Vasorelaxant Effect of Boesenbergia rotunda and Its Active Ingredients on an Isolated Coronary Artery
Abstract
1. Introduction
2. Results and Discussion
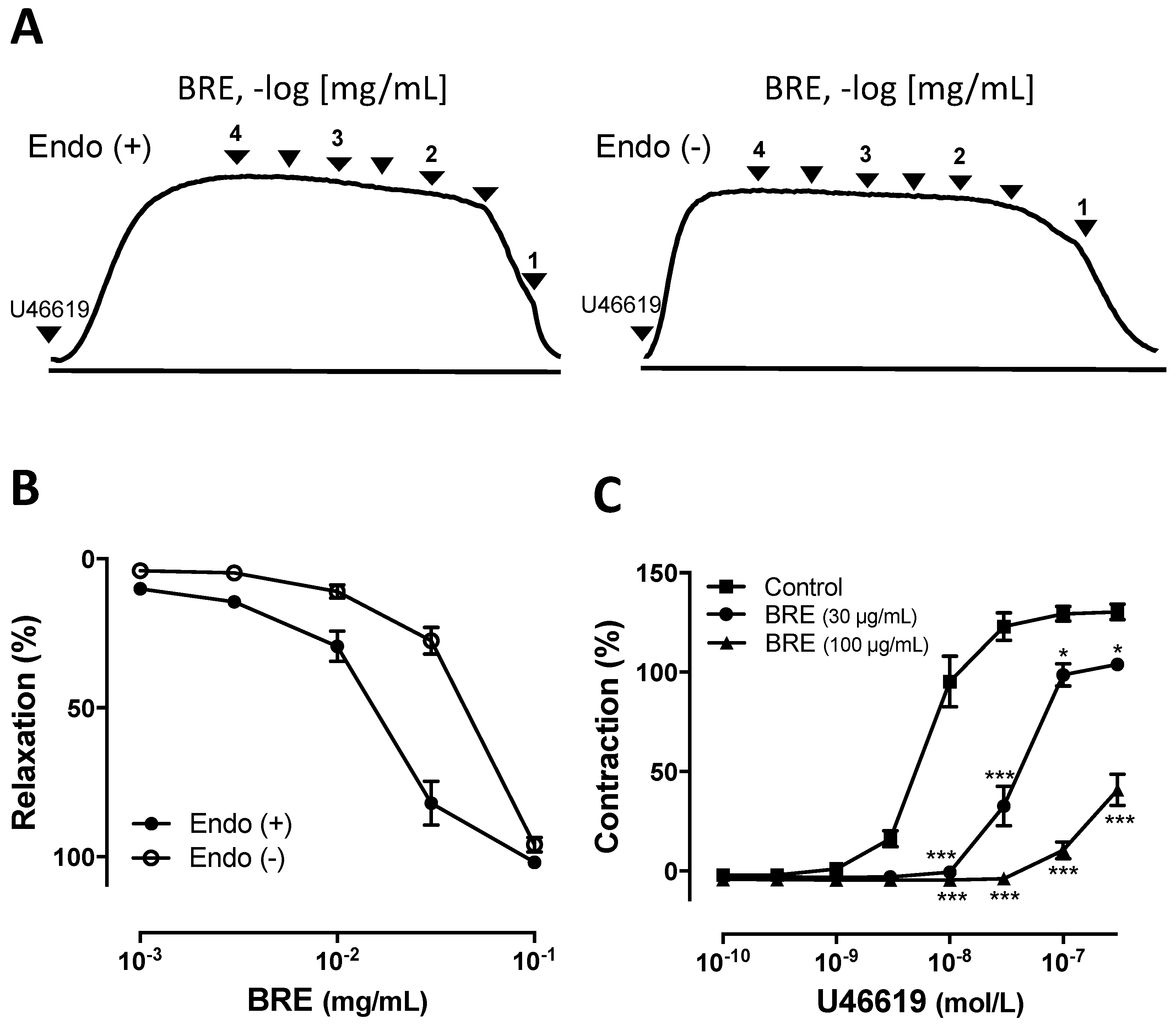
2.1. Vascular Relaxantion Induced by B. rotunda Rhizomes on Coronary Artery
2.2. Mechansim of BRE-Induced Vasorelaxation
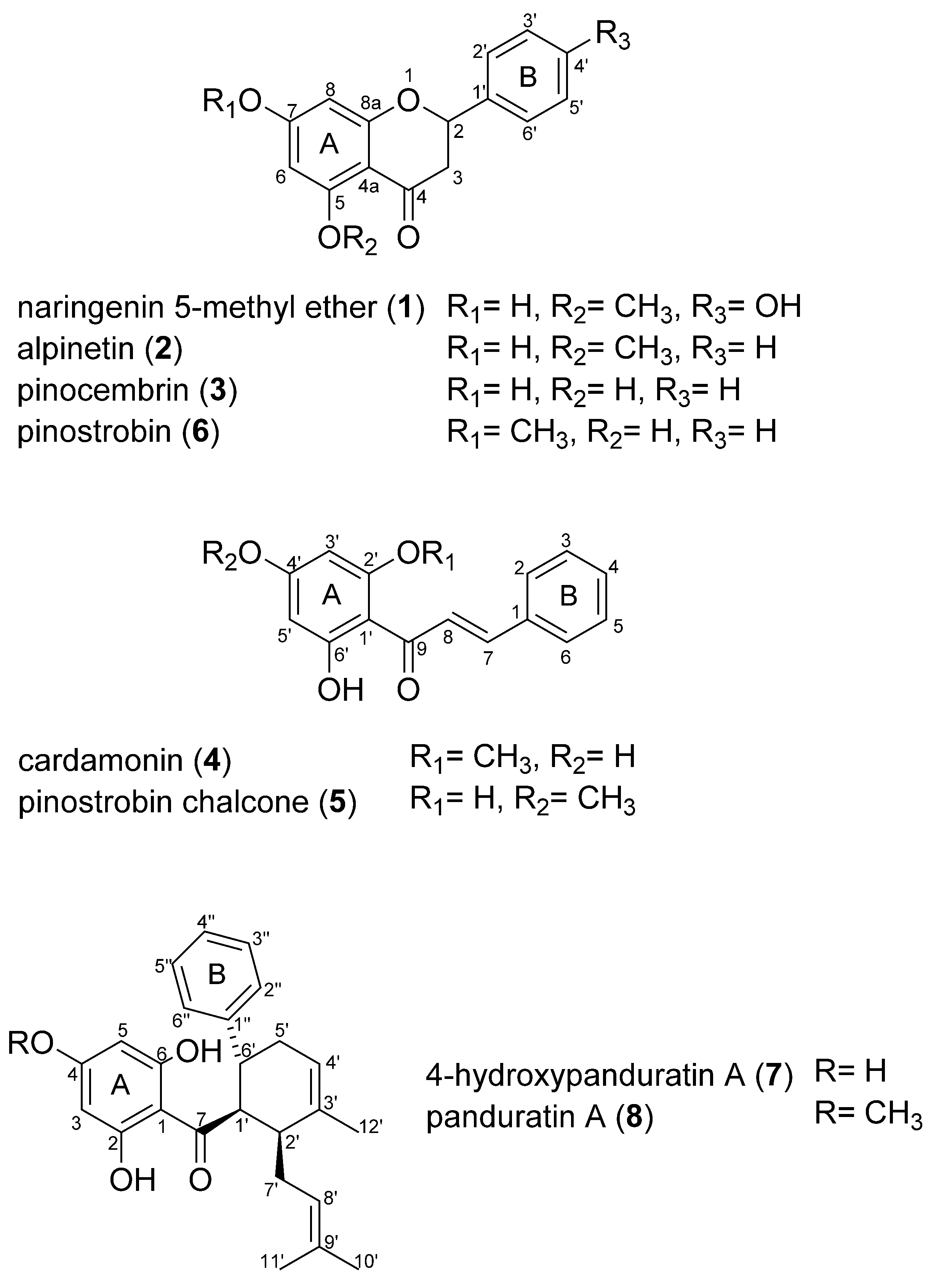
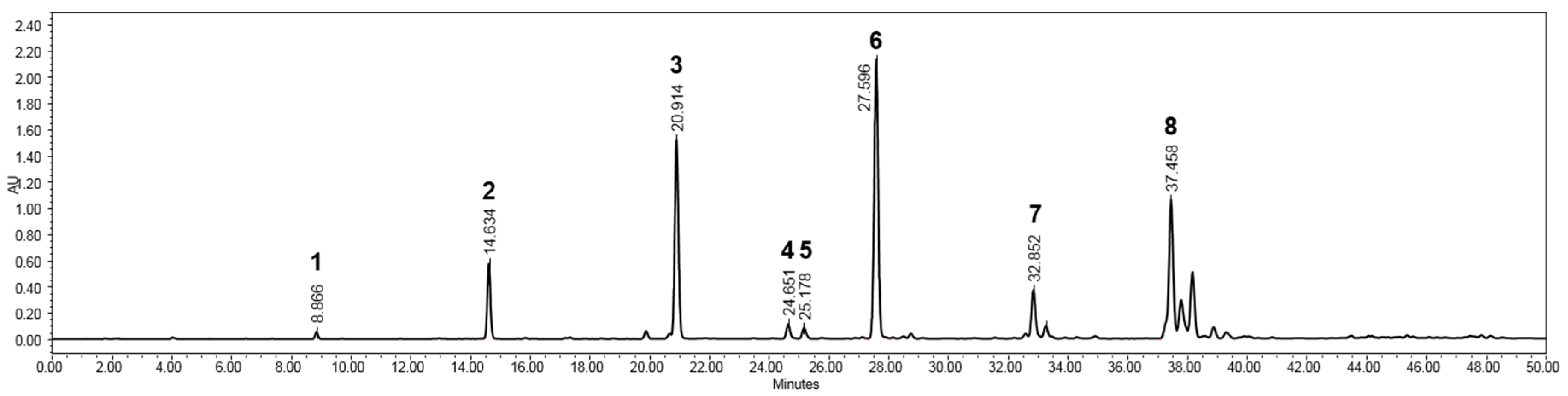
2.3. Isolation and Structural Elucidaton of Flavonoids in the Rhizomes of B. rotunda
2.4. Vasorelaxant Activity of Flavonoids from B. rotunda
3. Materials and Methods
3.1. Plant Material
3.2. Reagents and Chemicals
3.3. Extraction and Isolation of the Rhizomes of B. rotunda
3.4. HPLC-PDA and LC-MS Analysis
3.5. Vascular Reactivity
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BRE | Boesenbergia rotunda rhizomes methanol extract |
| HPLC-PDA | High performance liquid chromatography-photodiode array detector |
| LC-MS | Liquid chromatography-mass spectrometry |
| eNOS | Endothelial nitric oxide synthase |
| ODQ | 1H- [1,2,4]oxadiazole [4,3-alpha]quinoxalin-1-one |
| L-NA | L-Nitroarginine |
| TEA | Tetraethylammonium |
| cGMP | Cyclic guanosine monophosphate |
References
- Li, C.; Li, J.; Weng, X.; Lan, X.; Chi, X. Farnesoid X receptor agonist CDCA reduces blood pressure and regulates vascular tone in spontaneously hypertensive rats. J. Am. Soc. Hypertens. 2015, 9, 507–516.e7. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2013, 17, 2769–2782. [Google Scholar] [CrossRef] [PubMed]
- Oude Griep, L.M.; Verschuren, W.M.M.; Kromhout, D.; Ocké, M.C.; Geleijnse, J.M. Variety in fruit and vegetable consumption and 10-year incidence of CHD and stroke. Public Health Nutr. 2012, 15, 2280–2286. [Google Scholar] [CrossRef]
- Rienks, J.; Barbaresko, J.; Nothlings, U. Association of polyphenol biomarkers with cardiovascular disease and mortality risk: A systematic review and meta-analysis of observational studies. Nutrients 2017, 9, 415. [Google Scholar] [CrossRef] [PubMed]
- The Plant List. Available online: http://www.theplantlist.org/tpl1.1/record/kew-221874 (accessed on 30 November 2019).
- Eng-Chong, T.; Yean-Kee, L.; Chin-Fei, C.; Choon-Han, H.; Sher-Ming, W.; Li-Ping, C.T.; Gen-Teck, F.; Khalid, N.; Abd Rahman, N.; Karsani, S.A.; et al. Boesenbergia rotunda: From ethnomedicine to drug discovery. Evid. Based Complement. Alternat. Med. 2012, 2012, 473637. [Google Scholar] [CrossRef] [PubMed]
- Chahyadi, A.; Hartati, R.; Wirasutisna, K.R.; Elfahmi. Boesenbergia pandurata Roxb., an indonesian medicinal plant: Phytochemistry, biological activity, plant biotechnology. Procedia Chem. 2014, 13, 13–37. [Google Scholar]
- Shindo, K.; Kato, M.; Kinoshita, A.; Kobayashi, A.; Koike, Y. Analysis of antioxidant activities contained in the Boesenbergia pandurata Schult. rhizome. Biosci. Biotechnol. Biochem. 2006, 70, 2281–2284. [Google Scholar] [CrossRef]
- Tuchinda, P.; Reutrakul, V.; Claeson, P.; Pongprayoon, U.; Sematong, T.; Santisuk, T.; Taylor, W.C. Anti-inflammatory cyclohexenyl chalcone derivatives in Boesenbergia pandurata. Phytochemistry 2002, 59, 169–173. [Google Scholar] [CrossRef]
- Yun, J.M.; Kwon, H.; Hwang, J.K. In vitro anti-inflammatory activity of panduratin A isolated from Kaempferia pandurata in raw264.7 cells. Planta Med. 2003, 69, 1102–1108. [Google Scholar]
- Hwang, J.K.; Chung, J.Y.; Baek, N.I.; Park, J.H. Isopanduratin A from Kaempferia pandurata as an active antibacterial agent against cariogenic Streptococcus mutans. Int. J. Antimicrob. Agents 2004, 23, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Rukayadi, Y.; Lee, K.; Han, S.; Yong, D.; Hwang, J.K. In vitro activities of panduratin A against clinical Staphylococcus strains. Antimicrob. Agents Chemother. 2009, 53, 4529–4532. [Google Scholar] [CrossRef] [PubMed]
- Rukayadi, Y.; Han, S.; Yong, D.; Hwang, J.K. In vitro antibacterial activity of panduratin A against enterococci clinical isolates. Biol. Pharm. Bull. 2010, 33, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Cheenpracha, S.; Karalai, C.; Ponglimanont, C.; Subhadhirasakul, S.; Tewtrakul, S. Anti-HIV-1 protease activity of compounds from Boesenbergia pandurata. Bioorg. Med. Chem. 2006, 14, 1710–1714. [Google Scholar] [CrossRef] [PubMed]
- Kiat, T.S.; Pippen, R.; Yusof, R.; Ibrahim, H.; Khalid, N.; Rahman, N.A. Inhibitory activity of cyclohexenyl chalcone derivatives and flavonoids of fingerroot, Boesenbergia rotunda (L.), towards dengue-2 virus NS3 protease. Bioorg. Med. Chem. Lett. 2006, 16, 3337–3340. [Google Scholar] [CrossRef]
- Kirana, C.; Jones, G.P.; Record, I.R.; McIntosh, G.H. Anticancer properties of panduratin A isolated from Boesenbergia pandurata (Zingiberaceae). J. Nat. Med. 2007, 61, 131–137. [Google Scholar] [CrossRef]
- Yun, J.M.; Kweon, M.H.; Kwon, H.; Hwang, J.K.; Mukhtar, H. Induction of apoptosis and cell cycle arrest by a chalcone panduratin A isolated from Kaempferia pandurata in androgen-independent human prostate cancer cells PC3 and DU145. Carcinogenesis 2006, 27, 1454–1464. [Google Scholar] [CrossRef]
- Isa, N.M.; Abdul, A.B.; Abdelwahab, S.I.; Abdullah, R.; Sukari, M.A.; Kamalidehghan, B.; Hadi, A.H.A.; Mohan, S. Boesenbergin A, a chalcone from Boesenbergia rotunda induces apoptosis via mitochondrial dysregulation and cytochrome c release in A549 cells in vitro: Involvement of HSP70 and Bcl2/Bax signalling pathways. J. Funct. Foods 2013, 5, 87–97. [Google Scholar] [CrossRef]
- Shim, J.S.; Kwon, Y.Y.; Han, Y.S.; Hwang, J.K. Inhibitory effect of panduratin A on UV-induced activation of mitogen-activated protein kinases (MAPKs) in dermal fibroblast cells. Planta Med. 2008, 74, 1446–1450. [Google Scholar] [CrossRef]
- Shim, J.S.; Han, Y.S.; Hwang, J.K. The effect of 4-hydroxypanduratin A on the mitogen-activated protein kinase-dependent activation of matrix metalloproteinase-1 expression in human skin fibroblasts. J. Dermatol. Sci. 2009, 53, 129–134. [Google Scholar] [CrossRef]
- Lee, C.W.; Kim, H.S.; Kim, H.K.; Kim, J.W.; Yoon, J.H.; Cho, Y.; Hwang, J.K. Inhibitory effect of panduratin A isolated from Kaempferia panduarata Roxb. on melanin biosynthesis. Phytother. Res. 2010, 24, 1600–1604. [Google Scholar] [CrossRef]
- Abdelwahab, S.I.; Mohan, S.; Abdulla, M.A.; Sukari, M.A.; Abdul, A.B.; Taha, M.M.; Syam, S.; Ahmad, S.; Lee, K.H. The methanolic extract of Boesenbergia rotunda (L.) Mansf. and its major compound pinostrobin induces anti-ulcerogenic property in vivo: Possible involvement of indirect antioxidant action. J. Ethnopharmacol. 2011, 137, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, M.S.; Jo, K.; Lee, K.E.; Hwang, J.K. Therapeutic potential of panduratin A, LKB1-dependent AMP-activated protein kinase stimulator, with activation of PPARα/δ for the treatment of obesity. Diabetes Obes. Metab. 2011, 13, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Kim, M.S.; Sa, B.K.; Kim, M.B.; Hwang, J.K. Boesenbergia pandurata attenuates diet-induced obesity by activating AMP-activated protein kinase and regulating lipid metabolism. Int. J. Mol. Sci. 2012, 13, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Feletou, M.; Vanhoutte, P.M. Endothelium-derived hyperpolarizing factor: Where are we now? Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1215–1225. [Google Scholar] [CrossRef]
- Zeller, A.; Wenzl, M.V.; Beretta, M.; Stessel, H.; Russwurm, M.; Koesling, D.; Schmidt, K.; Mayer, B. Mechanisms underlying activation of soluble guanylate cyclase by the nitroxyl donor Angeli’s salt. Mol. Pharmacol. 2009, 76, 1115–1122. [Google Scholar] [CrossRef]
- Gupta, S.; McArthur, C.; Grady, C.; Ruderman, N.B. Role of endothelium-derived nitric oxide in stimulation of Na(+)-K(+)-ATPase activity by endothelin in rabbit aorta. Am. J. Physiol. 1994, 266, H577–H582. [Google Scholar] [CrossRef]
- Nelson, M.T.; Quayle, J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995, 268, C799–C822. [Google Scholar] [CrossRef]
- Jaipetch, T.; Kanghae, S.; Pancharoen, O.; Patrick, V.; Reutrakul, V.; Tuntiwachwuttikul, P.; White, A. Constituents of Boesenbergia pandurata (syn. Kaempferia pandurata): Isolation, crystal structure and synthesis of (±)-boesenbergin A. Aust. J. Chem. 1982, 35, 351–361. [Google Scholar]
- Trakoontivakorn, G.; Nakahara, K.; Shinmoto, H.; Takenaka, M.; Onishi-Kameyama, M.; Ono, H.; Yoshida, M.; Nagata, T.; Tsushida, T. Structural analysis of a novel antimutagenic compound, 4-hydroxypanduratin A, and the antimutagenic activity of flavonoids in a Thai spice, fingerroot (Boesenbergia pandurata Schult.) against mutagenic heterocyclic amines. J. Agric. Food Chem. 2001, 49, 3046–3050. [Google Scholar] [CrossRef]
- Win, N.N.; Awale, S.; Esumi, H.; Tezuka, Y.; Kadota, S. Bioactive secondary metabolites from Boesenbergia pandurata of Myanmar and their preferential cytotoxicity against human pancreatic cancer PANC-1 cell line in nutrient-deprived medium. J. Nat. Prod. 2007, 70, 1582–1587. [Google Scholar] [CrossRef]
- Pandji, C.; Grimm, C.; Wray, V.; Witte, L.; Proksch, P. Insecticidal constituents from four species of the Zingiberaceae. Phytochemistry 1993, 34, 415–419. [Google Scholar] [CrossRef]
- Tuntiwachwuttikul, P.; Pancharoen, O.; Reutrakul, V.; Byrne, L. (1’RS,2’SR,6’RS)-(2,6-dihydroxy-4-methoxyphenyl)-[3’-methyl-2’-(3’’-methylbut-2’’-enyl)-6’-phenyl-cyclohex-3’-enyl]methanone (panduratin A)-a constituent of the red rhizomers of a variety of Boesenbergia pandurata. Aust. J. Chem. 1984, 37, 449–453. [Google Scholar] [CrossRef]
- Agrawal, P.K. Carbon-13 NMR of Flavonoids, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Micha, R.; Penalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA 2017, 317, 912–924. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Oak, M.H.; Auger, C.; Belcastro, E.; Park, S.H.; Lee, H.H.; Schini-Kerth, V.B. Potential mechanisms underlying cardiovascular protection by polyphenols: Role of the endothelium. Free Radic. Biol. Med. 2018, 122, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.T.; Lau, C.W.; Chan, F.L.; Yao, X.; Chen, Z.Y.; He, Z.D.; Huang, Y. Vasorelaxant effects of cardamonin and alpinetin from Alpinia henryi K. Schum. J. Cardiovasc. Pharmacol. 2001, 37, 596–606. [Google Scholar] [CrossRef]
- Zhu, X.-M.; Fang, L.-H.; Li, Y.-J.; Du, G.-H. Endothelium-dependent and -independent relaxation induced by pinocembrin in rat aortic rings. Vascul. Pharmacol. 2007, 46, 160–165. [Google Scholar] [CrossRef]
- Li, L.; Yang, H.-G.; Yuan, T.-Y.; Zhao, Y.; Du, G.-H. Rho kinase inhibition activity of pinocembrin in rat aortic rings contracted by angiotensin II. Chin. J. Nat. Med. 2013, 11, 258–263. [Google Scholar] [CrossRef]
- Luna-Vazquez, F.J.; Ibarra-Alvarado, C.; Camacho-Corona, M.D.R.; Rojas-Molina, A.; Rojas-Molina, J.I.; Garcia, A.; Bah, M. Vasodilator activity of compounds isolated from plants used in Mexican traditional medicine. Molecules 2018, 23, 1474. [Google Scholar] [CrossRef]
- Sharma, K.; Lee, H.H.; Gong, D.S.; Park, S.H.; Yi, E.; Schini-Kerth, V.; Oak, M.H. Fine air pollution particles induce endothelial senescence via redox-sensitive activation of local angiotensin system. Environ. Pollut. 2019, 252, 317–329. [Google Scholar] [CrossRef] [PubMed]

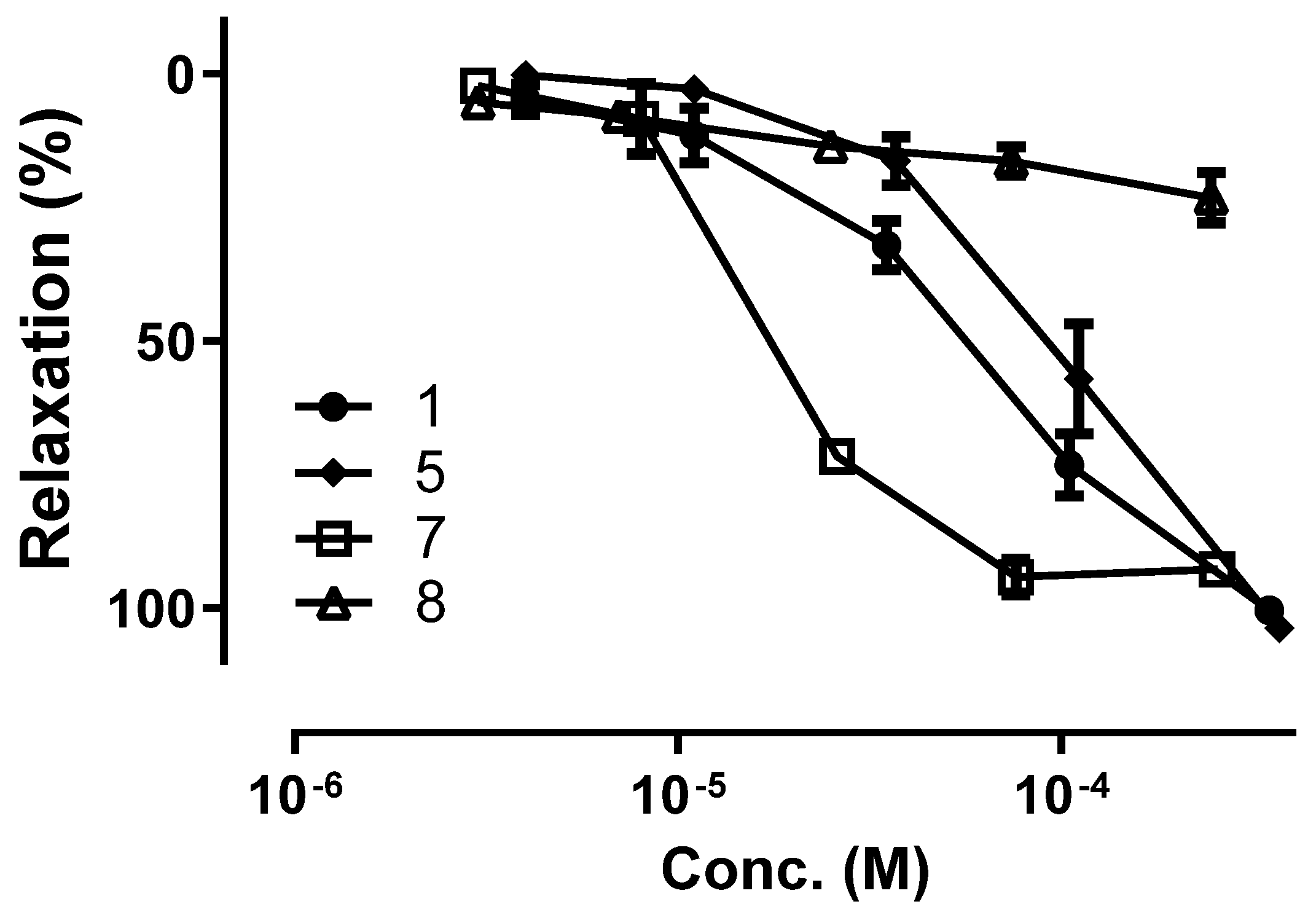
| Compound | EC50 (μM) | Emax (%) |
|---|---|---|
| Naringenin 5-methyl ether (1) | 52.3 ± 10.4 | 100.4 ± 1.9 |
| Alpinetin (2) | 85.6 ± 23.9 | 104.6 ± 8.9 |
| Pinocembrin (3) | 35.1 ± 15.3 | 107.2 ± 3.5 |
| Cardamonin (4) | 166.7 ± 35.5 | 65.4 ± 7.4 |
| Pinostrobin chalcone (5) | 92.1 ± 22.7 | 103.7 ± 3.7 |
| Pinostrobin (6) | 52.0 ± 14.3 | 89.9 ± 2.1 |
| 4-Hydroxypanduratin A (7) | 17.8 ± 2.5 | 92.7 ± 6.8 |
| Panduratin A (8) | EC50 ≥ 1000 | 23.1 ± 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, D.; Gong, D.-S.; Oh, S.H.; Sung, E.H.; Lee, S.O.; Kim, D.-W.; Oak, M.-H.; Kim, H.J. Vasorelaxant Effect of Boesenbergia rotunda and Its Active Ingredients on an Isolated Coronary Artery. Plants 2020, 9, 1688. https://doi.org/10.3390/plants9121688
Adhikari D, Gong D-S, Oh SH, Sung EH, Lee SO, Kim D-W, Oak M-H, Kim HJ. Vasorelaxant Effect of Boesenbergia rotunda and Its Active Ingredients on an Isolated Coronary Artery. Plants. 2020; 9(12):1688. https://doi.org/10.3390/plants9121688
Chicago/Turabian StyleAdhikari, Deepak, Dal-Seong Gong, Se Hee Oh, Eun Hee Sung, Seung On Lee, Dong-Wook Kim, Min-Ho Oak, and Hyun Jung Kim. 2020. "Vasorelaxant Effect of Boesenbergia rotunda and Its Active Ingredients on an Isolated Coronary Artery" Plants 9, no. 12: 1688. https://doi.org/10.3390/plants9121688
APA StyleAdhikari, D., Gong, D.-S., Oh, S. H., Sung, E. H., Lee, S. O., Kim, D.-W., Oak, M.-H., & Kim, H. J. (2020). Vasorelaxant Effect of Boesenbergia rotunda and Its Active Ingredients on an Isolated Coronary Artery. Plants, 9(12), 1688. https://doi.org/10.3390/plants9121688






