Anti-Inflammatory Activities of an Extract of In Vitro Grown Adventitious Shoots of Toona sinensis in LPS-Treated RAW264.7 and Propionibacterium acnes-Treated HaCaT Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Induction and Proliferation of Adventitious Shoots of T. sinensis
2.2. Preparation of TS Extract
2.3. DPPH Scavenging Activity Assay
2.4. Superoxide Dismutase (SOD) Activity Assay
2.5. Cell Cultures
2.6. Antibacterial Assay
2.7. Cell Viability Assay
2.8. Measurement of NO and PGE2
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Real-Time RT-PCR
2.11. Western Blot Analyses
2.12. Statistical Analysis
3. Results and Discussion
3.1. In Vitro Proliferation of Adventitious Shoots of T. sinensis
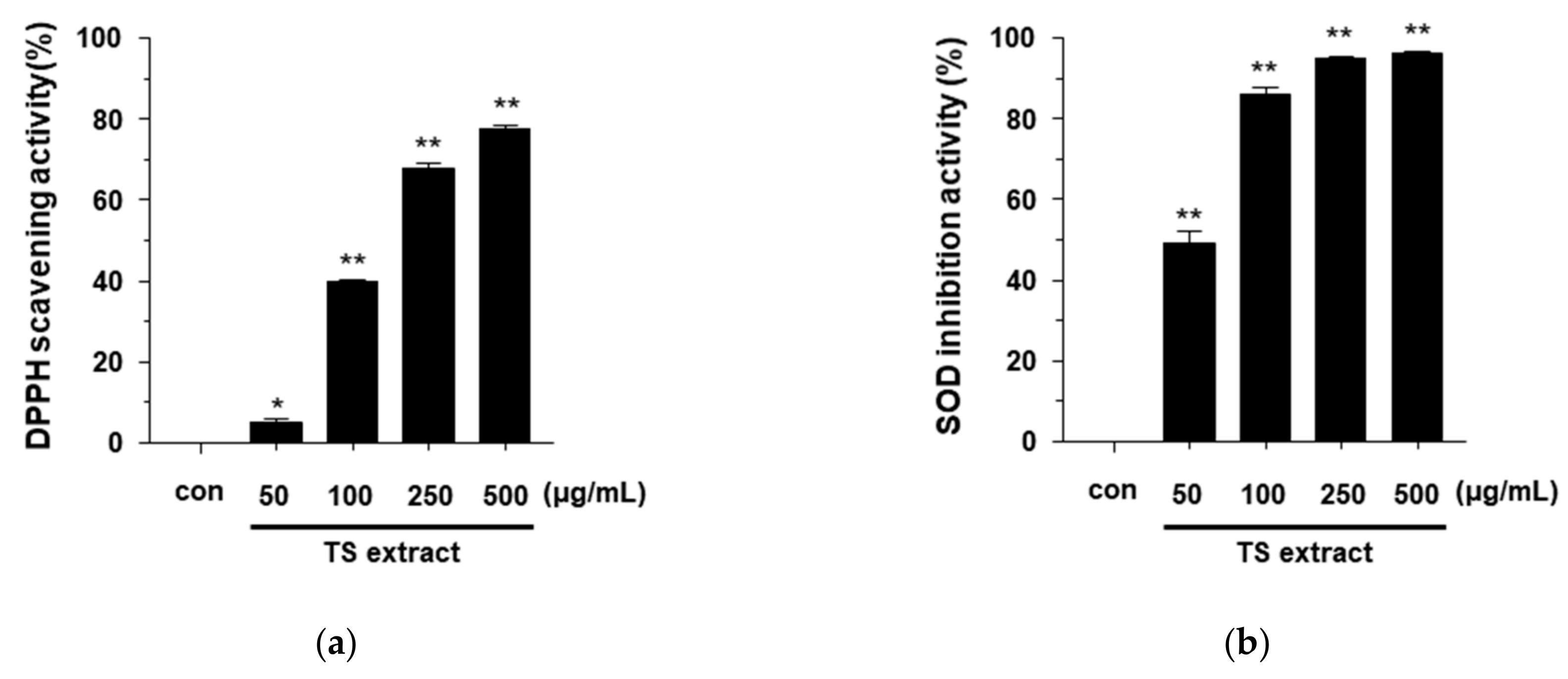
3.2. Anntioxidant Effects of TS Extract
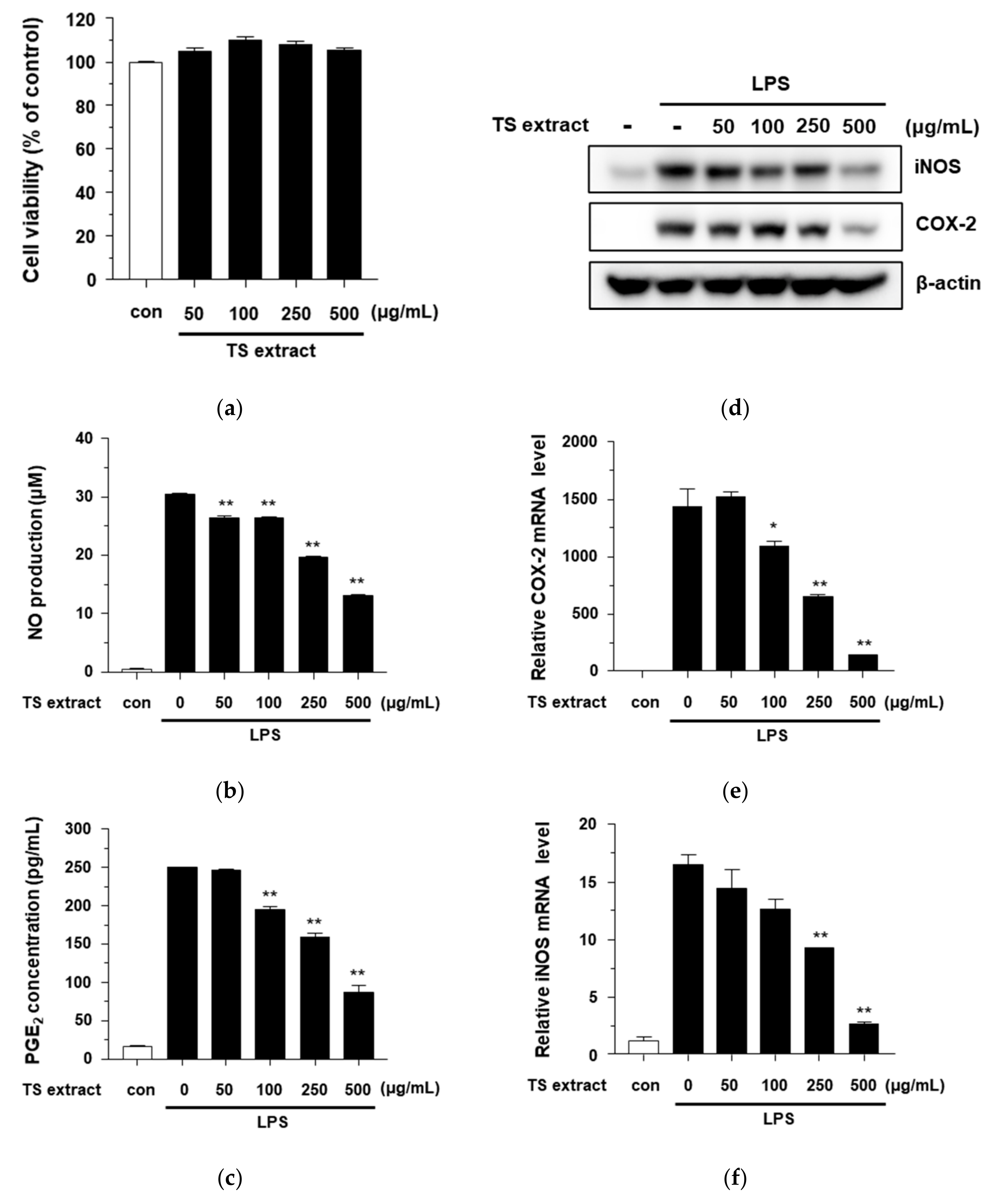
3.3. Cytotoxicity and Anti-inflammatiory Effects of TS Extract on RAW264.7 Cells
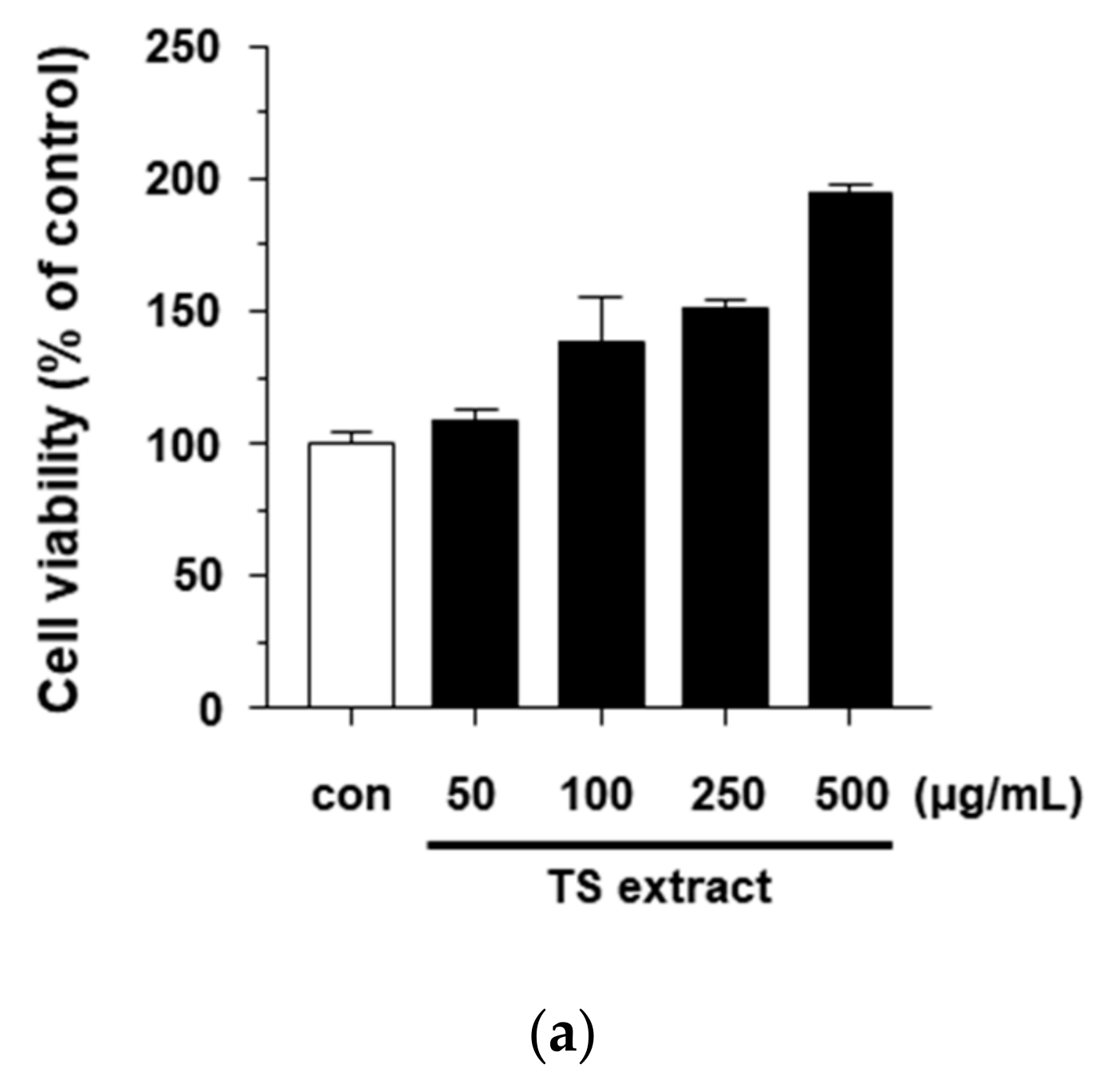
3.4. Antibacterial Activity of TS Extract Against P. acnes
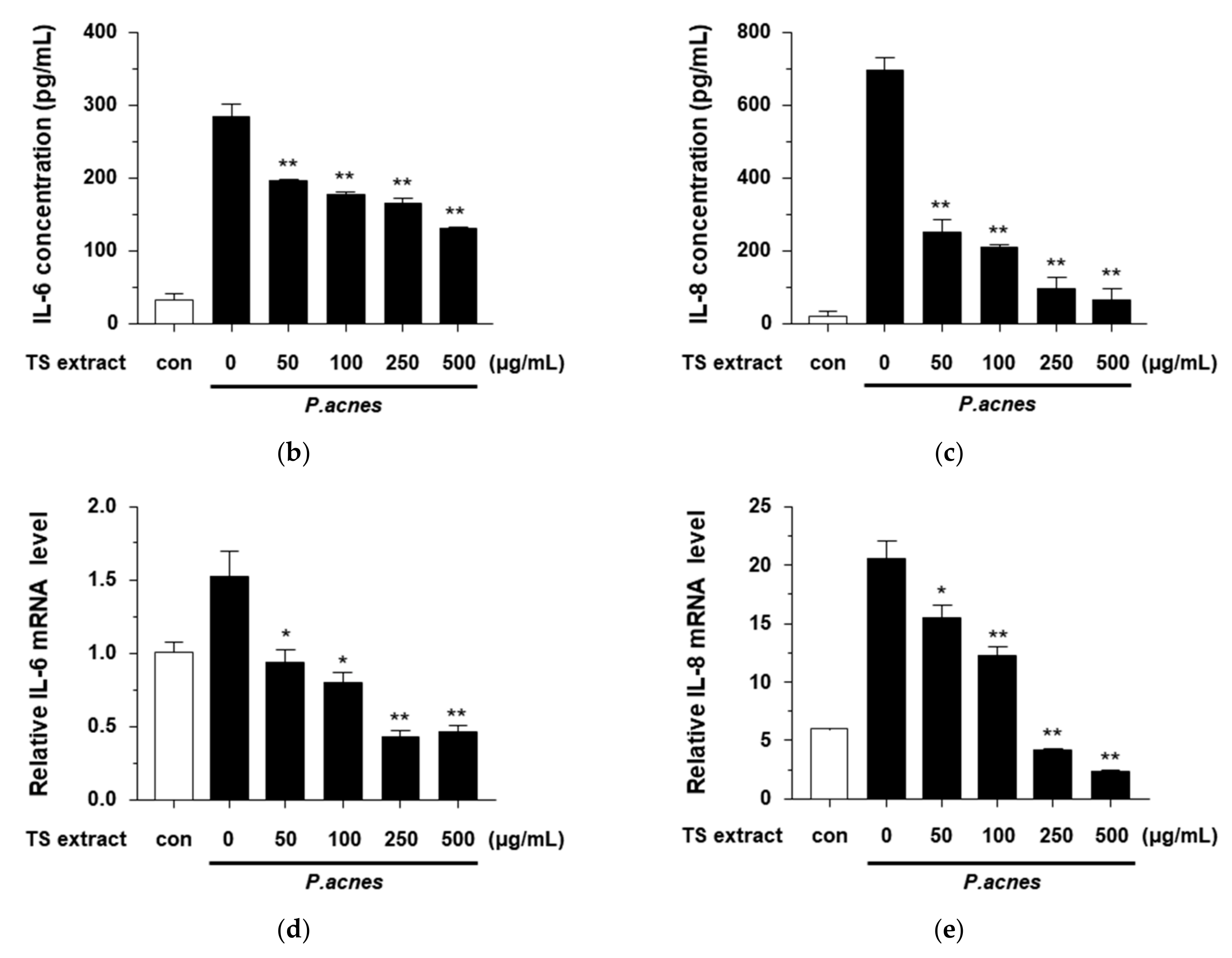
3.5. Anti-Inflammatory Effects of TS Extract in P. acnes-treated HaCaT Cells
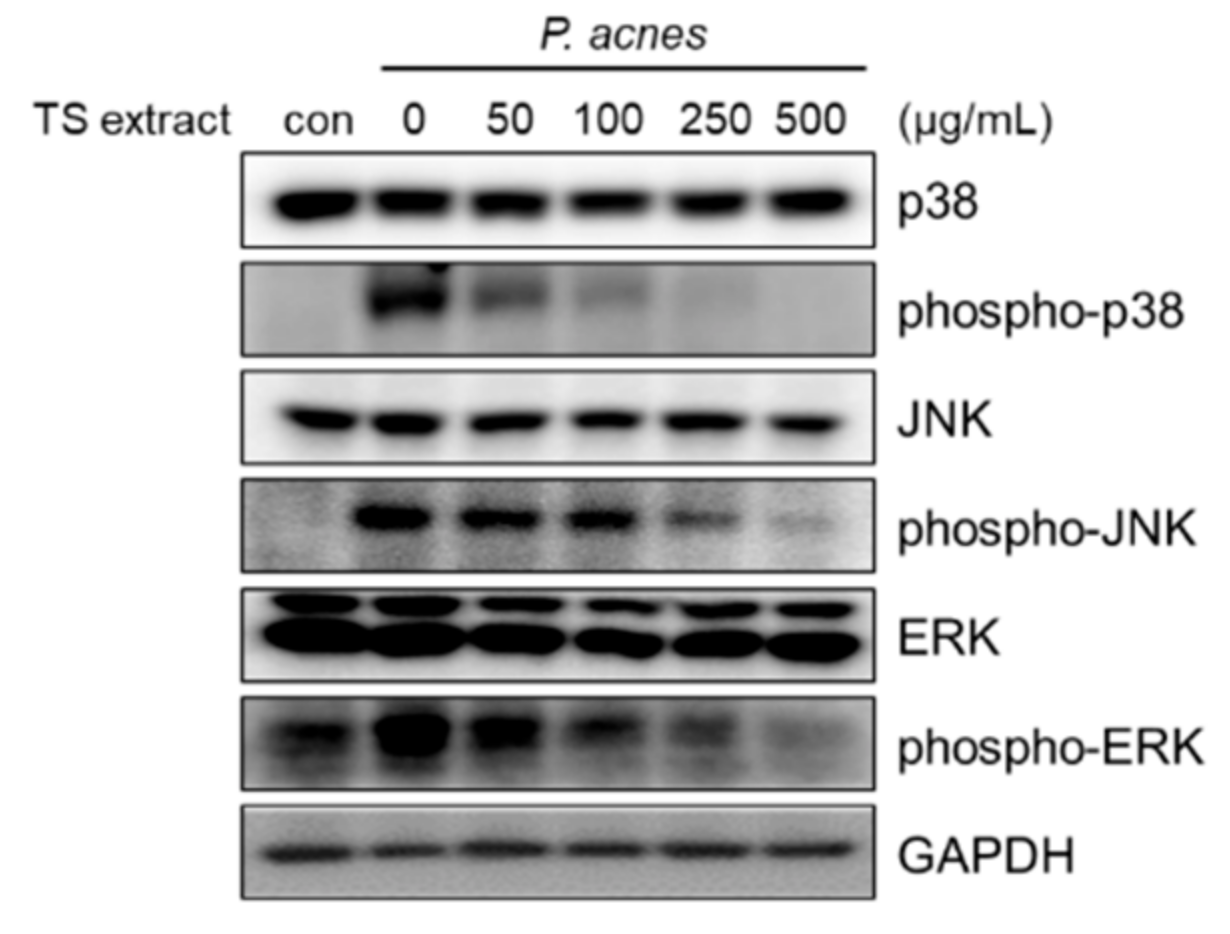
3.6. Regulatory Effects of TS Extract on Activated MAPK Signaling Pathway in P. acnes-Treated HaCaT Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tan, J.K.; Bhate, K. A global perspective on the epidemiology of acne. Br. J. Dermatol. 2015, 172, 3–12. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Zhu, X.; Mao, Y.; Guo, M.; Yu, H.; Hao, L.; Hua, Q.; Lu, Z.; Hong, M.; An, F. Enhancement of anti-acne effect of Scutellaria baicalensis extract by fermentation with symbiotic fungus Penicillium decumbens. J. Biosci. Bioeng. 2020, 130, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.M.; Liao, L.Y.; Li, L.; Yi, F.; Meng, H.; He, Y.F.; Guo, M.M. Skin inflammation induced by ambient particulate matter in China. Sci. Total Environ. 2019, 682, 364–373. [Google Scholar] [CrossRef]
- Wolkenstein, P.; Machovcová, A.; Szepietowski, J.C.; Tennstedt, D.; Veraldi, S.; Delarue, A. Acne prevalence and associations with lifestyle: A cross-sectional online survey of adolescents/young adults in 7 European countries. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 298–306. [Google Scholar] [CrossRef]
- Melnik, B.C. Linking diet to acne metabolomics, inflammation, and comedogenesis: An update. Clin. Cosmet. Investig. Dermatol. 2015, 8, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Jugeau, S.; Tenaud, I.; Knol, A.C.; Jarrousse, V.; Quereux, G.; Khammari, A.; Dreno, B. Induction of toll-like receptors by Propionibacterium acnes. Br. J. Dermatol. 2005, 153, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Vowels, B.R.; Yang, S.; Leyden, J.J. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: Implications for chronic inflammatory acne. Infect. Immun. 1995, 63, 3158–3165. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Tsai, T.H.; Chuang, L.T.; Li, Y.Y.; Zouboulis, C.C.; Tsai, P.J. Anti-bacterial and anti-inflammatory properties of capric acid against Propionibacterium acnes: A comparative study with lauric acid. J. Dermatol. Sci. 2014, 73, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Dessinioti, C.; Katsambas, A. Propionibacterium acnes and antimicrobial resistance in acne. Clin. Dermatol. 2017, 35, 163–167. [Google Scholar] [CrossRef]
- Sinha, P.; Srivastava, S.; Mishra, N.; Yadav, N.P. New perspectives on antiacne plant drugs: Contribution to modern therapeutics. Biomed. Res. Int. 2014, 2014, 301304. [Google Scholar] [CrossRef] [PubMed]
- Altpeter, F.; Springer, N.M.; Bartley, L.E.; Blechl, A.E.; Brutnell, T.P.; Citovsky, V.; Conrad, L.; Gelvin, S.B.; Jackson, D.; Kausch, A.P.; et al. Advancing crop transformation in the era of genome editing. Plant Cell. 2016, 28, 1510–1520. [Google Scholar] [CrossRef]
- Verpoorte, R.; van der Heijden, R.; Memelink, J. Engineering the plant cell factory for secondary metabolite production. Transgen. Res. 2000, 9, 323–343. [Google Scholar] [CrossRef]
- Debnath, M.; Malik, C.P.; Bisen, P.S. Micropropagation: A tool for the production of high quality plant-based medicines. Curr. Pharm. Biotechnol. 2006, 7, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.R.; Ravishankar, G.A. Plant cell cultures: Chemical factories of secundary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [PubMed]
- Fischer, R.; Vasilev, N.; Twyman, R.M.; Schillberg, S. High-value products from plants: The challenges of process optimization. Curr. Opin. Biotechnol. 2015, 32, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.; Jang, M.O.; Jin, Y.W.; Lee, E.K.; Loake, G.J. Plant cell culture strategies for the production of natural products. BMB Rep. 2016, 49, 149–158. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F.A. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Liao, J.W.; Yeh, J.Y.; Lin, Y.C.; Wei, M.M.; Chung, Y.C. Mutagenicity and safety evaluation of water extract of fermented Toona sinensis Roemor leaves. J. Food Sci. 2009, 74, T7–T13. [Google Scholar] [CrossRef]
- Truong, V.L.; Ko, S.Y.; Jun, M.; Jeong, W.S. Quercitrin from Toona sinensis (Juss.) M.Roem. Attenuates Acetaminophen-Induced Acute Liver Toxicity in HepG2 Cells and Mice through Induction of Antioxidant Machinery and Inhibition of Inflammation. Nutrients 2016, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.W.; Yang, R.Y.; Tsou, S.C.; Lo, C.S.; Ho, C.T.; Lee, T.C.; Wang, M. Analysis of antioxidant activity and antioxidant constituents of Chinese toon. J. Funct. Foods. 2009, 1, 253–259. [Google Scholar] [CrossRef]
- Wu, C.C.; Liu, C.H.; Chang, Y.P.; Hsieh, S.L. Effects of hot-water extract of Toona sinensis on immune response and resistance to Aeromonas hydrophila in Oreochromis mossambicus. Fish Shellfish Immunol. 2010, 29, 258–263. [Google Scholar] [CrossRef]
- Peng, W.; Liu, Y.; Hu, M.; Zhang, M.; Yang, J.; Liang, F.; Huang, Q.; Wu, C. Toona sinensis: A comprehensive review on its traditional usages, phytochemisty, pharmacology and toxicology. Rev. Bras. Farmacogn. 2019, 29, 111–124. [Google Scholar] [CrossRef] [PubMed]
- James, J.T.; Dubery, I.A. Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.) Urban. Molecules 2009, 14, 3922–3941. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, R.H.; Wang, M.; Xu, G.B.; Liao, S.G. Prodrugs of triterpenoids and their derivatives. Eur. J. Med. Chem. 2017, 131, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, M.A.; Yonamine, M.; Aniya, Y. Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally-induced liver injuries. Gen. Pharmacol. 1999, 32, 661–667. [Google Scholar] [CrossRef]
- Grange, P.A.; Chéreau, C.; Raingeaud, J.; Nicco, C.; Weill, B.; Dupin, N.; Batteux, F. Production of superoxide anions by keratinocytes initiates P. acnes-induced inflammation of the skin. PLoS Pathog. 2009, 5, e1000527. [Google Scholar] [CrossRef]
- Hussein, S.Z.; Mohd Yusoff, K.; Makpol, S.; Mohd Yusof, Y.A. Gelam honey attenuates carrageenan-induced rat paw inflammation via NF-κB pathway. PLoS ONE 2013, 8, e72365. [Google Scholar] [CrossRef]
- Bhat, Y.J.; Latief, I.; Hassan, I. Update on etiopathogenesis and treatment of Acne. Ind. J. Dermatol. Venereol. Leprol. 2017, 83, 298–306. [Google Scholar] [CrossRef]


| Gene | Sequence (5’ to 3’) | |
|---|---|---|
| COX-2 | Forward | CTCTACAACAACTCCATCCT |
| (mouse) | Reverse | ATTCTGCAGCCATTTCCTTC |
| iNOS | Forward | CGAAACGCTTCACTTCCAA |
| (mouse) | Reverse | TGAGCCTATATTGCTGTGGCT |
| β-actin | Forward | CGGTTCCGATGCCCTGAGGCTCTT |
| (mouse) | Reverse | CGTCACACTTCATGATGGAATTGA |
| IL-6 | Forward | AGCCACTCACCTCTTCAGAAC |
| (human) | Reverse | GCCTCTTTGCTGCTTTCACAC |
| IL-8 | Forward | CTGATTTCTGCAGCTCTGTG |
| (human) | Reverse | GGGTGGAAAGGTTTGGAGTATG |
| GAPDH | Forward | TGCACCACCAACTGCTTAGC |
| (human) | Reverse | GGCATGGACTGTGGTCATGAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.-J.; Park, I.-S.; Jie, E.Y.; Ahn, W.S.; Kim, S.-J.; Jeong, S.-I.; Yu, K.-Y.; Kim, S.W.; Jung, C.-H. Anti-Inflammatory Activities of an Extract of In Vitro Grown Adventitious Shoots of Toona sinensis in LPS-Treated RAW264.7 and Propionibacterium acnes-Treated HaCaT Cells. Plants 2020, 9, 1701. https://doi.org/10.3390/plants9121701
Lim H-J, Park I-S, Jie EY, Ahn WS, Kim S-J, Jeong S-I, Yu K-Y, Kim SW, Jung C-H. Anti-Inflammatory Activities of an Extract of In Vitro Grown Adventitious Shoots of Toona sinensis in LPS-Treated RAW264.7 and Propionibacterium acnes-Treated HaCaT Cells. Plants. 2020; 9(12):1701. https://doi.org/10.3390/plants9121701
Chicago/Turabian StyleLim, Hyeon-Ji, In-Sun Park, Eun Yee Jie, Woo Seok Ahn, Sang-Jun Kim, Seung-Il Jeong, Kang-Yeol Yu, Suk Weon Kim, and Chan-Hun Jung. 2020. "Anti-Inflammatory Activities of an Extract of In Vitro Grown Adventitious Shoots of Toona sinensis in LPS-Treated RAW264.7 and Propionibacterium acnes-Treated HaCaT Cells" Plants 9, no. 12: 1701. https://doi.org/10.3390/plants9121701
APA StyleLim, H.-J., Park, I.-S., Jie, E. Y., Ahn, W. S., Kim, S.-J., Jeong, S.-I., Yu, K.-Y., Kim, S. W., & Jung, C.-H. (2020). Anti-Inflammatory Activities of an Extract of In Vitro Grown Adventitious Shoots of Toona sinensis in LPS-Treated RAW264.7 and Propionibacterium acnes-Treated HaCaT Cells. Plants, 9(12), 1701. https://doi.org/10.3390/plants9121701





