Spider Silk Fibroin Protein Heterologously Produced in Rice Seeds Reduce Diabetes and Hypercholesterolemia in Mice
Abstract
1. Introduction
2. Results
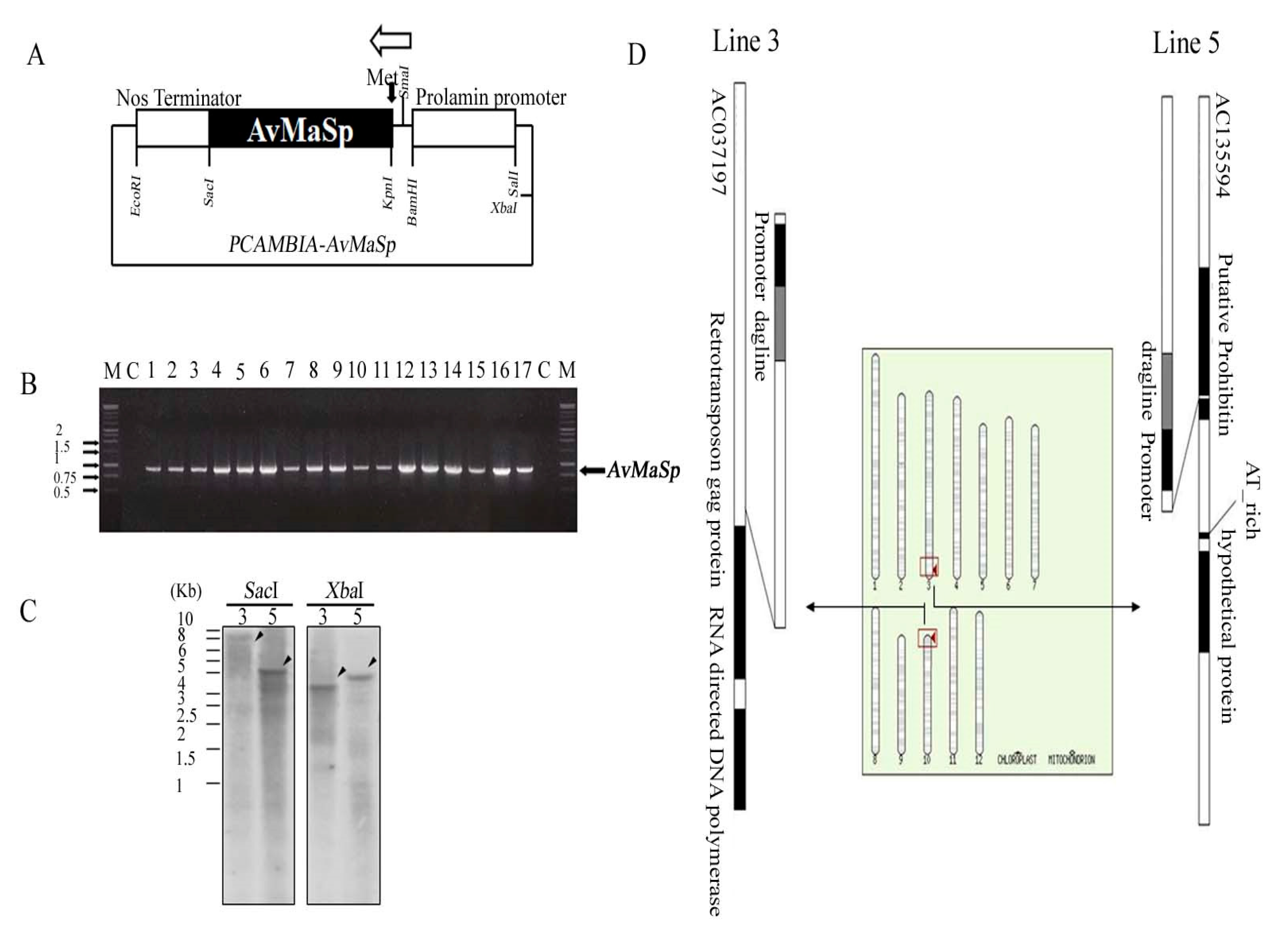
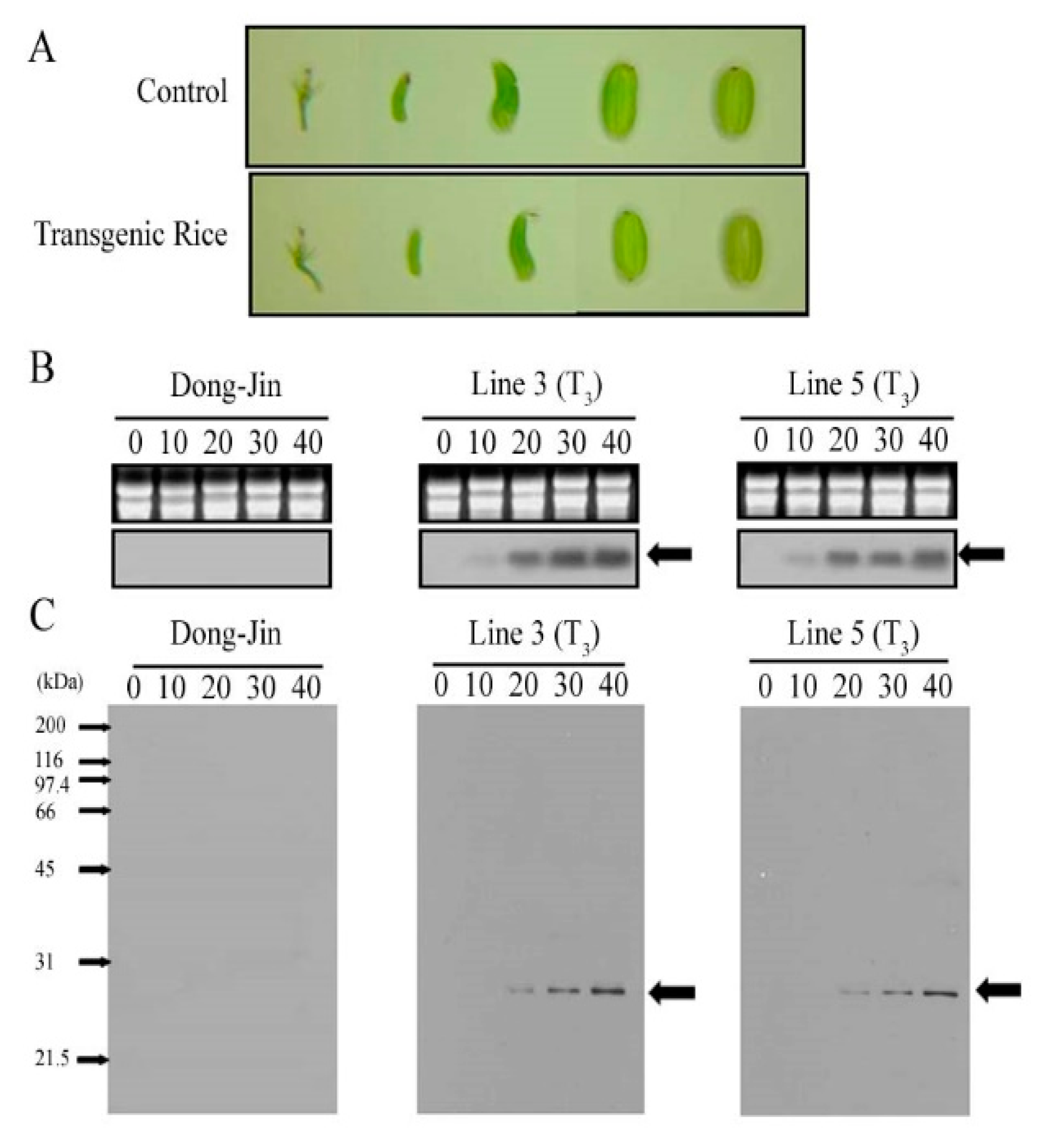
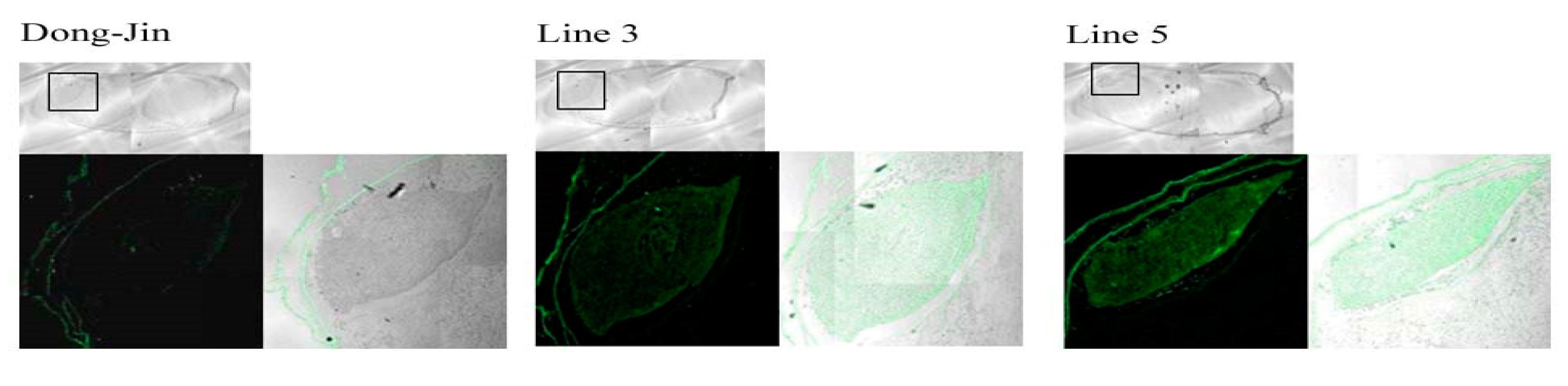
2.1. Generation and Characterization of a Transgenic Rice Line
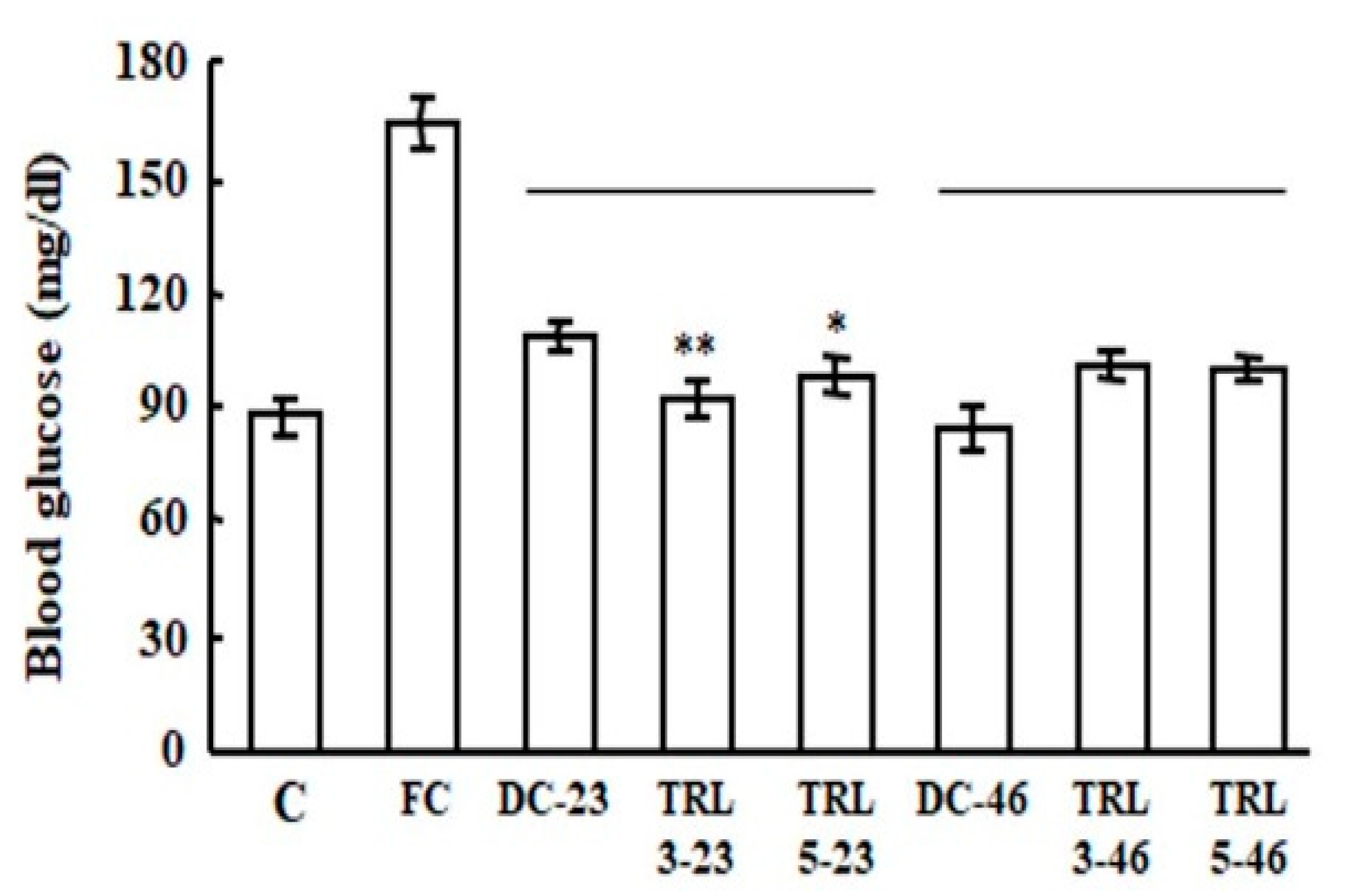
2.2. Effects of the Transgenic Rice on Mouse Liver Weights and Blood Glucose Levels
2.3. Effects of the Transgenic Rice on the Mouse Lipid Metabolism
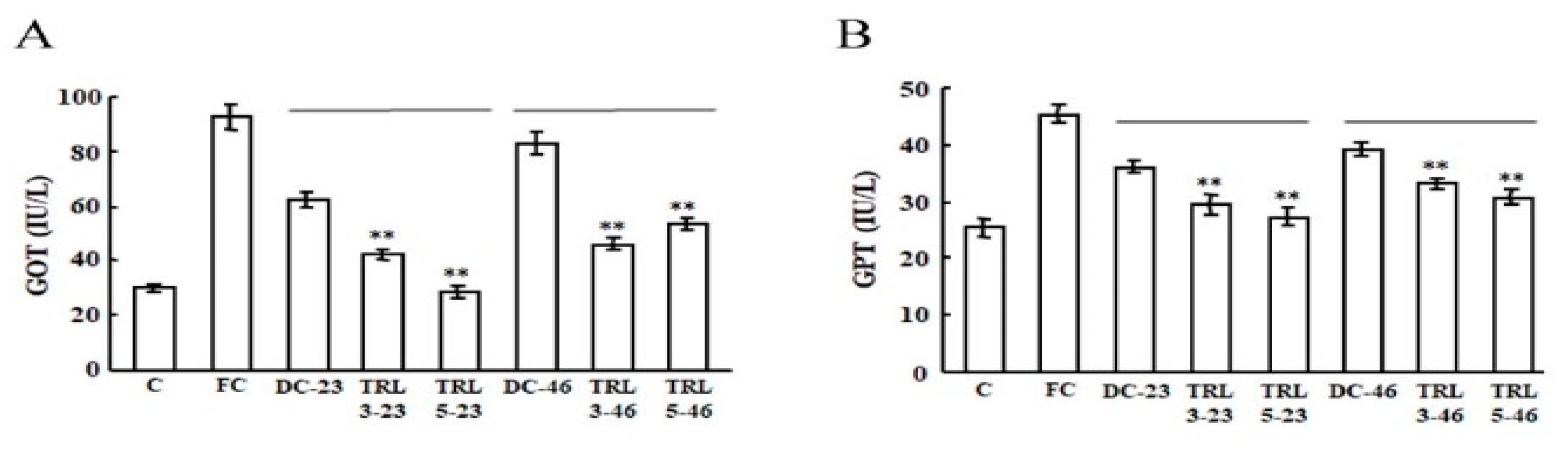
2.4. Effects of the Transgenic Rice on Mouse Liver Function
3. Discussion
4. Materials and Methods
4.1. Production of Transgenic Rice
4.2. DNA and RNA Gel Blot Analysis
4.3. Genome Analysis
4.4. Western Blot Analysis
4.5. Immunofluorescence Staining
4.6. Animals
4.7. Sample Collections
4.8. Analyses
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Scheibel, T. Spider silks: Recombinant synthesis, assembly, spinning, and engineering of synthetic proteins. Microb. Cell Factories 2004, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Vendrely, C.; Scheibel, T. Biotechnological Production of Spider-Silk Proteins Enables New Applications. Macromol. Biosci. 2007, 7, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A. Direct Ink Writing of 3D Functional Materials. Adv. Funct. Mater. 2006, 16, 2193–2204. [Google Scholar] [CrossRef]
- Sobajo, C.; Behzad, F.; Yuan, X.-F.; Hernandez, R.M. Silk: A Potential Medium for Tissue Engineering. Eplasty 2008, 8, e47. [Google Scholar] [PubMed]
- Foo, C.W.P.; Kaplan, D. Genetic engineering of fibrous proteins: Spider dragline silk and collagen. Adv. Drug Deliv. Rev. 2002, 54, 1131–1143. [Google Scholar] [CrossRef]
- Ayoub, N.A.; Garb, J.E.; Tinghitella, R.M.; Collin, M.A.; Hayashi, C.Y. Blueprint for a High-Performance Biomaterial: Full-Length Spider Dragline Silk Genes. PLoS ONE 2007, 2, e514. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Kim, B.Y.; Kim, D.-H.; Jin, B.R. Molecular cloning and characterization of the partial major ampullate silk protein gene from the spider Araneus ventricosus. J. Asia Pac. Èntomol. 2012, 15, 641–646. [Google Scholar] [CrossRef]
- Zhou, C. Fine organization of Bombyx mori fibroin heavy chain gene. Nucleic Acids Res. 2000, 28, 2413–2419. [Google Scholar] [CrossRef]
- Gao, J.; Bai, J.; Man, Q.; Liu, G. Mechanism of silk hydrates on modulating the blood glucose metabolism in rats with experimental diabetes. Wei Sheng Yan Jiu J. Hyg. Res. 2000, 29, 379–382. [Google Scholar]
- Hyun, C.-K.; Kim, I.-Y.; Frost, S.C. Soluble fibroin enhances insulin sensitivity and glucose metabolism in 3T3-L1 adipocytes. J. Nutr. 2004, 134, 3257–3263. [Google Scholar] [CrossRef]
- Park, K.-J.; Jin, H.-H.; Hyun, C.-K. Antigenotoxicity of peptides produced from silk fibroin. Process Biochem. 2002, 38, 411–418. [Google Scholar] [CrossRef]
- Jung, E.Y.; Lee, H.-S.; Kim, J.M.; Lee, K.-W.; Suh, H.J.; Lee, H.J. Feeding silk protein hydrolysates to C57BL/KsJ-db/db mice improves blood glucose and lipid profiles. Nutr. Res. 2010, 30, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Do, S.-G.; Park, J.-H.; Nam, H.; Kim, J.-B.; Lee, J.-Y.; Oh, Y.-S.; Suh, J.-G. Silk fibroin hydrolysate exerts an anti-diabetic effect by increasing pancreatic β cell mass in C57BL/KsJ-db/db mice. J. Vet. Sci. 2012, 13, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Kim, B.Y.; Kim, D.H.; Jin, B.R. Spider silk fibroin enhances insulin secretion and reduces blood glucose levels in diabetic mice. J. Asia Pac. Èntomol. 2014, 17, 907–909. [Google Scholar] [CrossRef]
- Gotoh, K.; Izumi, H.; Kanamoto, T.; Tamada, Y.; Nakashima, H. Sulfated Fibroin, a Novel Sulfated Peptide Derived from Silk, Inhibits Human Immunodeficiency Virus Replicationin Vitro. Biosci. Biotechnol. Biochem. 2000, 64, 1664–1670. [Google Scholar] [CrossRef]
- Igarashi, K.; Yoshioka, K.; Mizutani, K.; Miyakoshi, M.; Murakami, T.; Akizawa, T. Blood Pressure-Depressing Activity of a Peptide Derived from Silkworm Fibroin in Spontaneously Hypertensive Rats. Biosci. Biotechnol. Biochem. 2006, 70, 517–520. [Google Scholar] [CrossRef]
- Yonekura-Sakakibara, K.; Saito, K. Review: Genetically modified plants for the promotion of human health. Biotechnol. Lett. 2006, 28, 1983–1991. [Google Scholar] [CrossRef]
- Yang, L.; Wakasa, Y.; Takaiwa, F. Biopharming to Increase Bioactive Peptides in Rice Seed. J. AOAC Int. 2008, 91, 957–964. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, B.Y.; Kim, D.-H.; Jin, B.R. Recombinant spider silk fibroin protein produces a non-cytotoxic and non-inflammatory response. J. Asia Pac. Èntomol. 2016, 19, 1015–1018. [Google Scholar] [CrossRef]
- Acharya, C.; Ghosh, S.K.; Kundu, S.C. Silk fibroin protein from mulberry and non-mulberry silkworms: Cytotoxicity, biocompatibility and kinetics of L929 murine fibroblast adhesion. J. Mater. Sci. Mater. Electron. 2008, 19, 2827–2836. [Google Scholar] [CrossRef]
- Dams-Kozlowska, H.; Majer, A.; Tomasiewicz, P.; Lozinska, J.; Kaplan, D.L.; Mackiewicz, A. Purification and cytotoxicity of tag-free bioengineered spider silk proteins. J. Biomed. Mater. Res. Part A 2012, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Cao, G.; Renyu, X.; Zhonghua, P.; Xiaojian, Z.; Zhou, W.; Gong, C. Reducing blood glucose level in TIDMmice by orally administering the silk glands of transgenic hIGF-I silkworms. Biochem. Biophys. Res. Commun. 2011, 410, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.; Kushima, Y.; Muramatsu, K. Effect of sulfur containing amino acid and glycine on plasma cholesterol level in rats fed on a high cholesterol diet. Arg. Biol. Chem. Tokyo 1985, 49, 3455–3461. [Google Scholar]
- Hwang, E.; Kang, B.; Kim, B.; Lee, H.J. Protein quality evaluation and effect of plasma contents of acid hydrolysates of cocoon in rats fed by high cholesterol, high triglyceride, and high sucrose diet. J. Korean Soc. Food Sci. Nutr. 2011, 30, 1004–1009. [Google Scholar]
- Lee, S.H.; Cho, H.N.; Hyun, C.K.; Jew, S.S. Physiology functional characteristic of silk peptide. Food Sci. Ind. 2002, 35, 57–62. [Google Scholar]
- Lee, S.H.; Park, D.; Yang, G.; Bae, D.-K.; Yang, Y.-H.; Kim, T.K.; Kim, D.; Kyung, J.; Yeon, S.; Koo, K.C.; et al. Silk and silkworm pupa peptides suppress adipogenesis in preadipocytes and fat accumulation in rats fed a high-fat diet. Eur. J. Nutr. 2011, 51, 1011–1019. [Google Scholar] [CrossRef]
- Gatesy, J. Extreme Diversity, Conservation, and Convergence of Spider Silk Fibroin Sequences. Science 2001, 291, 2603–2605. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, B.Y.; Je, Y.H.; Woo, S.D.; Sohn, H.D.; Jin, B.R. Molecular cloning and expression of the C-terminus of spider flagelliform silk protein from Araneus ventricosus. J. Biosci. 2007, 32, 705–712. [Google Scholar] [CrossRef]
- Teulé, F.; Miao, Y.-G.; Sohn, B.-H.; Kim, Y.-S.; Hull, J.J.; Fraser, M.J.; Lewis, R.V.; Jarvis, D.L. Silkworms transformed with chimeric silkworm/spider silk genes spin composite silk fibers with improved mechanical properties. Proc. Natl. Acad. Sci. USA 2012, 109, 923–928. [Google Scholar] [CrossRef]
- Zhu, C.; Naqvi, S.; Gómez-Galera, S.; Pelacho, A.M.; Capell, T.; Christou, P. Transgenic strategies for the nutritional enhancement of plants. Trends Plant Sci. 2007, 12, 548–555. [Google Scholar] [CrossRef]
- Ye, X.; Al-Babill, S.; Klöti, A.; Zhang, J.; Lucca, P.; Beyer, P.; Potrykus, I. Engineering the provitamin A (beta-carotene) biosynthetic pathway into (varotenoid-free) rice. Science 2000, 287, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Paine, J.A.; Shipton, C.A.; Chaggar, S.; Howells, R.M.; Kennedy, M.J.; Vernon, G.; Wright, S.Y.; Hinchliffe, E.; Adams, J.L.; Silverstone, A.L.; et al. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat. Biotechnol. 2005, 23, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Jevnikar, A.M. Transgenic rice for allergy immunotherapy. Proc. Natl. Acad. Sci. USA 2005, 102, 17255–17256. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, F.; Hino, S.; Okajima, T.; Nadano, D.; Aoki, N.; Matsuda, T. Suppressive expression of the glutelin A1-collagen peptide fusion gene in hybrid rice lines with the mutant line, Low Glutelin Content-1 (LGC-1). Plant Biotechnol. 2008, 25, 465–471. [Google Scholar] [CrossRef]
- Xie, T.; Qiu, Q.; Zhang, W.; Ning, T.; Yang, W.; Zheng, C.; Wang, C.; Zhu, Y.; Yang, D. A biologically active rhIGF-1 fusion accumulated in transgenic rice seeds can reduce blood glucose in diabetic mice via oral delivery. Peptides 2008, 29, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Akama, K.; Kanetou, J.; Shimosaki, S.; Kawakami, K.; Tsuchikura, S.; Takaiwa, F. Seed-specific expression of truncated OsGAD2 produces GABA-enriched rice grains that influence a decrease in blood pressure in spontaneously hypertensive rats. Transgenic Res. 2009, 18, 865–876. [Google Scholar] [CrossRef] [PubMed]
- González-Ortiz, M.; Medina-Santillán, R.; Martínez-Abundis, E.; Von Drateln, C.R. Effect of glycine on insulin secretion and action in healthy first-degree relatives of type 2 diabetes mellitus patients. Horm. Metab. Res. 2001, 33, 358–360. [Google Scholar] [CrossRef]
- Gannon, M.C.; Nuttall, J.A.; Nuttall, F.Q. The metabolic response to ingested glycine1–3. Am. J. Clin. Nutr. 2002, 76, 1302–1307. [Google Scholar] [CrossRef]
- Alvarado-Vásquez, N.; Zamudio, P.; Cerón, E.; Vanda, B.; Zenteno, E.; Carvajal-Sandoval, G. Effect of glycine in streptozotocin-induced diabetic rats. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 134, 521–527. [Google Scholar] [CrossRef]
- Anuradha, C.V. Amino acid support in the prevention of diabetes and diabetic complications. Curr. Protein Pept. Sci. 2009, 10, 8–17. [Google Scholar] [CrossRef]
- Toki, S. Rapid and efficientAgrobacterium-mediated transformation in rice. Plant Mol. Biol. Rep. 1997, 15, 16–21. [Google Scholar] [CrossRef]
- Mohanty, A.; Sarma, N.; Tyagi, A.K. Agrobacterium-mediated high frequency transformation of an elite indica rice variety Pusa Basmati 1 and transmission of the transgenes to R2 progeny. Plant Sci. 1999, 147, 127–137. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, X.; Schmitt, K.; Hubert, R.; Navidi, W.; Arnheim, N. Whole genome amplification from a single cell: Implications for genetic analysis. Proc. Natl. Acad. Sci. USA 1992, 89, 5847–5851. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.T.; Lee, K.S.; Hur, Y.J.; Kim, B.Y.; Li, J.; Yu, S.; Jin, B.R.; Kim, D.H. Spider Silk Fibroin Protein Heterologously Produced in Rice Seeds Reduce Diabetes and Hypercholesterolemia in Mice. Plants 2020, 9, 1282. https://doi.org/10.3390/plants9101282
Yang WT, Lee KS, Hur YJ, Kim BY, Li J, Yu S, Jin BR, Kim DH. Spider Silk Fibroin Protein Heterologously Produced in Rice Seeds Reduce Diabetes and Hypercholesterolemia in Mice. Plants. 2020; 9(10):1282. https://doi.org/10.3390/plants9101282
Chicago/Turabian StyleYang, Won Tae, Kwang Sik Lee, Yeon Jae Hur, Bo Yeon Kim, Jianhong Li, Sibin Yu, Byung Rae Jin, and Doh Hoon Kim. 2020. "Spider Silk Fibroin Protein Heterologously Produced in Rice Seeds Reduce Diabetes and Hypercholesterolemia in Mice" Plants 9, no. 10: 1282. https://doi.org/10.3390/plants9101282
APA StyleYang, W. T., Lee, K. S., Hur, Y. J., Kim, B. Y., Li, J., Yu, S., Jin, B. R., & Kim, D. H. (2020). Spider Silk Fibroin Protein Heterologously Produced in Rice Seeds Reduce Diabetes and Hypercholesterolemia in Mice. Plants, 9(10), 1282. https://doi.org/10.3390/plants9101282





