Evaluation of the Antiwrinkle Activity of Enriched Isatidis Folium Extract and an HPLC–UV Method for the Quality Control of Its Cream Products
Abstract
1. Introduction
2. Results and Discussion
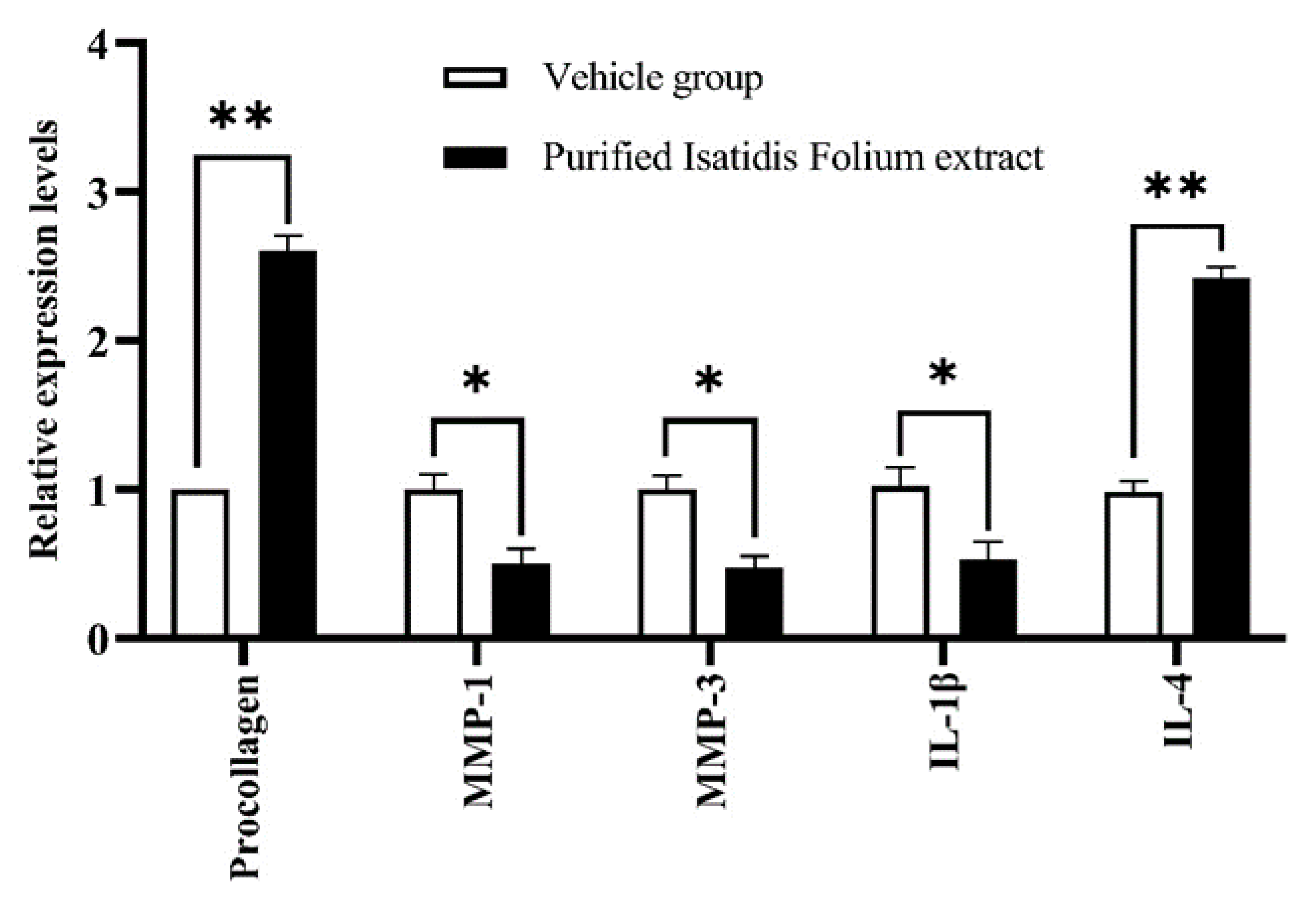
2.1. Antiwrinkle Activity
2.2. Structural Characterization of the Main Compounds from Enriched Isatidis Folium Extract
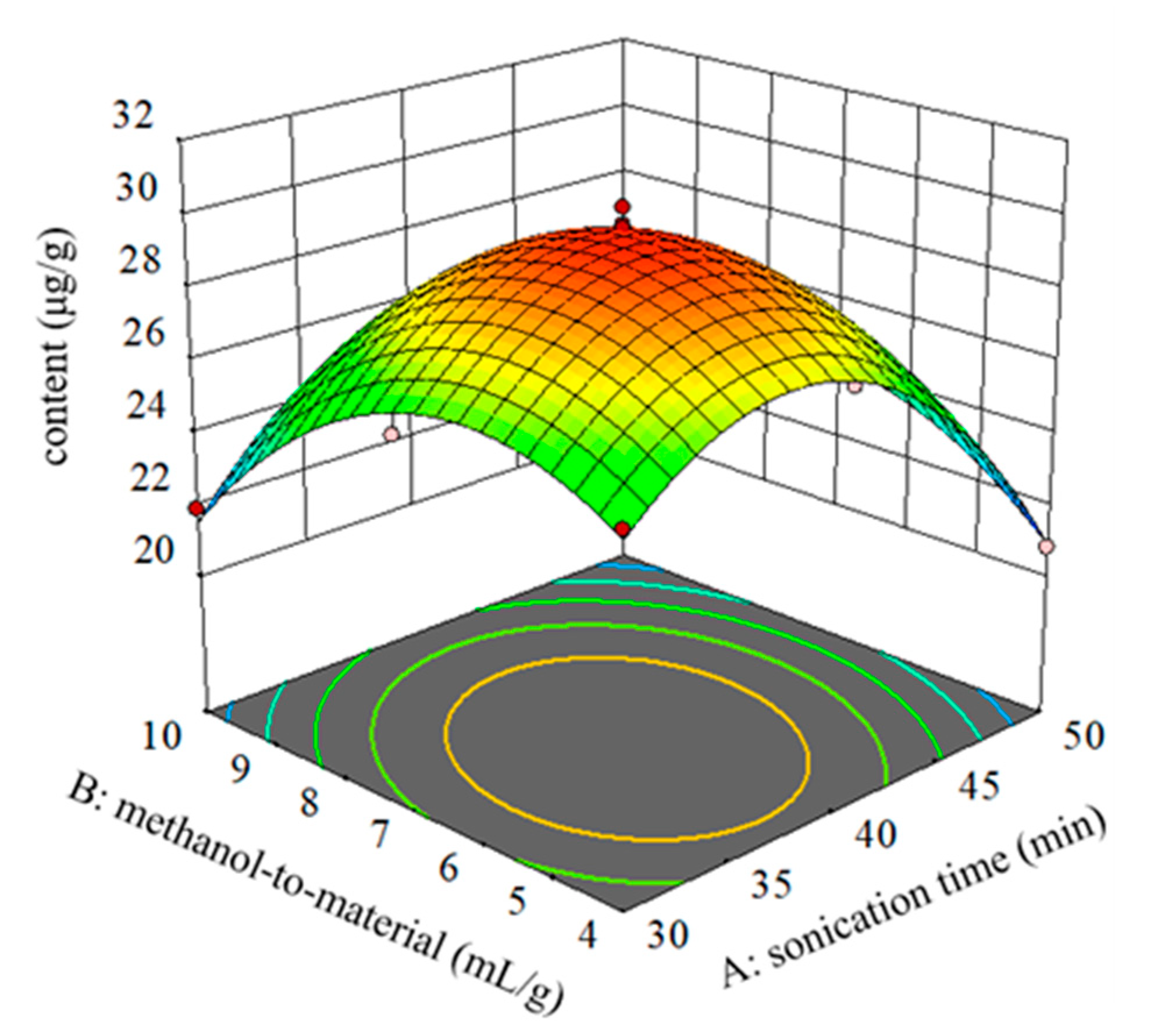
2.3. Optimizing the Analytical Method
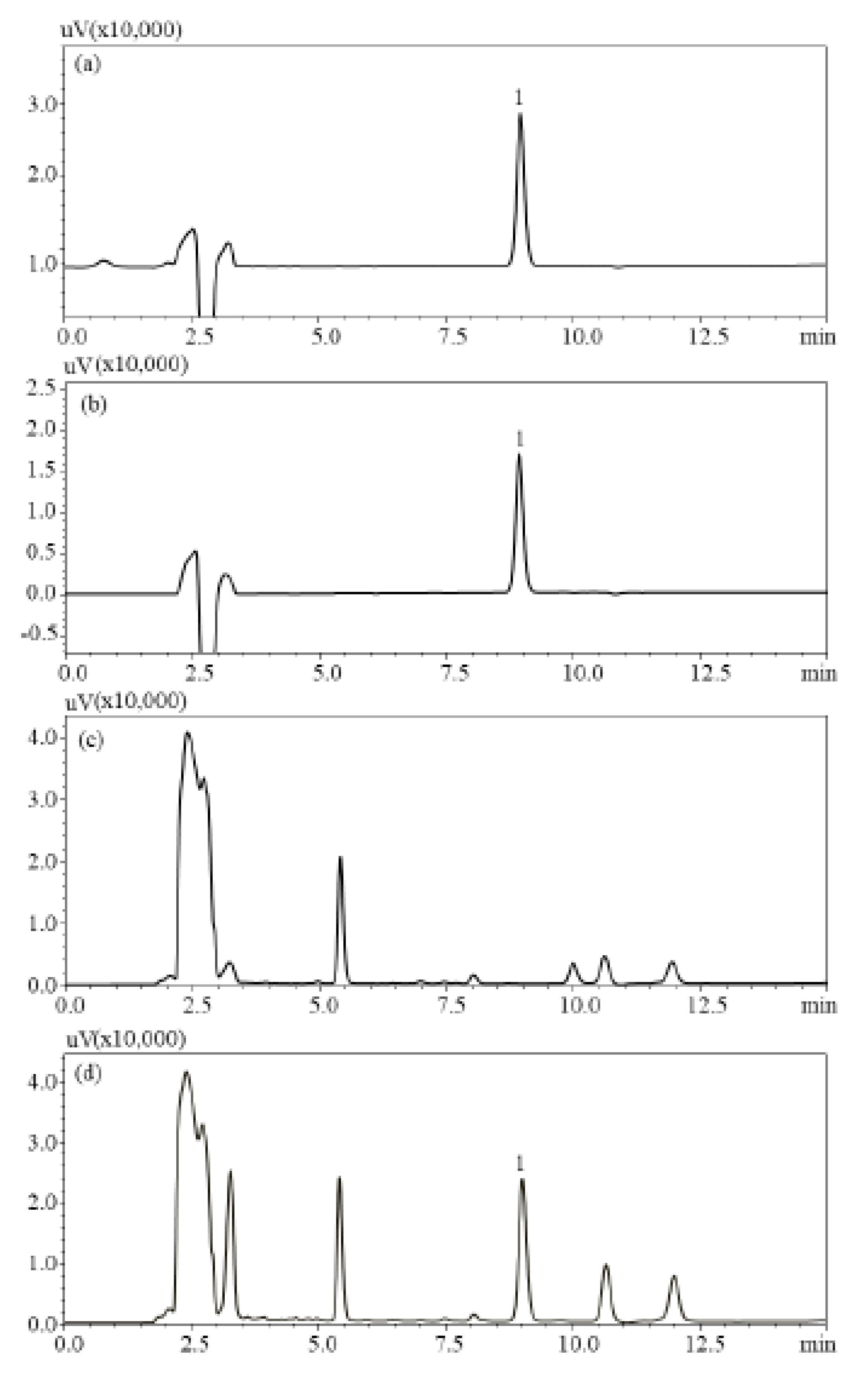
2.4. Method Validation
2.4.1. Linearity, LOD, and LOQ
2.4.2. Accuracy and Precision
2.4.3. Repeatability
2.4.4. Recoveries
2.5. Optimization of Sample Preparation for Extracting TMCA in Developed Antiwrinkle Cream Products
2.6. Method Application to Raw Materials and Products
3. Materials and Methods
3.1. Plant Material and Reagents
3.2. Preparing Enriched Isatidis Folium Extract and Cosmetic Creams
3.3. Preparation of Sample and Standard Solution
3.4. Optimizing Experimental Parameters
3.5. HPLC-PDA-ESI-MS/MS Analysis
3.6. Quality Control of Enriched Isatidis Folium Extract and Developed Antiwrinkle Cream Products
3.7. Cell Culture
3.8. Real-Time Reverse-Transcription Polymerase Chain Reaction (Real-Time RT-PCR)
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Trojahn, C.; Dobos, G.; Lichterfeld, A.; Blume-Peytavi, U.; Kottner, J. Characterizing facial skin ageing in humans: Disentangling extrinsic from intrinsic biological phenomena. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, Y.G.; Lee, H.-J.; Lim, S.J.; Nho, C.W. Youngiasides A and C isolated from Youngia denticulatum inhibit UVB-Induced MMP expression and promote type I procollagen production via repression of MAPK/AP-1/NF-κB and activation of AMPK/Nrf2 in HaCaT cells and human dermal fibroblasts. J. Agric. Food Chem. 2015, 63, 5428–5438. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Ngo, H.T.; Seo, S.A.; Park, B.; Zhang, M.; Yi, T.-H. Protective effect of dietary Alchemilla mollis on UVB-irradiated premature skin aging through regulation of transcription factor NFATc1 and Nrf2/ARE pathways. Phytomedicine 2018, 39, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, G.; Cao, F.; Fu, F.-F. Collection and evaluation of thirty-seven pomegranate germplasm resources. Appl. Biol. Chem. 2020, 63, 1–15. [Google Scholar] [CrossRef]
- Liu, S.; You, L.; Zhao, Y.; Chang, X. Hawthorn polyphenol extract inhibits UVB-induced skin photoaging by regulating MMP expression and type I procollagen production in mice. J. Agric. Food Chem. 2018, 66, 8537–8546. [Google Scholar] [CrossRef]
- Raffetto, J.D.; Khalil, R.A. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem. Pharmacol. 2008, 75, 346–359. [Google Scholar] [CrossRef]
- Pourrajab, F.; Forouzannia, S.K.; Tabatabaee, S.A. Molecular characteristics of bone marrow mesenchymal stem cells, source of regenerative medicine. Int. J. Cardiol. 2013, 163, 125–131. [Google Scholar] [CrossRef]
- Hornebeck, W.; Maquart, F.X. Proteolyzed matrix as a template for the regulation of tumor progression. Biomed. Pharmacother 2003, 57, 223–230. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Matsui, M.S.; Elmets, C.A.; Mukhtar, H. Polyphenolic antioxidant (-)-epigallocatechin-3-gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem. Photobiol. 1999, 69, 148–153. [Google Scholar] [CrossRef]
- Gong, M.; Zhai, X.; Yu, L.; Li, C.; Ma, X.; Shen, Q.; Han, Y.; Yang, D. ADSCs inhibit photoaging-and photocarcinogenesis-related inflammatory responses and extracellular matrix degradation. J. Cell. Biochem. 2020, 121, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Lee, S.-R.; Kim, M.-K.; Shin, C.-Y.; Lee, D.H.; Chung, J.H. Activation of peroxisome proliferator-activated receptor alpha improves aged and UV-irradiated skin by catalase induction. PLoS ONE 2016, 11, e0162628. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gong, D.; Zhu, Y.; Wei, W.; Sun, G. Quality consistency evaluation of Isatidis Folium combined with equal weight quantified ratio fingerprint method and determination of antioxidant activity. J. Chromatogr. B 2018, 1095, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Lü, H.T.; Liu, J.; Deng, R.; Song, J.Y. Preparative isolation and purification of indigo and indirubin from Folium isatidis by high-speed counter-current chromatography. Phytochem. Anal. 2012, 23, 637–641. [Google Scholar] [CrossRef]
- Zhang, Q.; Hong, B.; Zheng, L.; Wang, X.; Cai, D. Matrix solid-phase dispersion extraction followed by HPLC-diode array detection method for the determination of major constituents in a traditional Chinese medicine Folium isatidis (Da-qing-ye). J. Sep. Sci. 2012, 35, 2453–2459. [Google Scholar] [CrossRef]
- Liang, Z.; Li, B.; Liang, Y.; Su, Y.; Ito, Y. Separation and purification of two minor compounds from Radix isatidis by integrative MPLC and HSCCC with preparative HPLC. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 647–653. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Song, F.; Liu, Z.; Zheng, Z.; Xing, J.; Liu, S. Study of the ESI and APCI interfaces for the UPLC–MS/MS analysis of pesticides in traditional Chinese herbal medicine. Anal. Bioanal. Chem. 2014, 406, 1481–1491. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, Y.; Jin, J.; Dong, L.; Xu, F.; Chen, S.; Wang, Z.; Liang, G.; Shan, X. n-Butanol extract from Folium isatidis inhibits lipopolysaccharide-induced inflammatory cytokine production in macrophages and protects mice against lipopolysaccharide-induced endotoxic shock. Drug Des. Dev. Ther. 2015, 9, 5601. [Google Scholar] [CrossRef][Green Version]
- Wu, B.; Wang, L.; Jiang, L.; Dong, L.; Xu, F.; Lu, Y.; Jin, J.; Wang, Z.; Liang, G.; Shan, X. n-butanol extract from Folium isatidis inhibits the lipopolysaccharide-induced downregulation of CXCR1 and CXCR2 on human neutrophils. Mol. Med. Rep. 2018, 17, 179–185. [Google Scholar] [CrossRef]
- Saleem, H.; Htar, T.T.; Naidu, R.; Anwar, S.; Zengin, G.; Locatelli, M.; Ahemad, N. HPLC–PDA polyphenolic quantification, UHPLC–MS secondary metabolite composition, and in vitro enzyme inhibition potential of Bougainvillea glabra. Plants 2020, 9, 388. [Google Scholar] [CrossRef]
- Torres-Vega, J.; Gómez-Alonso, S.; Pérez-Navarro, J.; Pastene-Navarrete, E. Green extraction of alkaloids and polyphenols from Peumus boldus leaves with natural deep eutectic solvents and profiling by HPLC-PDA-IT-MS/MS and HPLC-QTOF-MS/MS. Plants 2020, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Song, H.; Xie, J.; Liu, T.; Zhao, X.; Chen, X.; He, X.; Wu, S.; Zhang, Y.; Zheng, X. Research progress in the biological activities of 3, 4, 5-trimethoxycinnamic acid (TMCA) derivatives. Eur. J. Med. Chem. 2019, 173, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Rouau, X.; Cheynier, V.; Surget, A.; Gloux, D.; Barron, C.; Meudec, E.; Louis-Montero, J.; Criton, M. A dehydrotrimer of ferulic acid from maize bran. Phytochemistry 2003, 63, 899–903. [Google Scholar] [CrossRef]
- Wang, Q.; Xiao, B.-X.; Pan, R.-L.; Liu, X.-M.; Liao, Y.-H.; Feng, L.; Cao, F.-R.; Chang, Q. An LC-MS/MS method for simultaneous determination of three Polygala saponin hydrolysates in rat plasma and its application to a pharmacokinetic study. J. Ethnopharmacol. 2015, 169, 401–406. [Google Scholar] [CrossRef]
- Mariani, C.; Braca, A.; Vitalini, S.; De Tommasi, N.; Visioli, F.; Fico, G. Flavonoid characterization and in vitro antioxidant activity of Aconitum anthora L.(Ranunculaceae). Phytochemistry 2008, 69, 1220–1226. [Google Scholar] [CrossRef]
- Gao, D.; Le Ba, V.; Rustam, R.; Cho, C.W.; Yang, S.Y.; Su, X.D.; Kim, Y.H.; Kang, J.S. Isolation of bioactive components with soluble epoxide hydrolase inhibitory activity from Stachys sieboldii MiQ. by ultrasonic-assisted extraction optimized using response surface methodology. Prep. Biochem. Biotechnol. 2020, 1–10. [Google Scholar] [CrossRef]
- Balli, D.; Bellumori, M.; Orlandini, S.; Cecchi, L.; Mani, E.; Pieraccini, G.; Mulinacci, N.; Innocenti, M. Optimized hydrolytic methods by response surface methodology to accurately estimate the phenols in cereal by HPLC-DAD: The case of millet. Food Chem. 2020, 303, 125393. [Google Scholar] [CrossRef]
- Cheng, X.; Cheng, Y.; Zhang, N.; Zhao, S.; Cui, H.; Zhou, H. Purification of flavonoids from Carex meyeriana Kunth based on AHP and RSM: Composition analysis, antioxidant, and antimicrobial activity. Ind. Crops Prod. 2020, 157, 112900. [Google Scholar] [CrossRef]
- Mei, Z.; Zhang, R.; Zhao, Z.; Zheng, G.; Xu, X.; Yang, D. Extraction process and method validation for bioactive compounds from Citrus reticulata cv. Chachiensis: Application of response surface methodology and HPLC–DAD. Acta Chromatogr. 2020. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, L.; Jin, W.; Li, C.; Wang, Y.; Wan, H.; Yang, J. Simultaneous optimization of the ultrasonic extraction method and determination of the antioxidant activities of hydroxysafflor yellow A and anhydrosafflor yellow B from safflower using a response surface methodology. Molecules 2020, 25, 1226. [Google Scholar] [CrossRef]
- Pandey, A.; Belwal, T.; Sekar, K.C.; Bhatt, I.D.; Rawal, R.S. Optimization of ultrasonic-assisted extraction (UAE) of phenolics and antioxidant compounds from rhizomes of Rheum moorcroftianum using response surface methodology (RSM). Ind. Crops Prod. 2018, 119, 218–225. [Google Scholar] [CrossRef]
- Lim, H.-S.; Choi, J.-C.; Song, S.-B.; Kim, M. Quantitative determination of carmine in foods by high-performance liquid chromatography. Food Chem. 2014, 158, 521–526. [Google Scholar] [CrossRef] [PubMed]
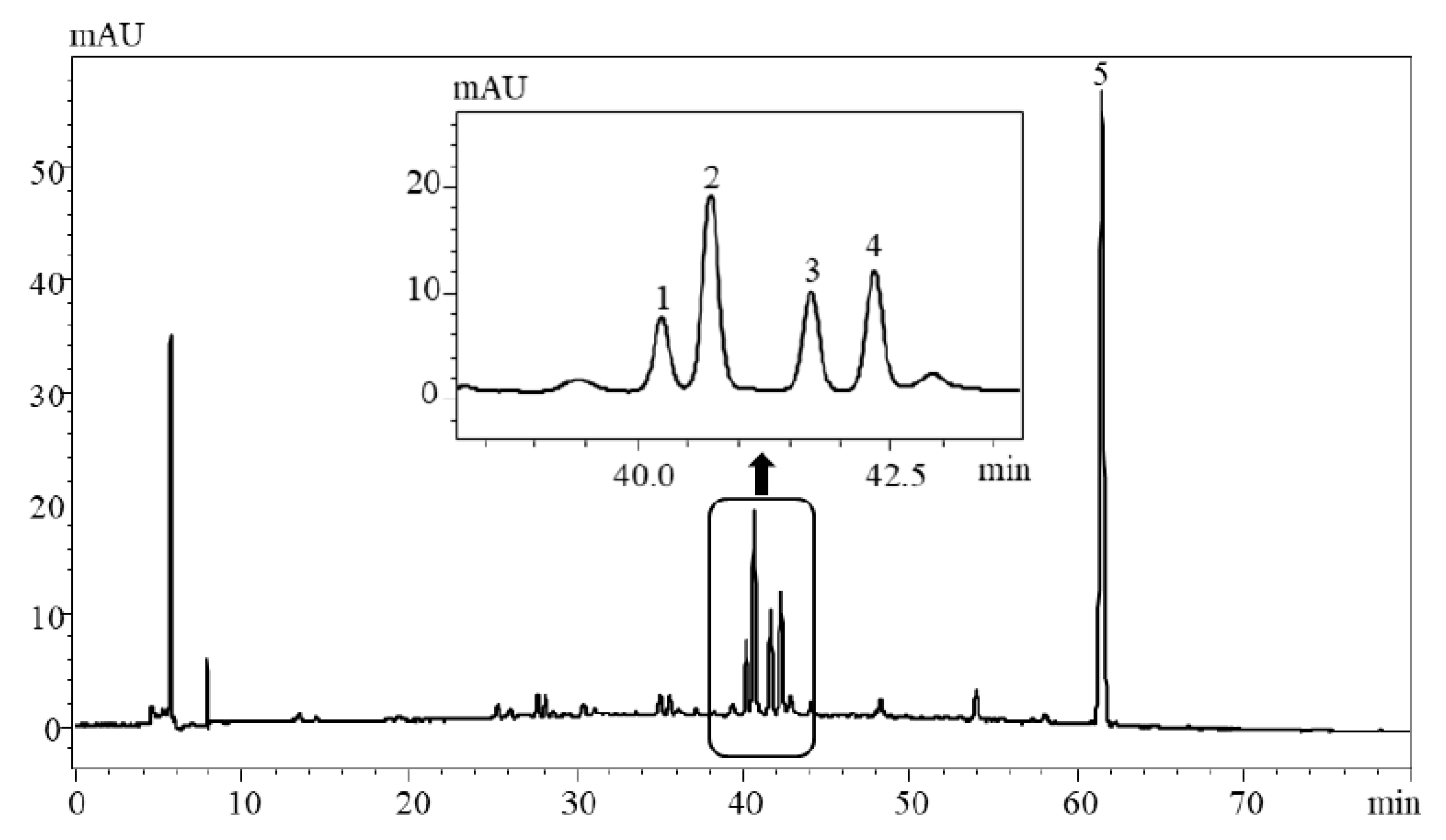
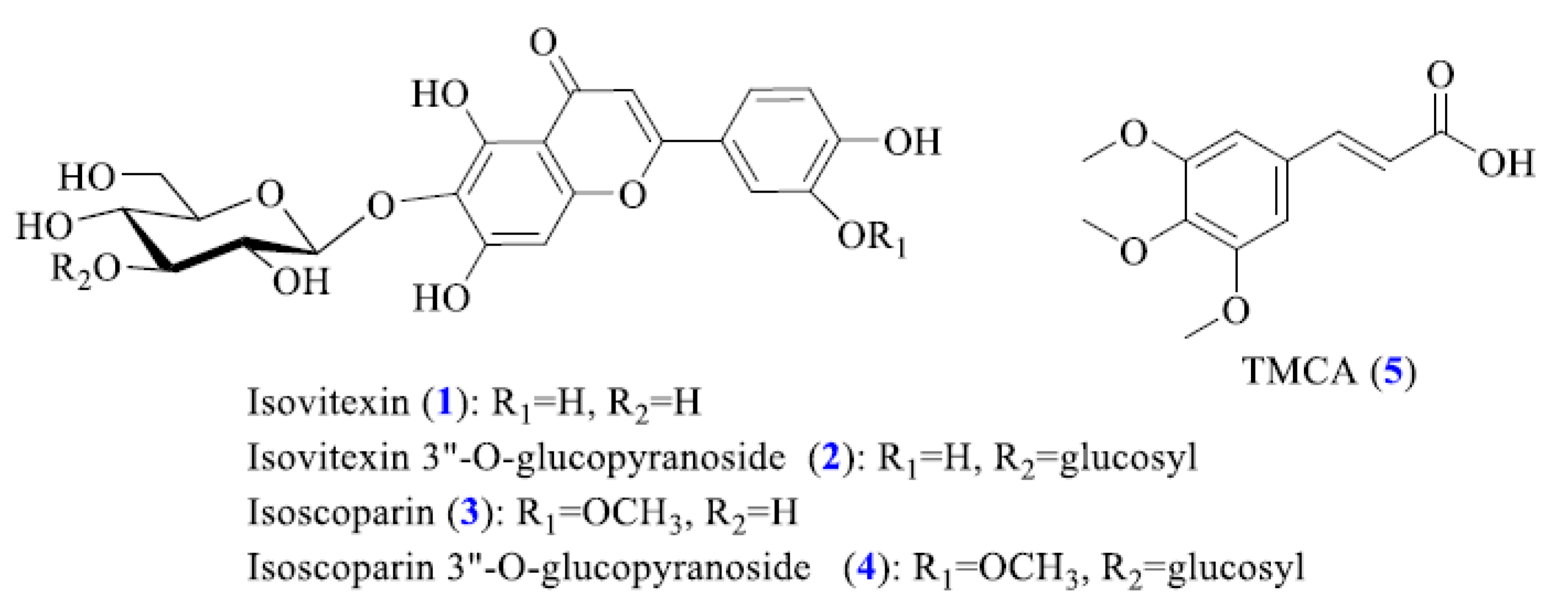
| No. | Rt a (min) | UV λ (nm) | Precursorion (m/z) | Production (m/z) | Molecular Formula | Identification |
|---|---|---|---|---|---|---|
| 1 | 40.19 | 213, 269, 336 | 431.05 (M-H)− | 311.12 (M-H-C4H9O4)− | C21H20O10 | isovitexin |
| 2 | 40.82 | 213, 269, 338 | 592.65 (M-H)− | 311.12 (M-Glcb-C4H9O4-H)− 431.00 (M-Glc-H)− | C27H30O15 | isovitexin-3″-O-glucopyranoside |
| 3 | 41.78 | 209, 270, 346 | 461.20 (M-H)− | 341.28 (M-H-C4H9O4)− | C22H22O11 | isoscoparin |
| 4 | 42.35 | 208, 270, 348 | 624.03 (M-H)− | 341.28 (M-Glc-C4H9O4-H)− 461.12 (M-Glc–H)− | C28H32O16 | isoscoparin-3″-O-glucopyranoside |
| 5 | 62.43 | 229,302 | 239.00 (M-H)+ | 239.00 (M + H)+ | C12H14O5 | TMCAc |
| Run | A: Sonication Time (min) | B: Methanol-to-Material Ratio (mL/g) | Content of TMCA Y (μg/g) |
|---|---|---|---|
| 1 | 40 (0) | 7 (0) | 30.2 |
| 2 | 40 (0) | 4 (−1) | 27.3 |
| 3 | 40 (0) | 7 (0) | 29.7 |
| 4 | 40 (0) | 7 (0) | 29.6 |
| 5 | 40 (0) | 10 (1) | 25.2 |
| 6 | 50 (+1) | 10 (+1) | 21.3 |
| 7 | 40 (0) | 7 (0) | 29.4 |
| 8 | 50 (+1) | 4 (−1) | 20.8 |
| 9 | 40 (0) | 7 (0) | 29.7 |
| 10 | 30 (−1) | 10 (+1) | 21.9 |
| 11 | 50 (1) | 7(0) | 24.2 |
| 12 | 30 (−1) | 4 (−1) | 25.8 |
| 13 | 30 (−1) | 7 (0) | 26.0 |
| Sample | Batch Number | Concentration |
|---|---|---|
| The powder of enriched Isatidis Folium extract | B-1 | 126.32 ± 0.32 mg/g |
| B-2 | 127.45 ± 0.21 mg/g | |
| B-3 | 126.61 ± 0.01 mg/g | |
| Anti-wrinkle cream products | C-1 | 29.2 ± 0.01 μg/g |
| C-2 | 28.9 ± 0.02 μg/g | |
| C-3 | 29.6 ± 0.01 μg/g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, D.; Cho, C.W.; Kim, C.T.; Jeong, W.S.; Kang, J.S. Evaluation of the Antiwrinkle Activity of Enriched Isatidis Folium Extract and an HPLC–UV Method for the Quality Control of Its Cream Products. Plants 2020, 9, 1586. https://doi.org/10.3390/plants9111586
Gao D, Cho CW, Kim CT, Jeong WS, Kang JS. Evaluation of the Antiwrinkle Activity of Enriched Isatidis Folium Extract and an HPLC–UV Method for the Quality Control of Its Cream Products. Plants. 2020; 9(11):1586. https://doi.org/10.3390/plants9111586
Chicago/Turabian StyleGao, Dan, Chong Woon Cho, Cheong Taek Kim, Won Seok Jeong, and Jong Seong Kang. 2020. "Evaluation of the Antiwrinkle Activity of Enriched Isatidis Folium Extract and an HPLC–UV Method for the Quality Control of Its Cream Products" Plants 9, no. 11: 1586. https://doi.org/10.3390/plants9111586
APA StyleGao, D., Cho, C. W., Kim, C. T., Jeong, W. S., & Kang, J. S. (2020). Evaluation of the Antiwrinkle Activity of Enriched Isatidis Folium Extract and an HPLC–UV Method for the Quality Control of Its Cream Products. Plants, 9(11), 1586. https://doi.org/10.3390/plants9111586






