Transcriptome Analysis of Alternative Splicing Events Induced by Arbuscular Mycorrhizal Fungi (Rhizophagus irregularis) in Pea (Pisum sativum L.) Roots
Abstract
1. Introduction
2. Results
2.1. Bioinformatics Analysis of RNA Sequencing Data
2.2. Detection of Differentially Expressed Genes in Response to AM Fungi Inoculation
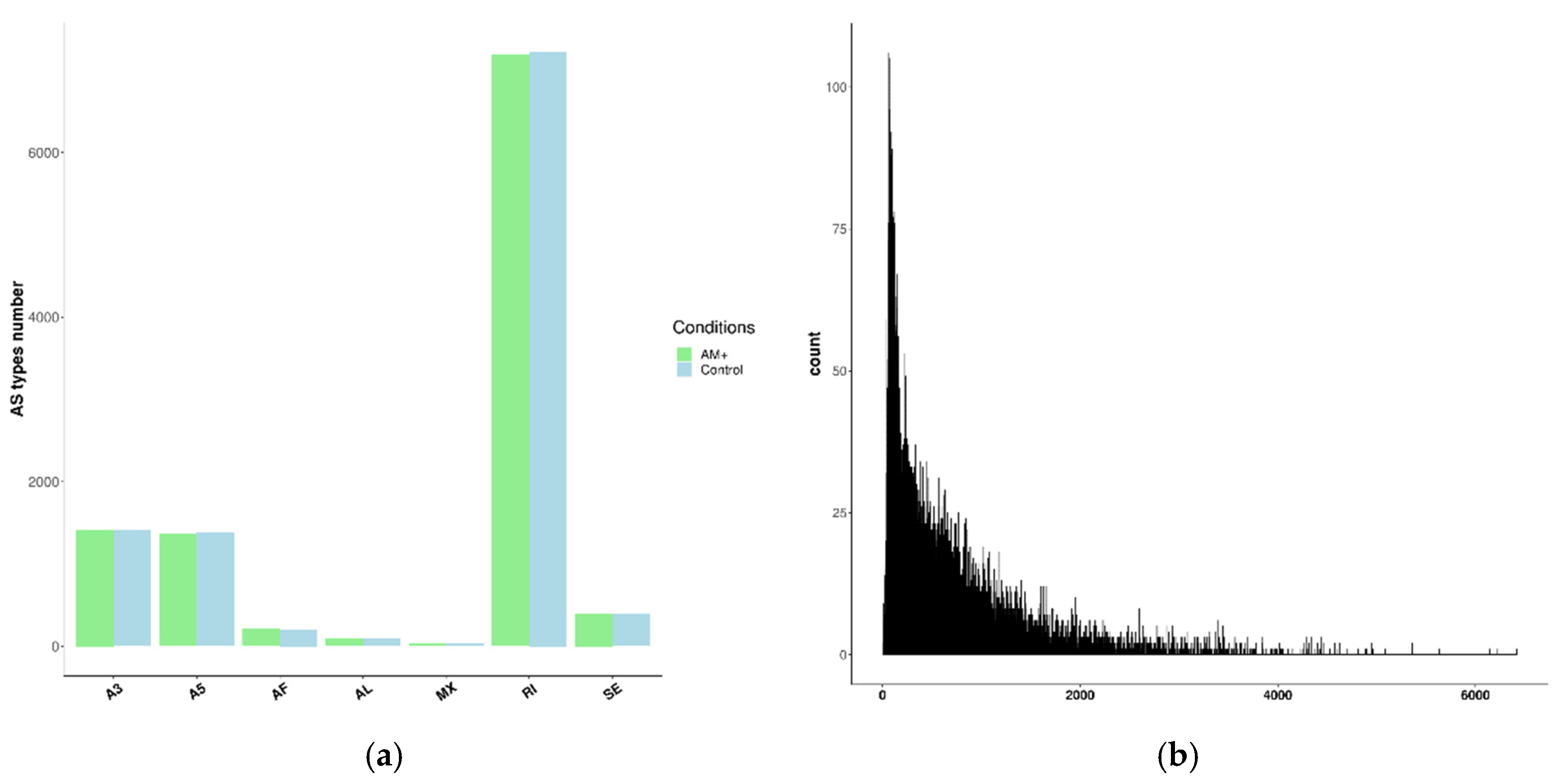
2.3. Description of the Landscape of Alternative Splicing in the Control and Mycorrhizal Roots in P. sativum
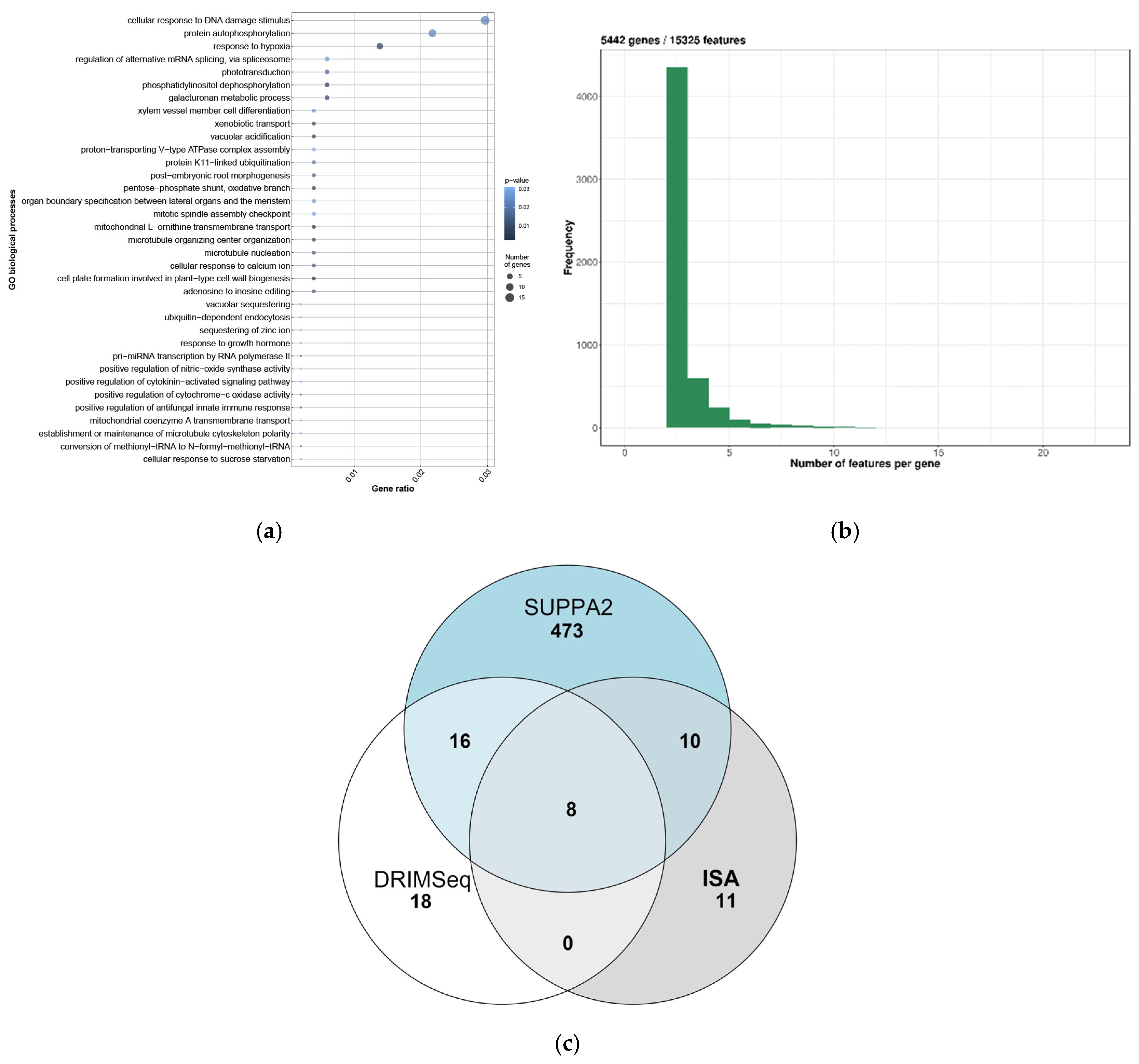
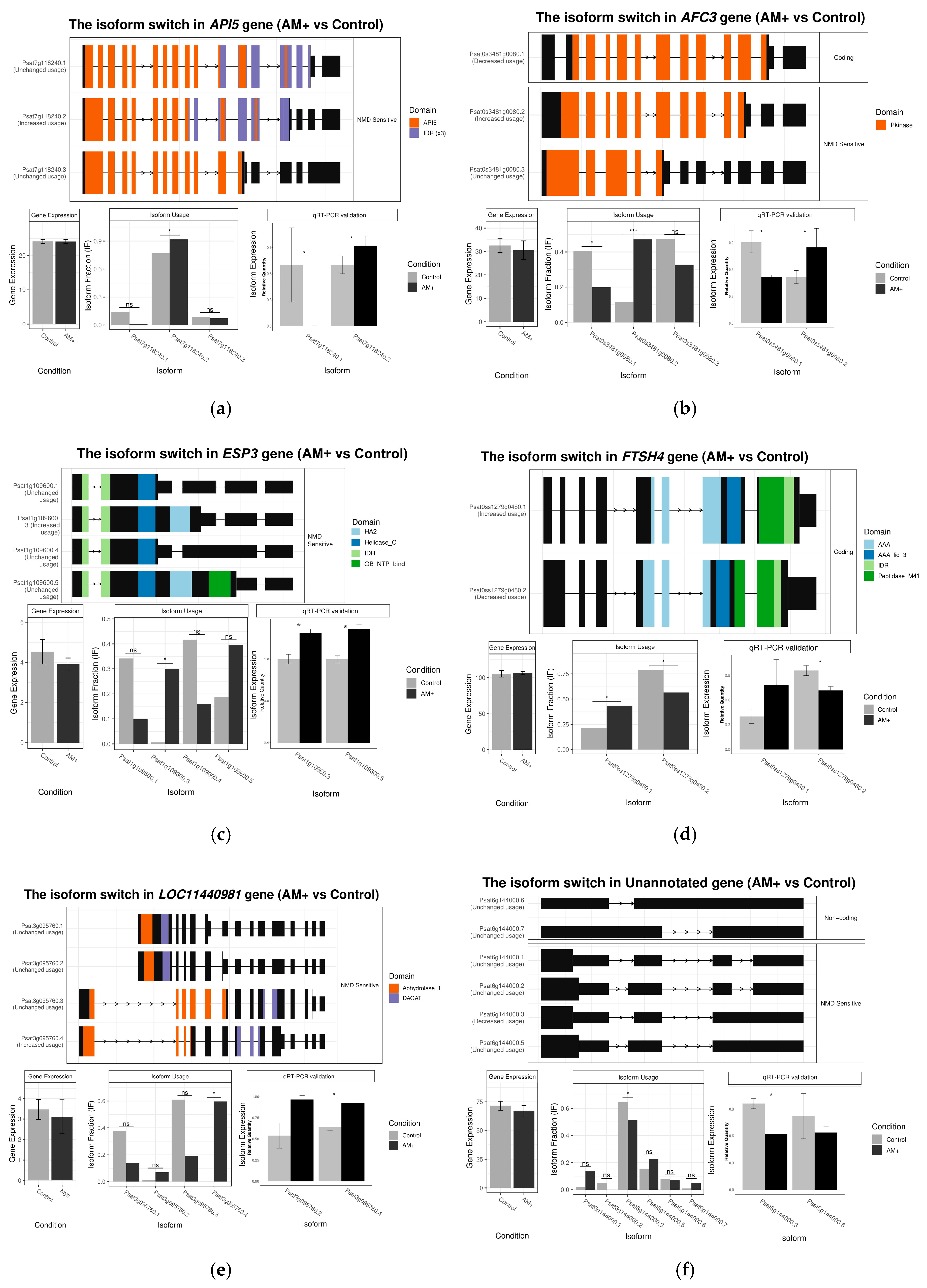
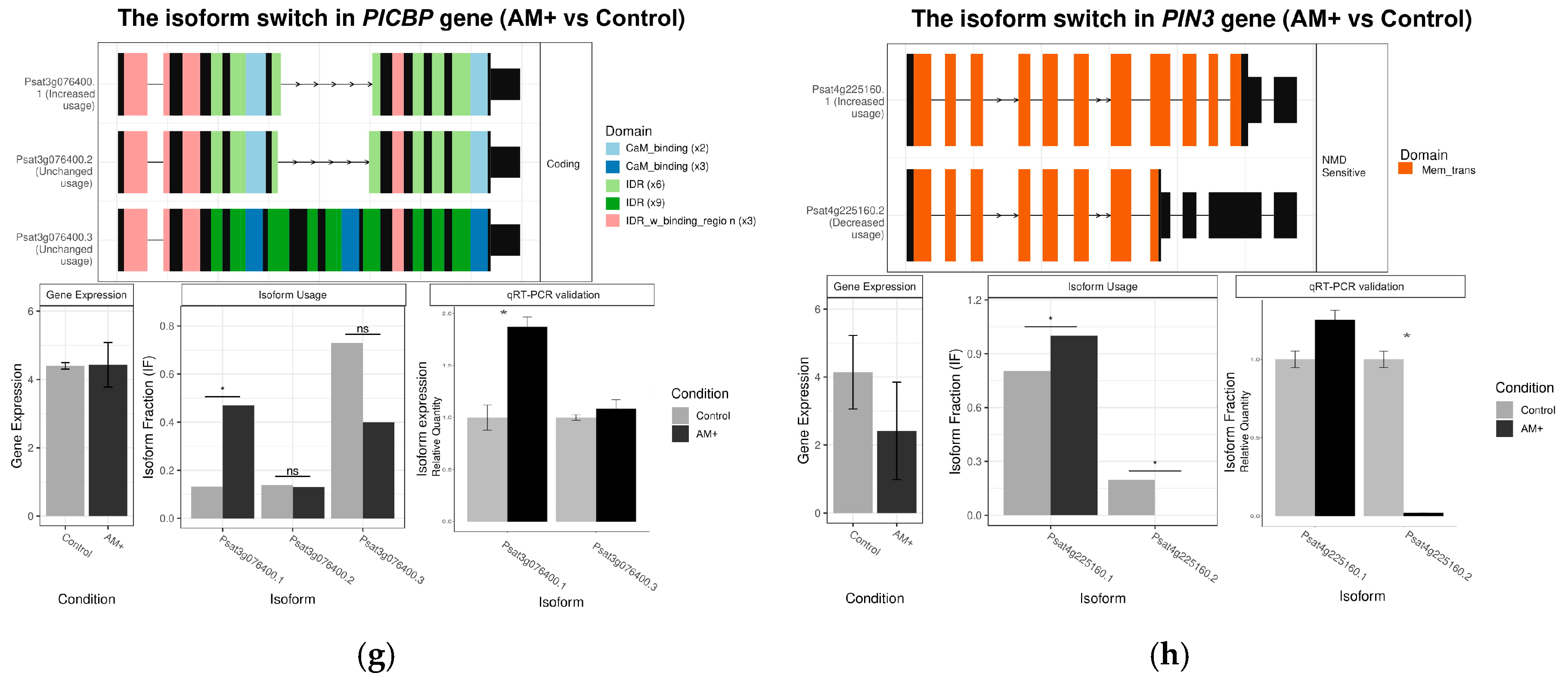
2.4. Identification and Analysis of Differential AS Events in Mycorrhizal Roots
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Experimental Conditions
4.2. RNA Extraction, cDNA Synthesis, qRT-PCR and Sequencing
4.3. Bioinformatic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oldroyd, G.E.D. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant. Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C.; Oldroyd, G.E.D. Plant signalling in symbiosis and immunity. Nature 2017, 543, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.H.; Tirichine, L.; Jurkiewicz, A.; Sullivan, J.T.; Heckmann, A.B.; Bek, A.S.; Ronson, C.W.; James, E.K.; Stougaard, J. The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nat. Commun. 2010, 1, 10. [Google Scholar] [CrossRef]
- Roy, S.; Liu, W.; Nandety, R.S.; Crook, A.; Mysore, K.S.; Pislariu, C.I.; Frugoli, J.; Dickstein, R.; Udvardi, M.K. Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. Plant. Cell 2020, 32, 15–41. [Google Scholar] [CrossRef]
- Küster, H.; Vieweg, M.F.; Manthey, K.; Baier, M.C.; Hohnjec, N.; Perlick, A.M. Identification and expression regulation of symbiotically activated legume genes. Phytochemistry 2007, 68, 8–18. [Google Scholar] [CrossRef]
- Dursun, N.M.; Nouri, E.; Reinhardt, D. The symbiosis of Medicago truncatula with arbuscular mycorrhizal fungi. In The Model Legume Medicago Truncatula; de Bruijn, F., Ed.; Wiley: Hoboken, NJ, USA, 2020; pp. 471–484. ISBN 978-1-119-40916-8. [Google Scholar]
- Tromas, A.; Parizot, B.; Diagne, N.; Champion, A.; Hocher, V.; Cissoko, M.; Crabos, A.; Prodjinoto, H.; Lahouze, B.; Bogusz, D.; et al. Heart of endosymbioses: Transcriptomics reveals a conserved genetic program among arbuscular mycorrhizal, actinorhizal and legume-rhizobial symbioses. PLoS ONE 2012, 7, e44742. [Google Scholar] [CrossRef]
- Camps, C.; Jardinaud, M.; Rengel, D.; Carrère, S.; Hervé, C.; Debellé, F.; Gamas, P.; Bensmihen, S.; Gough, C. Combined genetic and transcriptomic analysis reveals three major signalling pathways activated by Myc-LCO s in Medicago truncatula. New Phytol. 2015, 208, 224–240. [Google Scholar] [CrossRef]
- Larrainzar, E.; Riely, B.K.; Kim, S.C.; Carrasquilla-Garcia, N.; Yu, H.-J.; Hwang, H.-J.; Oh, M.; Kim, G.B.; Surendrarao, A.K.; Chasman, D.; et al. Deep sequencing of the Medicago truncatula root transcriptome reveals a massive and early interaction between nodulation factor and ethylene signals. Plant. Physiol. 2015, 169, 233–265. [Google Scholar] [CrossRef]
- Reddy, A.S.N.; Marquez, Y.; Kalyna, M.; Barta, A. Complexity of the alternative splicing landscape in plants. Plant. Cell 2013, 25, 3657–3683. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Rio, D.C. Mechanisms and regulation of alternative pre-mRNA splicing. Annu. Rev. Biochem. 2015, 84, 291–323. [Google Scholar] [CrossRef] [PubMed]
- Iñiguez, L.P.; Ramírez, M.; Barbazuk, W.B.; Hernández, G. Identification and analysis of alternative splicing events in Phaseolus vulgaris and Glycine max. BMC Genom. 2017, 18, 650. [Google Scholar] [CrossRef] [PubMed]
- Trincado, J.L.; Entizne, J.C.; Hysenaj, G.; Singh, B.; Skalic, M.; Elliott, D.J.; Eyras, E. SUPPA2: Fast, accurate, and uncertainty-aware differential splicing analysis across multiple conditions. Genome Biol. 2018, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Khokhar, W.; Jabre, I.; Reddy, A.S.N.; Byrne, L.J.; Wilson, C.M.; Syed, N.H. Alternative splicing and protein diversity: Plants versus animals. Front. Plant. Sci. 2019, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Rayson, S.; Arciga-Reyes, L.; Wootton, L.; De Torres Zabala, M.; Truman, W.; Graham, N.; Grant, M.; Davies, B. A role for nonsense-mediated mRNA decay in plants: Pathogen responses are induced in Arabidopsis thaliana NMD mutants. PLoS ONE 2012, 7, e31917. [Google Scholar] [CrossRef]
- Gracz, J. Alternative splicing in plant stress response. BTA 2016, 1, 9–17. [Google Scholar] [CrossRef]
- Laloum, T.; Martín, G.; Duque, P. Alternative splicing control of abiotic stress responses. Trends Plant. Sci. 2018, 23, 140–150. [Google Scholar] [CrossRef]
- Shang, X.; Cao, Y.; Ma, L. Alternative splicing in plant genes: A means of regulating the environmental fitness of plants. IJMS 2017, 18, 432. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, H.; Jin, L.; Chen, T.; Wang, L.; Kang, H.; Hong, Z.; Zhang, Z. Splice variants of the SIP1 transcripts play a role in nodule organogenesis in Lotus japonicus. Plant. Mol. Biol. 2013, 82, 97–111. [Google Scholar] [CrossRef]
- Combier, J.P.; de Billy, F.; Gamas, P.; Niebel, A.; Rivas, S. Trans-regulation of the expression of the transcription factor MtHAP2-1 by a uORF controls root nodule development. Genes Dev. 2008, 22, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Huisman, R.; Hontelez, J.; Mysore, K.S.; Wen, J.; Bisseling, T.; Limpens, E. A symbiosis-dedicated SYNTAXIN OF PLANTS 13II isoform controls the formation of a stable host–microbe interface in symbiosis. New Phytol. 2016, 211, 1338–1351. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Oztas, O.; Zhang, X.; Wu, X.; Stonoha, C.; Wang, E.; Wang, B.; Wang, D. A symbiotic SNARE protein generated by alternative termination of transcription. Nat. Plants 2016, 2, 15197. [Google Scholar] [CrossRef] [PubMed]
- Bourcy, M.; Brocard, L.; Pislariu, C.I.; Cosson, V.; Mergaert, P.; Tadege, M.; Mysore, K.S.; Udvardi, M.K.; Gourion, B.; Ratet, P. Medicago truncatula DNF2 is a PI-PLC-XD-containing protein required for bacteroid persistence and prevention of nodule early senescence and defense-like reactions. New Phytol. 2013, 197, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Gagete, A.P.; Riera, M.; Franco, L.; Rodrigo, M.I. Functional analysis of the isoforms of an ABI3-like factor of Pisum sativum generated by alternative splicing. J. Exp. Bot. 2009, 60, 1703–1714. [Google Scholar] [CrossRef]
- Alves-Carvalho, S.; Aubert, G.; Carrère, S.; Cruaud, C.; Brochot, A.-L.; Jacquin, F.; Klein, A.; Martin, C.; Boucherot, K.; Kreplak, J.; et al. Full-length de novo assembly of RNA-seq data in pea (Pisum sativum L.) provides a gene expression atlas and gives insights into root nodulation in this species. Plant. J. 2015, 84, 1–19. [Google Scholar] [CrossRef]
- Kreplak, J.; Madoui, M.-A.; Cápal, P.; Novák, P.; Labadie, K.; Aubert, G.; Bayer, P.E.; Gali, K.K.; Syme, R.A.; Main, D.; et al. A reference genome for pea provides insight into legume genome evolution. Nat. Genet. 2019, 51, 1411–1422. [Google Scholar] [CrossRef]
- Messinese, E.; Mun, J.-H.; Yeun, L.H.; Jayaraman, D.; Rougé, P.; Barre, A.; Lougnon, G.; Schornack, S.; Bono, J.-J.; Cook, D.R.; et al. A novel nuclear protein interacts with the symbiotic DMI3 calcium- and calmodulin-dependent protein kinase of Medicago truncatula. MPMI 2007, 20, 912–921. [Google Scholar] [CrossRef]
- Floss, D.S.; Gomez, S.K.; Park, H.-J.; MacLean, A.M.; Müller, L.M.; Bhattarai, K.K.; Lévesque-Tremblay, V.; Maldonado-Mendoza, I.E.; Harrison, M.J. A transcriptional program for arbuscule degeneration during AM symbiosis is regulated by MYB1. Curr. Biol. 2017, 27, 1206–1212. [Google Scholar] [CrossRef]
- Kalo, P. Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science 2005, 308, 1786–1789. [Google Scholar] [CrossRef]
- Gobbato, E.; Wang, E.; Higgins, G.; Bano, S.A.; Henry, C.; Schultze, M.; Oldroyd, G.E. RAM1 and RAM2 function and expression during Arbuscular Mycorrhizal Symbiosis and Aphanomyces euteiches colonization. Plant. Signal. Behav. 2013, 8, e26049. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, D.; Rezzonico, E.; MacDonald-Comber Petétot, J.; Somerville, C.; Poirier, Y. Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. Plant. Cell 2002, 14, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Sablok, G.; Powell, B.; Braessler, J.; Yu, F.; Min, X.J. Comparative landscape of alternative splicing in fruit plants. Curr. Plant. Biol. 2017, 9–10, 29–36. [Google Scholar] [CrossRef]
- Filichkin, S.A.; Mockler, T.C. Unproductive alternative splicing and nonsense mRNAs: A widespread phenomenon among plant circadian clock genes. Biol. Direct. 2012, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.R.; Ritchie, W.; Wong, J.J.-L.; Schmitz, U.; Middleton, R.; An, X.; Mohandas, N.; Rasko, J.E.J.; Blobel, G.A. A dynamic intron retention program in the mammalian megakaryocyte and erythrocyte lineages. Blood 2016, 127, e24–e34. [Google Scholar] [CrossRef] [PubMed]
- Shirasawa, K.; Sasaki, K.; Hirakawa, H.; Isobe, S. Genomic region associated with pod color variation in pea (Pisum sativum). bioRxiv Genom. 2020. [Google Scholar] [CrossRef]
- Zorin, E.A.; Kulaeva, O.A.; Afonin, A.M.; Zhukov, V.A.; Tikhonovich, I.A. Analysis of alternative splicing events in the root tips and nodules of Pisum sativum L. Ecol. Genet. 2019, 17, 53–63. [Google Scholar] [CrossRef]
- Lecourieux, D.; Ranjeva, R.; Pugin, A. Calcium in plant defence-signalling pathways. New Phytol. 2006, 171, 249–269. [Google Scholar] [CrossRef]
- Cheval, C.; Aldon, D.; Galaud, J.-P.; Ranty, B. Calcium/calmodulin-mediated regulation of plant immunity. BBA 2013, 1833, 1766–1771. [Google Scholar] [CrossRef]
- Ranty, B.; Aldon, D.; Galaud, J.-P. Plant calmodulins and calmodulin-related proteins: Multifaceted relays to decode calcium signals. Plant. Signal. Behav. 2006, 1, 96–104. [Google Scholar] [CrossRef]
- Shaul, O. Unique aspects of plant nonsense-mediated mRNA decay. Trends Plant. Sci. 2015, 20, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Recchia, G.H.; Konzen, E.R.; Cassieri, F.; Caldas, D.G.G.; Tsai, S.M. Arbuscular mycorrhizal symbiosis leads to differential regulation of drought-responsive genes in tissue-specific root cells of common bean. Front. Microbiol. 2018, 9, 1339. [Google Scholar] [CrossRef] [PubMed]
- Foo, E.; Plett, J.M.; Lopez-Raez, J.A.; Reid, D. Editorial: The role of plant hormones in plant-microbe symbioses. Front. Plant. Sci. 2019, 10, 1391. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Medina, M.J.; Steinkellner, S.; Vierheilig, H.; Ocampo Bote, J.A.; García Garrido, J.M. Abscisic acid determines arbuscule development and functionality in the tomato arbuscular mycorrhiza. New Phytol. 2007, 175, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, M.; Gutjahr, C.; Couzigou, J.-M.; Zouine, M.; Lauressergues, D.; Timmers, A.; Audran, C.; Bouzayen, M.; Bécard, G.; Combier, J.-P. Auxin perception is required for arbuscule development in arbuscular mycorrhizal symbiosis. Plant. Physiol. 2014, 166, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.L.P.; Perrine-Walker, F.; Wasson, A.P.; Mathesius, U. The control of auxin transport in parasitic and symbiotic root-microbe interactions. Plants 2015, 4, 606–643. [Google Scholar] [CrossRef]
- Fusconi, A. Regulation of root morphogenesis in arbuscular mycorrhizae: What role do fungal exudates, phosphate, sugars and hormones play in lateral root formation? Ann. Bot. 2014, 113, 19–33. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Zhang, F.; Zhang, D.-J.; Srivastava, A.; Wu, Q.-S.; Zou, Y.-N. Mycorrhiza stimulates root-hair growth and IAA synthesis and transport in trifoliate orange under drought stress. Sci. Rep. 2018, 8, 1978. [Google Scholar] [CrossRef]
- Rodriguez, L.; Gonzalez-Guzman, M.; Diaz, M.; Rodrigues, A.; Izquierdo-Garcia, A.C.; Peirats-Llobet, M.; Fernandez, M.A.; Antoni, R.; Fernandez, D.; Marquez, J.A.; et al. C2-domain abscisic acid-related proteins mediate the interaction of PYR/PYL/RCAR abscisic acid receptors with the plasma membrane and regulate abscisic acid sensitivity in Arabidopsis. Plant. Cell 2014, 26, 4802–4820. [Google Scholar] [CrossRef]
- Charpentier, M.; Sun, J.; Wen, J.; Mysore, K.S.; Oldroyd, G.E.D. Abscisic acid promotion of arbuscular mycorrhizal colonization requires a component of the PROTEIN PHOSPHATASE 2A complex. Plant. Physiol. 2014, 166, 2077–2090. [Google Scholar] [CrossRef]
- Lopato, S.; Kalyna, M.; Dorner, S.; Kobayashi, R.; Krainer, A.R.; Barta, A. atSRp30, one of two SF2/ASF-like proteins from Arabidopsis thaliana, regulates splicing of specific plant genes. Genes Dev. 1999, 13, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Savaldi-Goldstein, S.; Aviv, D.; Davydov, O.; Fluhr, R. Alternative splicing modulation by a LAMMER kinase impinges on developmental and transcriptome expression. Plant. Cell 2003, 15, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, M.; Demura, T.; Sugiyama, M. Arabidopsis ROOT INITIATION DEFECTIVE1, a DEAH-Box RNA Helicase involved in pre-mRNA splicing, is essential for plant development. Plant. Cell 2013, 25, 2056–2069. [Google Scholar] [CrossRef] [PubMed]
- Shtark, O.Y.; Sulima, A.S.; Zhernakov, A.I.; Kliukova, M.S.; Fedorina, J.V.; Pinaev, A.G.; Kryukov, A.A.; Akhtemova, G.A.; Tikhonovich, I.A.; Zhukov, V.A. Arbuscular mycorrhiza development in pea (Pisum sativum L.) mutants impaired in five early nodulation genes including putative orthologs of NSP1 and NSP2. Symbiosis 2016, 68, 129–144. [Google Scholar] [CrossRef]
- Cranenbrouck, S.; Voets, L.; Bivort, C.; Renard, L.; Strullu, D.-G.; Declerck, S. Methodologies for in Vitro Cultivation of Arbuscular Mycorrhizal Fungi with Root Organs. In Vitro Culture of Mycorrhizas. In Soil Biology; Declerck, S., Ed.; Springer: Berlin, Germany, 2005; Volume 4, pp. 341–375. [Google Scholar]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche de methods d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Dobin, A.; Gingeras, T.R. Mapping RNA-seq reads with STAR. Curr. Protoc. Bioinform. 2015, 51. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Nowicka, M.; Robinson, M.D. DRIMSeq: A Dirichlet-multinomial framework for multivariate count outcomes in genomics. F1000Res 2016, 5, 1356. [Google Scholar] [CrossRef]
- Vitting-Seerup, K.; Sandelin, A. IsoformSwitchAnalyzeR: Analysis of changes in genome-wide patterns of alternative splicing and its functional consequences. Bioinformatics 2019, 35, 4469–4471. [Google Scholar] [CrossRef]
- Bryant, D.M.; Johnson, K.; DiTommaso, T.; Tickle, T.; Couger, M.B.; Payzin-Dogru, D.; Lee, T.J.; Leigh, N.D.; Kuo, T.-H.; Davis, F.G.; et al. A tissue-mapped Axolotl de novo transcriptome enables identification of limb regeneration factors. Cell Rep. 2017, 18, 762–776. [Google Scholar] [CrossRef]
- Adrian, A.; Rahnenfuhrer, J. topGO; Bioconductor: Buffalo, NY, USA, 2017. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Use R! Springer: Cham, Switzerland, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Zhukov, V.A.; Zhernakov, A.I.; Kulaeva, O.A.; Ershov, N.I.; Borisov, A.Y.; Tikhonovich, I.A. De novo assembly of the pea (Pisum sativum L.) nodule transcriptome. Int. J. Genom. 2015, 2015, 695947. [Google Scholar] [CrossRef]

| Sample Name | No. Reads before Trimming | No. Reads after Trimming | % Uniquely Mapped Reads | % of Reads Mapped to Multiple Loci |
|---|---|---|---|---|
| Control.1 | 21,904,071 | 21,700,136 | 92.70 | 2.01 |
| Control.2 | 30,815,955 | 30,517,632 | 94.05 | 2.05 |
| Control.3 | 25,026,957 | 24,787,916 | 94.72 | 2.07 |
| AM.1 | 25,985,674 | 25,711,178 | 93.24 | 2.07 |
| AM.2 | 25,226,401 | 24,959,410 | 93.39 | 2.11 |
| AM.3 | 26,840,632 | 26,515,644 | 94.35 | 2.08 |
| GT/AG | GC/AG | AT/AC | Non-Canonical | |
|---|---|---|---|---|
| Control | 15,475,479 | 160,638 | 16,588 | 37,918 |
| AM+ | 15,174,737 | 154,958 | 15,490 | 36,487 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorin, E.A.; Afonin, A.M.; Kulaeva, O.A.; Gribchenko, E.S.; Shtark, O.Y.; Zhukov, V.A. Transcriptome Analysis of Alternative Splicing Events Induced by Arbuscular Mycorrhizal Fungi (Rhizophagus irregularis) in Pea (Pisum sativum L.) Roots. Plants 2020, 9, 1700. https://doi.org/10.3390/plants9121700
Zorin EA, Afonin AM, Kulaeva OA, Gribchenko ES, Shtark OY, Zhukov VA. Transcriptome Analysis of Alternative Splicing Events Induced by Arbuscular Mycorrhizal Fungi (Rhizophagus irregularis) in Pea (Pisum sativum L.) Roots. Plants. 2020; 9(12):1700. https://doi.org/10.3390/plants9121700
Chicago/Turabian StyleZorin, Evgeny A., Alexey M. Afonin, Olga A. Kulaeva, Emma S. Gribchenko, Oksana Y. Shtark, and Vladimir A. Zhukov. 2020. "Transcriptome Analysis of Alternative Splicing Events Induced by Arbuscular Mycorrhizal Fungi (Rhizophagus irregularis) in Pea (Pisum sativum L.) Roots" Plants 9, no. 12: 1700. https://doi.org/10.3390/plants9121700
APA StyleZorin, E. A., Afonin, A. M., Kulaeva, O. A., Gribchenko, E. S., Shtark, O. Y., & Zhukov, V. A. (2020). Transcriptome Analysis of Alternative Splicing Events Induced by Arbuscular Mycorrhizal Fungi (Rhizophagus irregularis) in Pea (Pisum sativum L.) Roots. Plants, 9(12), 1700. https://doi.org/10.3390/plants9121700





