus genome. J. Exp. Bot. 2005, 56, 2573–2585. [Google Scholar] [CrossRef]
- Wang, F.; Dong, Y.X.; Tang, X.Z.; Tu, T.L.; Zhao, B.; Sui, N.; Fu, D.L.; Zhang, X.S. Comparative Transcriptome Analysis Revealing the Effect of Light on Anthocyanin Biosynthesis in Purple Grains of Wheat. J. Agric. Food Chem. 2018, 66, 3465–3476. [Google Scholar] [CrossRef]
- Baker, R.L.; Leong, W.F.; Brock, M.T.; Rubin, M.J.; Markelz, R.J.C.; Welch, S.; Maloof, J.N.; Weinig, C. Integrating transcriptomic network reconstruction and eQTL analyses reveals mechanistic connections between genomic architecture and Brassica rapa development. PLoS Genet. 2019, 15. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Liu, M.; Chen, H.; Zheng, Y.; Liu, Y.; Wang, X.; Yang, S.; Zhou, M.; Lin, J. INDUCER OF CBF EXPRESSION 1 is a male fertility regulator impacting anther dehydration in Arabidopsis. PLoS Genet. 2018, 14. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Gong, Y.; Jin, S.; Zhu, Q. Molecular analysis of a UDP-glucose: Flavonoid 3-O-glucosyltransferase (UFGT) gene from purple potato (Solanum tuberosum). Mol. Biol. Rep. 2011, 38, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gong, Q.; Ni, X.; Zhou, Y.; Gao, Z. UFGT: The Key Enzyme Associated with the Petals Variegation in Japanese Apricot. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
| Cultivars | Leaf Color Parameter | Chlorophyll a | Chlorophyll b | Anthocyanin | Carotenoid |
|---|---|---|---|---|---|
| CH | L* | −0.999 * | −0.962 | 0.945 | −0.999 * |
| a* | −0.971 | −1.000 ** | 0.999 * | −0.948 | |
| b* | 0.886 | 0.757 | −0.719 | 0.920 | |
| BM | L * | −0.972 | −0.889 | 0.782 | −0.942 |
| a* | −0.914 | −0.984 | 1.000 * | −0.951 | |
| b * | 0.899 | 0.772 | −0.631 | 0.849 | |
| BO | L* | −0.735 | −0.722 | 0.971 | −0.794 |
| a* | −0.906 | −0.898 | 0.998* | −0.941 | |
| b* | 0.190 | 0.208 | 0.469 | 0.098 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Li, J.; Yin, H.; Fan, Z.; Li, X. Integrated Physiological and Transcriptomic Analyses Reveal a Regulatory Network of Anthocyanin Metabolism Contributing to the Ornamental Value in a Novel Hybrid Cultivar of Camellia japonica. Plants 2020, 9, 1724. https://doi.org/10.3390/plants9121724
Pan L, Li J, Yin H, Fan Z, Li X. Integrated Physiological and Transcriptomic Analyses Reveal a Regulatory Network of Anthocyanin Metabolism Contributing to the Ornamental Value in a Novel Hybrid Cultivar of Camellia japonica. Plants. 2020; 9(12):1724. https://doi.org/10.3390/plants9121724
Chicago/Turabian StylePan, Liqin, Jiyuan Li, Hengfu Yin, Zhengqi Fan, and Xinlei Li. 2020. "Integrated Physiological and Transcriptomic Analyses Reveal a Regulatory Network of Anthocyanin Metabolism Contributing to the Ornamental Value in a Novel Hybrid Cultivar of Camellia japonica" Plants 9, no. 12: 1724. https://doi.org/10.3390/plants9121724
APA StylePan, L., Li, J., Yin, H., Fan, Z., & Li, X. (2020). Integrated Physiological and Transcriptomic Analyses Reveal a Regulatory Network of Anthocyanin Metabolism Contributing to the Ornamental Value in a Novel Hybrid Cultivar of Camellia japonica. Plants, 9(12), 1724. https://doi.org/10.3390/plants9121724





