Reaching Natural Growth: Light Quality Effects on Plant Performance in Indoor Growth Facilities
Abstract
1. Introduction
2. Results
2.1. Light Treatments
2.2. Plant Growth and Biomass Allocation
2.3. Leaf Pigmentation
2.4. Photosynthesis and Leaf Respiration
2.5. Principal Component Analysis (PCA)
3. Discussion
3.1. Light Quality Effects on Morphology
3.2. Light Quality Effect on Leaf Pigmentation
3.3. Light Quality Effects on Photosynthesis
3.4. Principal Component Analysis
4. Materials and Methods
4.1. Plant Material and Pre-Growing Conditions
4.2. Control and Light Quality Treatments
4.3. Climatic Growth Conditions
4.4. Morphological Parameters
4.5. Chlorophyll Fluorescence and Chlorophyll Content
4.6. Leaf Gas Exchange
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Junker, A.; Muraya, M.; Weigelt-Fischer, K.; Arana-Ceballos, F.; Klukas, C.; Melchinger, A.; Meyer, R.; Riewe, D.; Altmann, T. Optimizing experimental procedures for quantitative evaluation of crop plant performance in high throughput phenotyping systems. Front. Plant Sci. 2015, 5, 770. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, M.; Stahl, A.; Rudloff, J.; Wittkop, B.; Showdon, R. Not a load of rubbish: Simulated field trials in large-scale containers. Plantcell Environ. 2016, 39, 2064–2073. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Fiorani, F.; Pieruschka, R.; Wojciechowski, T.; van der Putten, W.; Kleyer, M.; Schurr, U.; Postma, J. Pampered inside, pestered outside? Differences and similarities between plant growing in controlled conditions and in the field. New Phytol. 2016, 213, 383–855. [Google Scholar] [CrossRef] [PubMed]
- Thiel, S.; Döhring, T.; Köfferlein, M.; Kosak, A.; Peter, M.; Seidlitz, H. A phytotron for plant stress research: How far can artificial lighting compare to natural sunlight? J. Plant Physiol. 1995, 148, 456–463. [Google Scholar] [CrossRef]
- Fujiwara, K.; Sawada, T. Design and development of an LED-Artificial sunlight source system prototype capable of controlling relative spectral power distribution. J. Light Vis. Environ. 2006, 30, 170–176. [Google Scholar] [CrossRef]
- Cocetta, G.; Casciani, D.; Bulgari, R.; Musante, F.; Kolton, A.; Rossi, M.; Ferrante, A. Light use efficiency for vegetables production in protected and indoor environments. Eur. Phys. J. Plus 2017, 132, 43. [Google Scholar] [CrossRef]
- Miao, Y.; Wang, X.; Gao, L.; Chen, Q.; QU, M. Blue light is more essential than red light for maintaining the activities of photosystem II and I and photosystem electron transport capacity in cucumber leaves. J. Integr. Agric. 2016, 15, 87–100. [Google Scholar] [CrossRef]
- Hogewoning, S.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Furuyama, S.; Ishigami, Y.; Hikosaka, S.; Goto, E. Effects of blue/ red ratio and light intensity on photomorphogenesis and photosynthesis of red leaf lettuce. Acta Hortic. 2014, 1037, 317–322. [Google Scholar] [CrossRef]
- Hernandez, R.; Eguchi, T.; Deveci, M.; Kubota, C. Tomato seedlings physiological respones under different percentages of blue and red photon flux ratios using LEDs and cool white fluorescent lamps. Sci. Hortic. 2016, 213, 270–280. [Google Scholar] [CrossRef]
- Piovene, C.; Orsini, F.; Bosi, S.; Sanoubar, R.; Bregola, V.; Dinelli, G.; Gianquinto, G. Optimal red:blue ratio in led lighting for nutraceutical indoor horticulture. Sci. Hortic. 2015, 193, 202–208. [Google Scholar] [CrossRef]
- Shengxin, C.; Chunxia, L.; Xuyang, Y.; Song, C.; Xuelei, J.; Xiaoyin, L.; Zhigang, X.; Rongzhan, G. Morphological, photosynthetic, and physiological responses of rapeseed leaf to different combinations of red and blue lights at the rosette stage. Front. Plant Sci. 2016, 7, 1144. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.; Leal-Costa, M.; Tavares, E.; Lage, C.; Esquibel, M. The effect of light quality on leaf production and development of in vitro-cultured plants of Alternanthera brasiliana Kuntze. Environ. Exp. Bot. 2011, 70, 43–50. [Google Scholar] [CrossRef]
- Hernandez, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Kim, J.; Glick, R.; Melis, A. Dynamic of photosystem stoichiometry adjustment by light quality in chloroplasts. Plant Physiol. 1993, 102, 181–190. [Google Scholar] [CrossRef]
- Clavijo-Herrera, J.; van Santen, E.; Gómez, C. Growth, Water-use efficiency, stomatal conductance, and nitrogen uptake of two lettuce cultivars grown under different percentages of blue and red light. Horticulturae 2019, 4, 16. [Google Scholar] [CrossRef]
- Pennisi, G.; Sanyé-Mengual, E.; Orisini, F.; Crepaldi, A.; Nicola, S.; Ochoa, J.; Fernandez, J.; Gianquinto, G. Modelling environmental burdens of indoor-grown vegetables and herbs as affected by red and blue LED lighting. Sustainability 2019, 11, 4063. [Google Scholar] [CrossRef]
- Schuerger, A.; Brown, C.; Stryjewski, E. Anatomical features of pepper plants (Capsicum annuum L.) Grown under red light-emitting diodes supplemented with blue or far-red light. Ann. Bot. 1997, 79, 273–282. [Google Scholar] [CrossRef]
- Bae, G.; Choi, G. Decoding of lights signals by plants phytochromes and their interacting proteins. Annu. Rev. Plant Biol. 2008, 59, 281–311. [Google Scholar] [CrossRef]
- Zhen, S.; van Iersel, M. Far-red light needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef]
- Hogewoning, S.; Trouwborst, G.; Meinen, E.; van Ieperen, W. Finding the optimal growth-light spectrum for greenhouse crops. Acta Hortic. 2012, 956, 357–363. [Google Scholar] [CrossRef]
- Dougher, T.; Bugbee, B. Differences in the response of Wheat, Soybean and Lettuce to reduced Blue radiation. Photochem. Photobiol. 2001, 73, 199–207. [Google Scholar] [CrossRef]
- Hogewoning, S.; Douwstra, P.; Trouwborst, G.; van Ieperen, W.; Harbison, J. An artificial solar spectrum substantially alters plants development compared with usual climate room irradiance spectra. J. Exp. Bot. 2010, 61, 1267–1276. [Google Scholar] [CrossRef]
- Terfa, M.; Solhaug, K.; Gislerød, H.; Olsem, J.; Torre, S. A high proportion of blue light increases the photosynthesis capacity and leaf formation rate of Rosa x hybrida but does not affect time to flower opening. Physiol. Plant. 2013, 148, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.; Terfa, M.; Olsen, J.; Torre, S. Red and blue light effects on morphology and flowering of Petunia x hybrida. Sci. Hortic. 2015, 184, 171–178. [Google Scholar] [CrossRef]
- Lin, C.; Yang, H.; Guo, H.; Modckler, T.; Chen, J.; Cashmore, A. Enhancement of blue-light sensitivity of Arabidopsis seedlings by a blue light receptor cryptochrome 2. Proc. Natl. Acad. Sci. USA 1998, 95, 2686–2690. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Van Labeke, M. Long-term effects of Red- and Blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front. Plant Sci. 2017, 8, 917. [Google Scholar] [CrossRef]
- Yorio, N.; Goins, G.; Kagie, H. Improving spinach, radish and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. Hortscience 2001, 36, 380–383. [Google Scholar] [CrossRef]
- Nhut, D.; Takamura, T.; Watanabe, H.; Okamoto, K.; Tanaka, M. Responses of strawberry plantlets in vitro under super bright red and blue light-emitting (LEDs). Plant Celltissue Organ Cult. 2003, 73, 43–52. [Google Scholar] [CrossRef]
- Barnes, C.; Bugbee, B. Morphological responses of wheat to blue light. J. Plant Physiol. 1992, 139, 339–342. [Google Scholar] [CrossRef]
- Dougher, T.; Bugbee, B. Is blue light good or bad for plants? Life Support Biosph. Sci. 1998, 5, 129–136. [Google Scholar] [PubMed]
- Vialet-Chabrand, S.; Matthews, J.; Simkin, A.; Raines, C.; Lawson, T. Importance of fluctuations in light on plants photosynthetic acclimation. Plant Physiol. 2017, 173, 2163–2179. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, R.; Ohashi-kaneko, K.; Fujiwara, K.; Goto, E.; Kurata, K. Photosynthesis characteristics of rice leaves grown under red light with or without supplemental blue light. Plant Cell Physiol. 2004, 45, 1870–1874. [Google Scholar] [CrossRef]
- Hogewoning, S.; Trouwborst, G.; Engbers, G.; Harbison, J.; van Ieperen, W.; Ruijsch, J.; Schapendok, A.; Pot, C.; van Kooten, O. Plant physiological acclimation to irradiation by light-emitting diodes (LEDs). Acta Hortic. 2007, 761, 183–191. [Google Scholar] [CrossRef]
- Matsuda, R.; Ohashi-kaneko, K.; Fujiwara, K.; Kurata, K. Analysis of the relationship between blue-light photon flux density and the photosynthetic properties of spinach (Spinacia oleracea L.) leaves with regard to the acclimation of photosynthesis to growth irradiance. Soil Sci. Plant Nutr. 2007, 53, 459–465. [Google Scholar] [CrossRef]
- Chiang, C.; Olsen, J.; Basler, D.; Bånkestad, D.; Hoch, G. Latitude and weather influences on sun light quality and the relationship to tree growth. Forest 2019, 10, 610. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic quesion of why the leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Pfannschmidt, T. Acclimation to varying light qualities: Toward the functional relationship of state transitions and adjustment of photosystem stoichiometry. J. Phycol. 2005, 41, 723–725. [Google Scholar] [CrossRef]
- Chow, W.; Melis, A.; Anderson, J. Adjustments of photo-system stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proc. Natl. Acad. Sci. USA 1990, 87, 7502–7506. [Google Scholar] [CrossRef]
- Atkin, O.; Evans, J.; Siebke, K. Relationship between the inhibition of leaf respiration by light and enhancement of leaf dark respiration following light treatment. Aust. J. Plant Physiol. 1998, 25, 437–443. [Google Scholar] [CrossRef]
- Annunziata, M.; Alpelt, F.; Carillo, P.; Krause, U.; Feil, R.; Mengin, V.; Lauxmann, M.; Nikoloski, Z.; Stitt, M.; Lunn, J. Getting back to nature: A reality check for experiments in controlled environments. J. Exp. Bot. 2017, 68, 4463–4477. [Google Scholar] [CrossRef] [PubMed]
- Sager, J.; Smith, W.; Edwards, J.; Cyr, K. Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. Am. Soc. Agric. Eng. 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor and Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Wellburn, A. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Lobo, F.; Barros, M.; Dalmagro, H.; Dalmolin, A.; Pereira, W.; Souza, E.; Vourlitis, G.; Rodriguez, C. Fitting net photosynthetic light-response curves with Microsoft Excel- a critical look at the models. Photosynthetica 2013, 51, 445–456. [Google Scholar] [CrossRef]
- Eilers, P.; Peeters, J. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol. Model. 1988, 42, 199–215. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
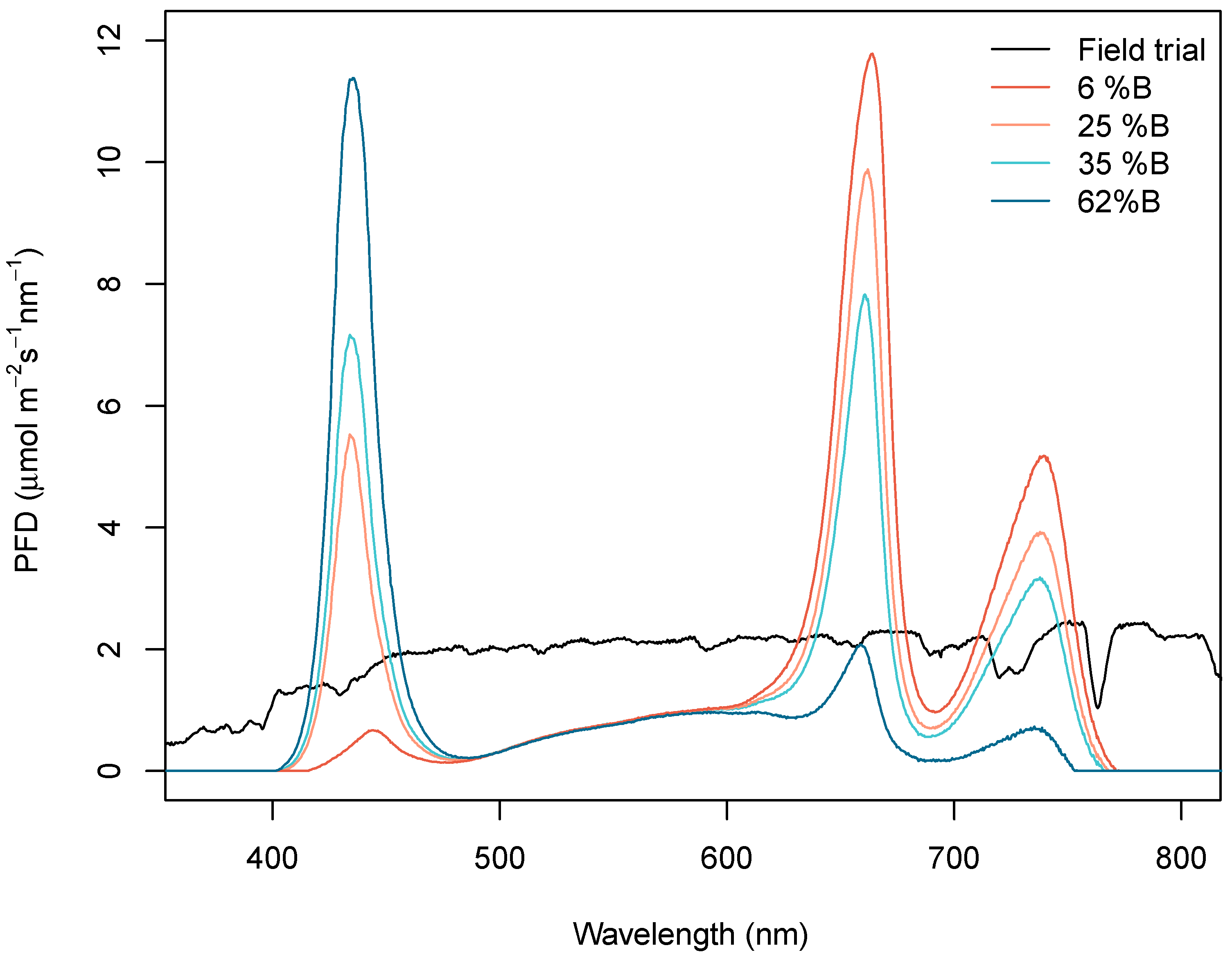
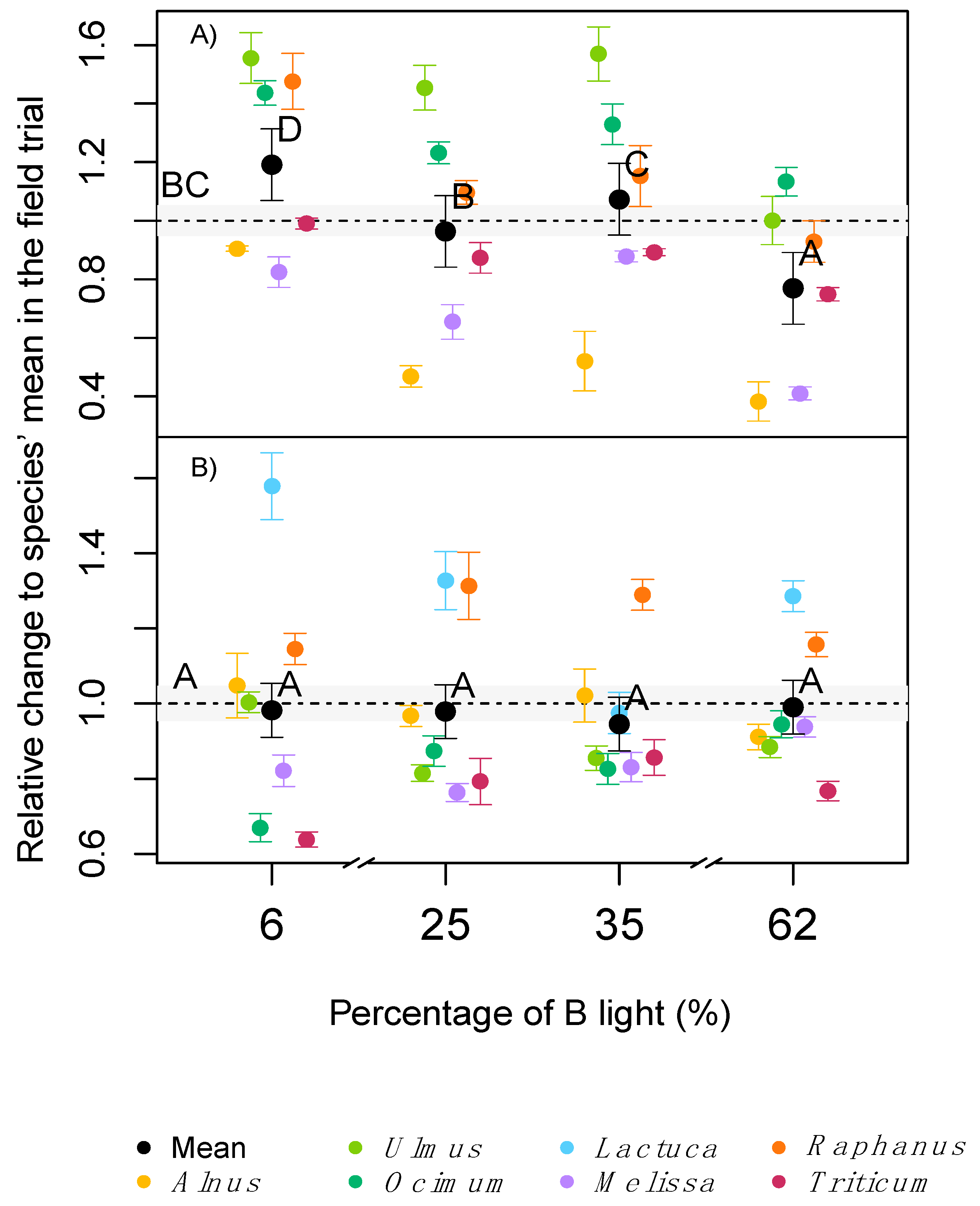



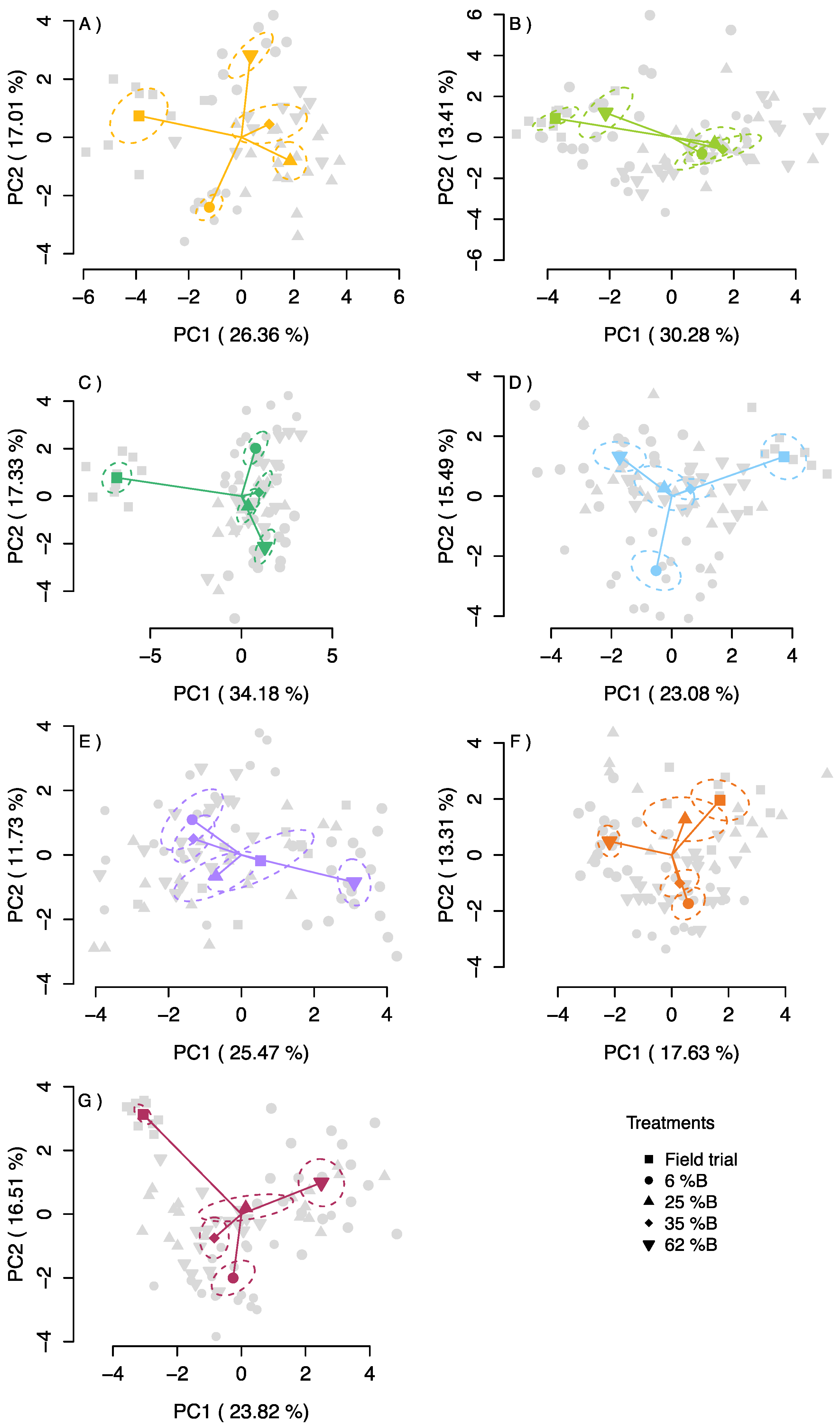
| Treatment\Characteristic | Field Trial | 6% B | 25% B | 35% B | 62% B |
|---|---|---|---|---|---|
| Blue (%) | 28 | 6 | 25 | 35 | 62 |
| Green (%) | 36 | 16 | 16 | 16 | 16 |
| Red (%) | 36 | 78 | 59 | 49 | 22 |
| R:FR ratio | 1.1 | 1.8 | 1.8 | 1.8 | 1.8 |
| Type of Factor | Fix Factors | Random Factors | ||
|---|---|---|---|---|
| Factor | Light Quality | Species | Light Quality × Specie | Replicate |
| Variable | ||||
| Biomass and Morphology | ||||
| Height * | <2.2 × 10−16 | <2.2 × 10−16 | <2.2 × 10−16 | 5 × 10−4 |
| Dry weight leaves | 1.16 × 10−5 | <2.2 × 10−16 | <2.2 × 10−16 | 1.5 × 10−3 |
| Dry weight shoot ** | 1.03 × 10−8 | <2.2 × 10−16 | <2.37 × 10−14 | - |
| Dry weight roots | 1.26 × 10−5 | <2.2 × 10−16 | <2.2 × 10−16 | <2.2 × 10−16 |
| Total dry weight | 8.74 × 10−5 | <2.2 × 10−16 | <2.2 × 10−16 | <2.2 × 10−16 |
| Root to Shoot ratio | 1.39 × 10−11 | <2.2 × 10−16 | <2.2 × 10−16 | <2.2 × 10−16 |
| SLA | 0.1024 | <2.2 × 10−16 | <2.2 × 10−16 | 7.9 × 10−3 |
| Chlorophyll | ||||
| Chlorophyll a (mg g−1) | 4.90 × 10−7 | <2.2 × 10−16 | 3.47 × 10−14 | <2.2 × 10−16 |
| Chlorophyll b (mg g−1) | <2.2 × 10−16 | <2.2 × 10−16 | <2.2 × 10−16 | 5.62 × 10−14 |
| Chl a:b ratio ** | 1.85 × 10−5 | <2.2 × 10−16 | 5.98 × 10−6 | - |
| Carotenoids (mg g−1) | 1.49 × 10−13 | <2.2 × 10−16 | 2.78 × 10−13 | <2.2 × 10−16 |
| Fv/Fm ** | 2.53 × 10−8 | <2.2 × 10−16 | 0.003297 | − |
| Standardised light | ||||
| Max photosynthesis ** | 0.03074 | 4.42 × 10−5 | 3.09 × 10−8 | - |
| Quantum yield ** | 2.44 × 10−6 | 1.94 × 10−12 | - | - |
| Dark respiration ** | 0.4026571 | 9.16 × 10−12 | 6.89 × 10−5 | − |
| Compensation point | 0.008619 | <2.2 × 10−16 | 5.48 × 10−11 | <2.2 × 10−16 |
| In−situ’ light | ||||
| Max photosynthesis ** | 6.52 × 10−6 | 1.25 × 10−12 | - | - |
| Quantum yield ** | 6.45 × 10−6 | 1.93 × 10−7 | - | - |
| Dark respiration ** | - | 4.06 × 10−6 | - | - |
| Compensation point | 0.3041 | 4.19 × 10−16 | 1.74 × 10−5 | <2.2 × 10−16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, C.; Bånkestad, D.; Hoch, G. Reaching Natural Growth: Light Quality Effects on Plant Performance in Indoor Growth Facilities. Plants 2020, 9, 1273. https://doi.org/10.3390/plants9101273
Chiang C, Bånkestad D, Hoch G. Reaching Natural Growth: Light Quality Effects on Plant Performance in Indoor Growth Facilities. Plants. 2020; 9(10):1273. https://doi.org/10.3390/plants9101273
Chicago/Turabian StyleChiang, Camilo, Daniel Bånkestad, and Günter Hoch. 2020. "Reaching Natural Growth: Light Quality Effects on Plant Performance in Indoor Growth Facilities" Plants 9, no. 10: 1273. https://doi.org/10.3390/plants9101273
APA StyleChiang, C., Bånkestad, D., & Hoch, G. (2020). Reaching Natural Growth: Light Quality Effects on Plant Performance in Indoor Growth Facilities. Plants, 9(10), 1273. https://doi.org/10.3390/plants9101273






