Leaf Age and Position Effects on Quantum Yield and Photosynthetic Capacity in Hemp Crowns
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Leaf Gas Exchange and Light Absorption Measurements
4.2. Photosynthetically Active Radiation Measurements
4.3. An/Ci Curve Fitting
4.4. An/Qp Curve Fitting
4.5. Parameterizations of Leaf Photosynthetic Longevity
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burgess, A.J.; Retkute, R.; Herman, T.; Murchie, E.H. Exploring relationships between canopy architecture, light distribution, and photosynthesis in contrasting rice genotypes using 3D canopy reconstruction. Front. Plant Sci. 2017, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Gspaltl, M.; Bauerle, W.L.; Binkley, D.; Sterba, H. Leaf area and light use efficiency patterns of Norway spruce under different thinning regimes and age classes. For. Ecol. Manag. 2013, 288, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Bielczynski, L.W.; Lacki, M.K.; Hoefnagels, I.; Gambin, A.; Crocea, R. Leaf and plant age affects photosynthetic performance and photoprotective capacity. Plant Physiol. 2017, 175, 1634–1648. [Google Scholar] [CrossRef]
- Hikosaka, K.; Terashima, I.; Katoh, S. Effects of leaf age, nitrogen nutrition and photon flux density on the distribution of nitrogen among leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Oecologia 1994, 97, 451–457. [Google Scholar] [CrossRef]
- Wright, I.J.; Michell, A.C.; Leishman, R.; Cassia, A.; Read, A.B.; Westoby, M. Gradients of light availability and leaf traits with leaf age and canopy position in 28 Australian shrubs and trees. Funct. Plant Biol. 2006, 33, 407–419. [Google Scholar] [CrossRef]
- Han, Q.; Kawasaki, T.; Nakano, T.; Chiba, Y. Leaf-age effects on seasonal variability in photosynthetic parameters and its relationships with leaf mass per area and leaf nitrogen concentration within a Pinus densiflora crown. Tree Physiol. 2008, 28, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, A.; Mulkey, S.S.; Samaniego, M.; Wright, S.J. Decline of photosynthetic capacity with leaf age and position in two tropical pioneer tree species. Am. J. Bot. 2002, 89, 1925–1932. [Google Scholar] [CrossRef]
- Werner, C.; Ryel, R.J.; Correia, O.; Beyschlag, W. Effects of photoinhibition on whole-plant carbon gain assessed with a photosynthesis model. Plant Cell Environ. 2001, 24, 27–40. [Google Scholar] [CrossRef]
- Whitewoods, C.D.; Coen, E. Growth and development of three-dimensional plant form. Curr. Biol. 2017, 27, R910–R918. [Google Scholar] [CrossRef]
- Ehleringer, J.; Björkman, O. Quantum yields for CO2 uptake in C3 and C4 plants. Plant Physiol. 1977, 59, 86–90. [Google Scholar] [CrossRef]
- Osborne, B.; Garrett, M. Quantum yields for CO2 uptake in some diploid and tetraploid plant species. Plant Cell Environ. 1983, 6, 135–144. [Google Scholar] [CrossRef]
- Long, S.P.; Postl, W.F.; Bolhár Nordenkampf, H.R. Quantum yields for uptake of carbon dioxide in C3 vascular plants of contrasting habitats and taxonomic groupings. Planta 1993, 189, 226–234. [Google Scholar] [CrossRef]
- Long, S.P.; Humphries, S.; Falkowski, P.G. Photoinhibition of photosynthesis in nature. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1994, 45, 633–662. [Google Scholar] [CrossRef]
- Long, S.P.; Zhu, X.G.; Naidu, S.I.; Ort, D.R. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 2006, 29, 315–330. [Google Scholar] [CrossRef]
- Evans, J.R. Nitrogen and photosynthesis in the flag leaf of wheat (Triticum aestivum L.). Plant Physiol. 1983, 72, 297–302. [Google Scholar] [CrossRef]
- Osmond, C.B.; Förster, B. Photoinhibition: Then and now. In Photoprotection, Photoinhibition, Gene Regulation and Environment—Advances in Photosynthesis and Respiration; Demmig-Adams, B., Adams, W.W., Mattoo, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 21, pp. 11–22. [Google Scholar] [CrossRef]
- Raven, J.A. Flight or flight: The economics of repair and avoidance of photoinhibition of photosynthesis. Funct. Ecol. 1989, 3, 5–19. [Google Scholar] [CrossRef]
- Kitajima, A.; Mulkey, S.S.; Wright, S.J. Decline of photosynthetic capacity with leaf age in relation to leaf longevities for five tropical canopy tree species. Am. J. Bot. 1997, 85, 702–708. [Google Scholar] [CrossRef]
- Wilson, K.B.; Baldocchi, D.D.; Hanson, P.J. Leaf age affects the seasonal pattern of photosynthetic capacity and net ecosystem exchange of carbon in a deciduous forest. Plant Cell Environ. 2001, 24, 571–583. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Cescatti, A.; Rodeghiero, M.; Tosens, T. Complex adjustments of photosynthetic potentials and internal diffusion conductance to current and previous light availabilities and leaf age in Mediterranean evergreen species. Plant Cell Environ. 2006, 29, 1159–1178. [Google Scholar] [CrossRef]
- Warren, C. Why does photosynthesis decrease with needle age in Pinus pinaster? Trees 2006, 20, 157–164. [Google Scholar] [CrossRef]
- Bondada, B.R.; Oosterhuis, D.M. Decline in photosynthesis as related to alterations in chloroplast ultrastructure of a cotton leaf during ontogeny. Photosynthetica 1998, 35, 467–471. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, G.; Zhu, X.-G. Optimal crop canopy architecture to maximize canopy photosynthetic CO2 uptake under elevated CO2—A theoretical study using a mechanistic model of canopy photosynthesis. Funct. Plant Biol. 2013, 40, 109–124. [Google Scholar] [CrossRef]
- Miner, G.S.; Bauerle, W.L. Seasonal responses of photosynthetic parameters in maize and sunflower and their relationship with leaf functional traits. Plant Cell Environ. 2019, 42, 1561–1574. [Google Scholar] [CrossRef]
- Parry, M.A.J.; Reynolds, M.; Salvucci, M.E.; Raines, C.; Andralojc, P.J.; Zhu, X.G.; Rice, G.D.; Condon, A.G.; Furbank, R.T. Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J. Exp. Bot. 2011, 62, 453–467. [Google Scholar] [CrossRef]
- Flexas, J.; Carriquí, M. Photosynthesis and photosynthetic efficiencies along the terrestrial plant’s phylogeny: Lessons for improving crop photosynthesis. Plant J. 2019. [Google Scholar] [CrossRef]
- Witkowski, E.T.F.; Lamont, B.B.; Walton, C.S.; Radford, S. Leaf demography, sclerophylla and ecophysiology of two Banksias with contrasting leaf life spans. Aust. J. Bot. 1992, 40, 849–862. [Google Scholar] [CrossRef]
- Ackerly, D.D.; Bazzaz, F.A. Leaf dynamics, self-shading and carbon gain in seedlings of a tropical pioneer tree. Oecologia 1995, 101, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Bauerle, W.L.; Weston, D.J.; Bowden, J.D.; Dudley, J.B.; Toler, J.E. Leaf absorptance of photosynthetically active radiation in relation to chlorophyll meter estimates among woody plant species. Sci. Hort. 2004, 101, 169–178. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I. Visible and near-infrared reflectance techniques for diagnosing plant physiological status. Trends Plant Sci. 1998, 3, 151–156. [Google Scholar] [CrossRef]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Bauerle, W.L.; Oren, R.; Way, D.A.; Qian, S.S.; Stoy, P.C.; Thornton, P.E.; Bowden, J.D.; Hoffman, F.M.; Reynolds, R.F. Photoperiodic regulation of the seasonal pattern of photosynthetic capacity and the implications for carbon cycling. Proc. Nat. Acad. Sci. USA 2012, 22, 8612–8617. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Duursma, R.A. Plantecophys—An R package for analyzing and modelling leaf gas exchange data. PLoS ONE 2015, 10, e0143346. [Google Scholar] [CrossRef] [PubMed]
- Wullschleger, S.D. Biochemical limitations of carbon assimilation in C3 plants—A retrospective analysis of the A/Ci curves of 109 species. J. Exp. Bot. 1993, 44, 907–920. [Google Scholar] [CrossRef]
- Jordan, D.B.; Ogren, W.L. The CO2/O2 specificity of ribulose 1,5-bisphosphate concentration, pH and temperature. Planta 1984, 161, 308–313. [Google Scholar] [CrossRef]
- Brooks, A.; Farquhar, G.D. Effects of temperature on the CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta 1985, 165, 397–406. [Google Scholar] [CrossRef]
- Parsons, R.; Weyers, J.D.B.; Lawson, T.; Godber, I.M. Rapid and straightforward estimates of photosynthetic characteristics using a portable gas exchange system. Photosynthesis 1997, 34, 265–279. [Google Scholar] [CrossRef]
- Sharp, R.E.; Matthews, M.A.; Boyer, J.S. Kok effect and the quantum yield of photosynthesis: Light partially inhibits dark respiration. Plant Physiol. 1984, 75, 95–101. [Google Scholar] [CrossRef]
- Singsaas, E.L.; Ort, D.R.; DeLucia, E.H. Variation in measured values of photosynthetic quantum yield in ecophysiological studies. Oecologia 2001, 128, 15–23. [Google Scholar] [CrossRef]
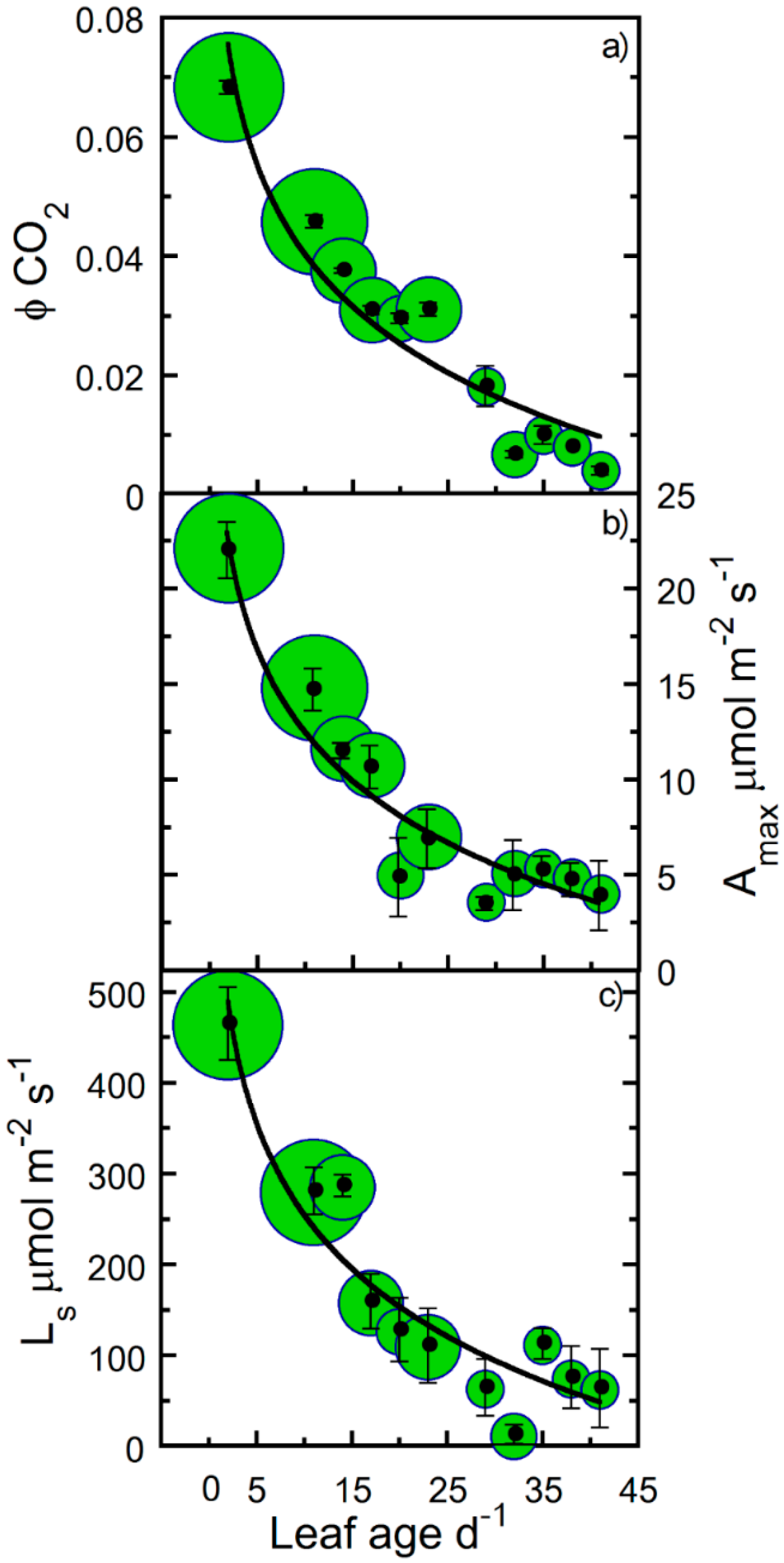
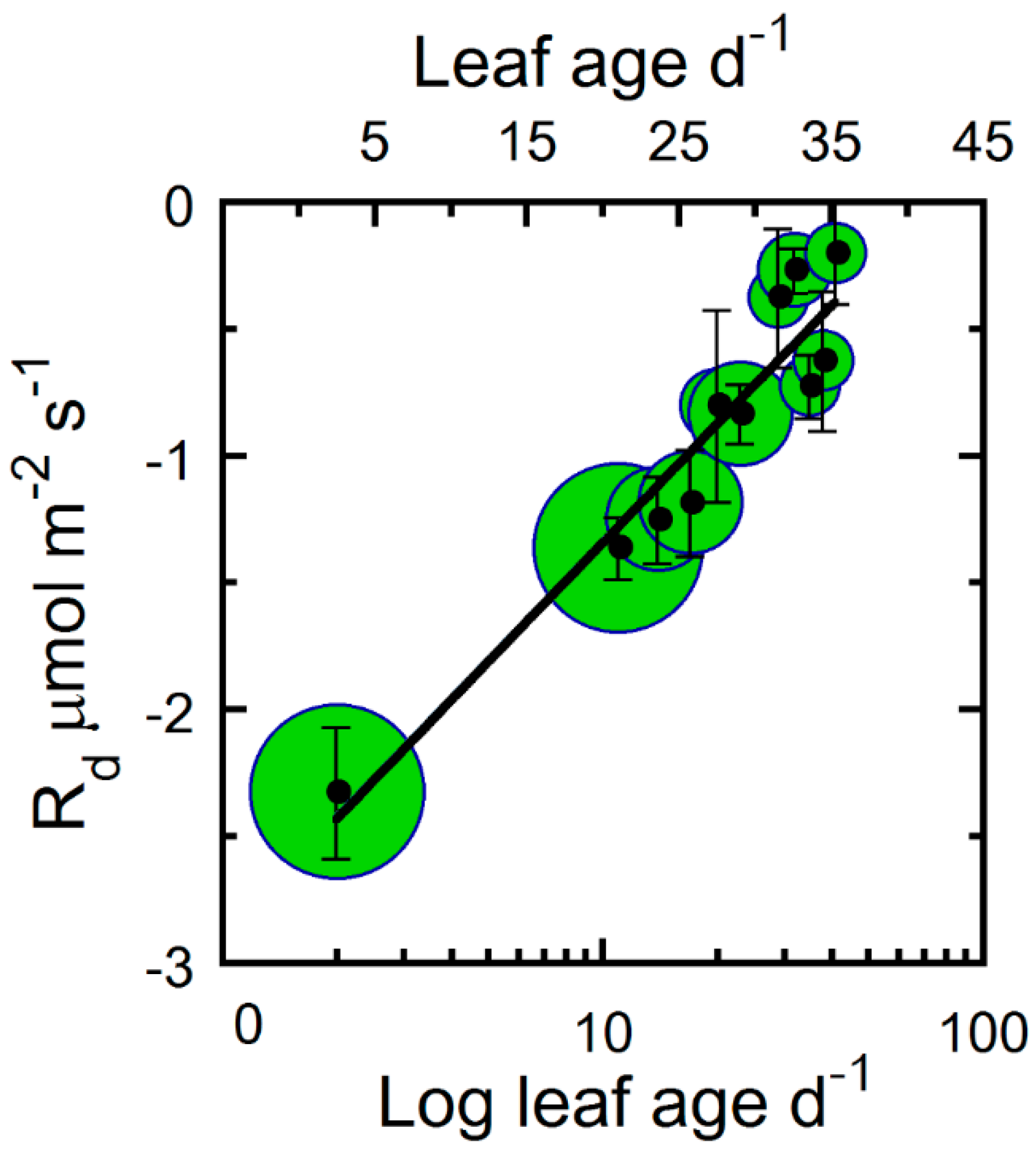
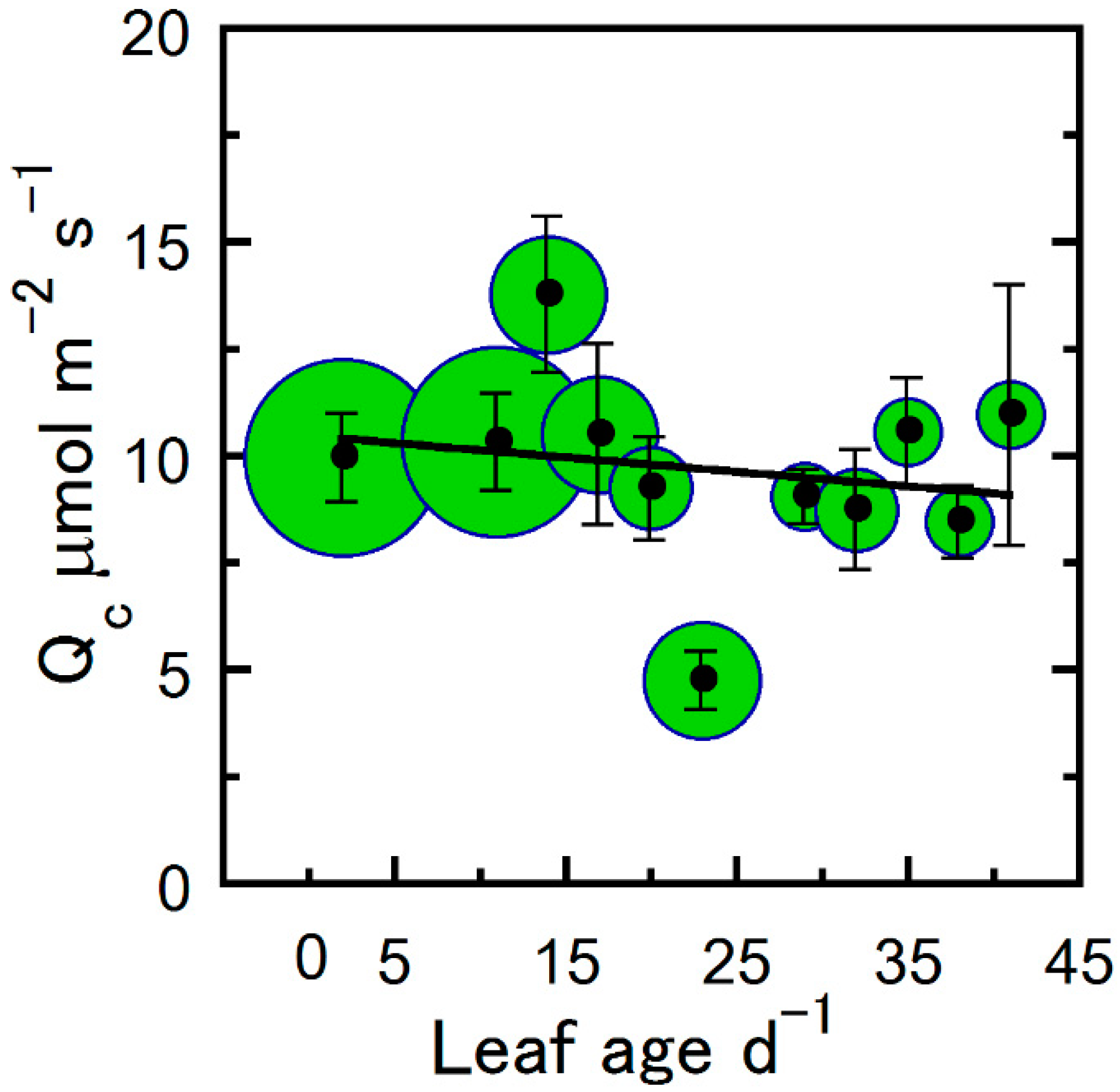
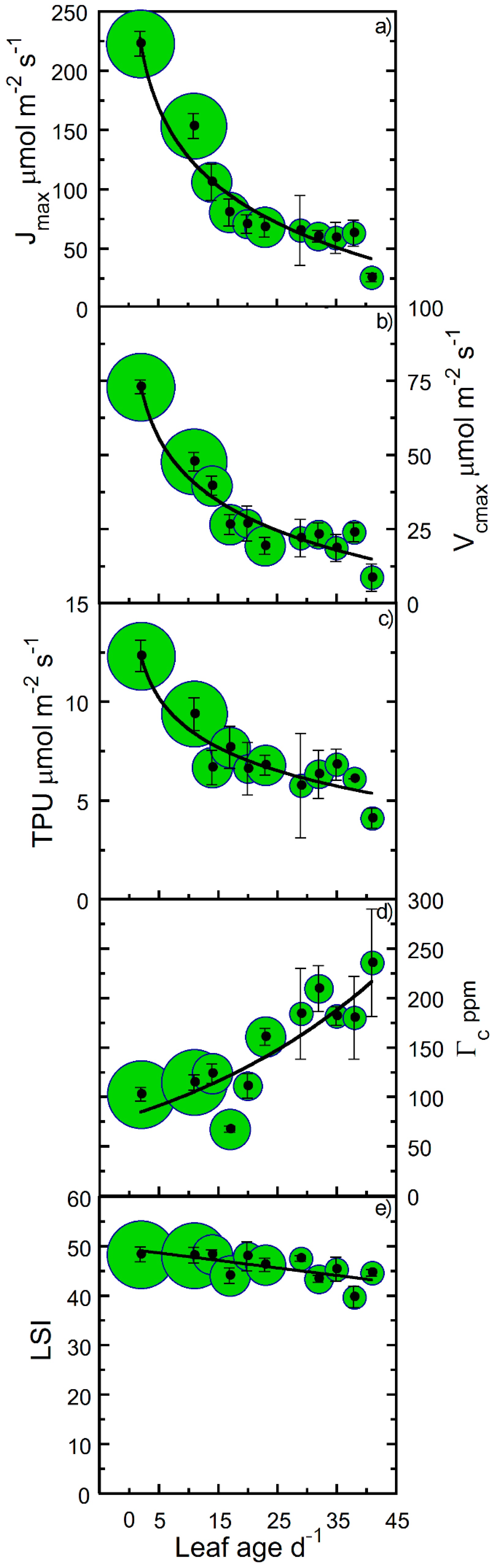
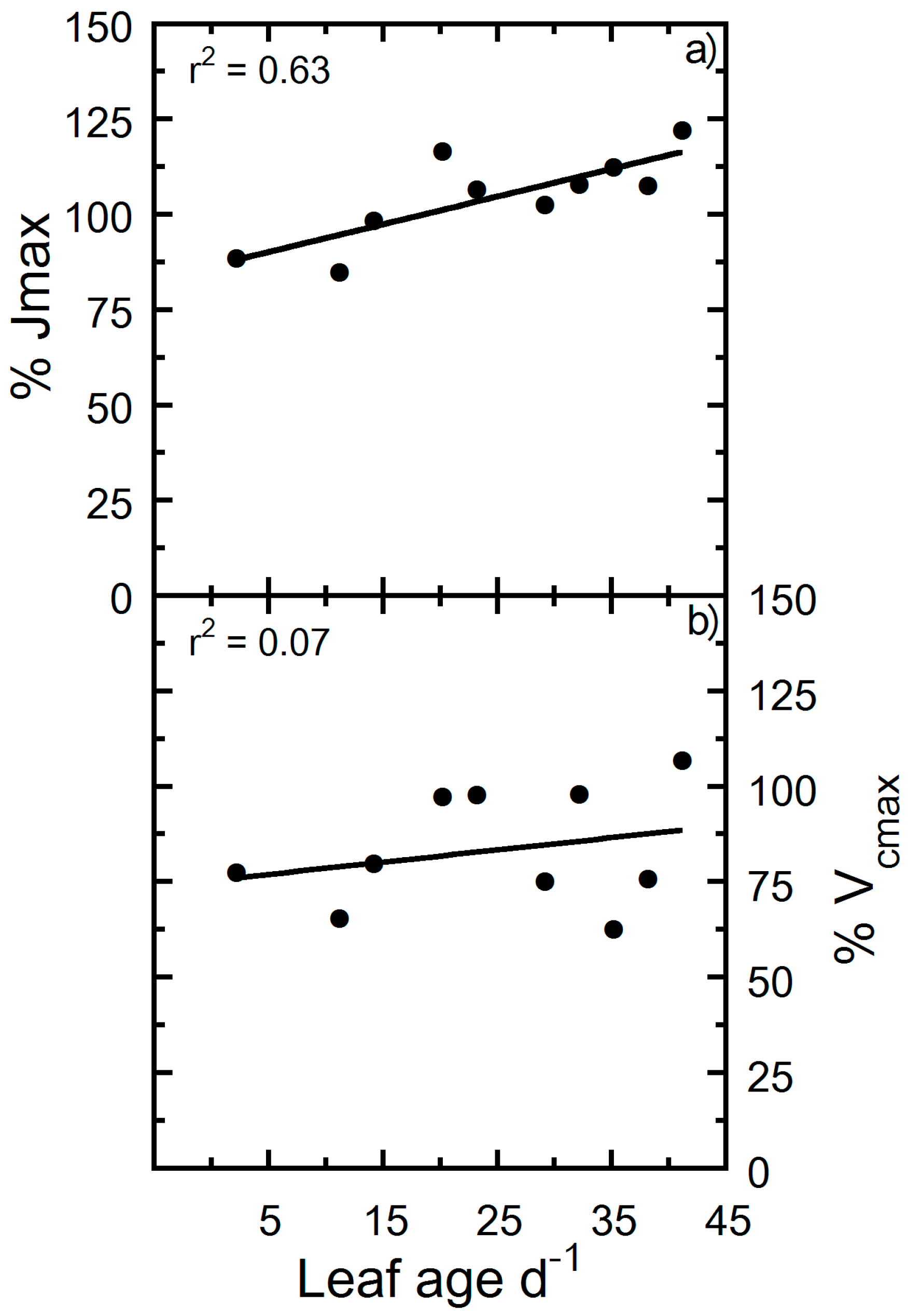
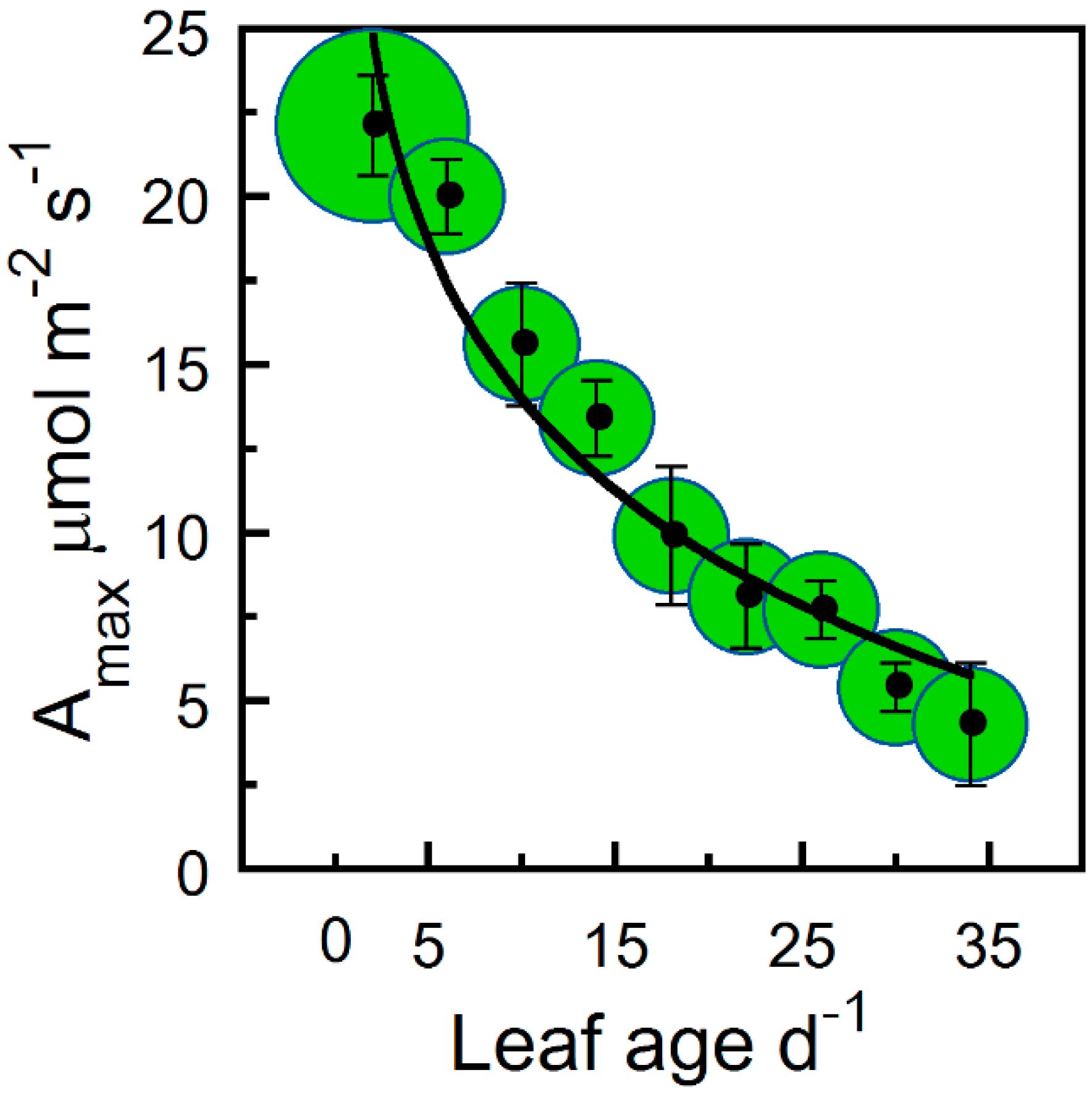
| Correlated Variable | r | p |
|---|---|---|
| Rd vs. Age | 0.95 | <0.00000 |
| Amax vs. Age | 0.96 | <0.00000 |
| Ls vs. Age | 0.95 | <0.00000 |
| ϕCO2 vs. Age | 0.94 | <0.00000 |
| Qc vs. Age | 0.04 | 0.60 |
| LSI vs. Age | 0.57 | 0.07 |
| Jmax vs. Age | 0.96 | <0.00000 |
| Vcmax vs. Age | 0.96 | <0.00000 |
| TPU vs. Age | 0.93 | 0.00003 |
| Γc vs. Age | 0.66 | 0.03 |
| Parameter | (−, +) | 50% | 75% | Equation Type |
|---|---|---|---|---|
| Amax | − | 9 | 25 | † |
| Ls | − | 8 | 20 | † |
| ϕCO2 | − | 8 | 22 | † |
| Rd | − | 9 | 25 | * |
| Qc | − | >100 | >100 | * |
| Jmax | − | 9 | 27 | † |
| Vcmax | − | 9 | 28 | † |
| TPU | − | 21 | 93 | † |
| Γc | + | 19 | 24 | † |
| LSI | − | >100 | >100 | * |
| Parameter | r2 | Slope*ln(x) | +y-Intercept | Equation Type |
|---|---|---|---|---|
| Rd | 0.91 | 0.68 * | −2.898 | * |
| Amax | 0.92 | −6.419 | 27.471 | † |
| Ls | 0.89 | −145.9 | 588.31 | † |
| ϕCO2 | 0.91 | −0.022 | 0.091 | † |
| Qc | 0.04 | −0.456 * | 11.001 | * |
| LSI | 0.32 | −0.152 * | 49.334 | * |
| Jmax | 0.93 | −61.47 | 269.8 | † |
| Vcmax | 0.92 | −19.65 | 87.833 | † |
| TPU | 0.93 | −2.312 | 13.956 | † |
| Γc | 0.63 | 3.546 | 67.292 | † |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauerle, W.L.; McCullough, C.; Iversen, M.; Hazlett, M. Leaf Age and Position Effects on Quantum Yield and Photosynthetic Capacity in Hemp Crowns. Plants 2020, 9, 271. https://doi.org/10.3390/plants9020271
Bauerle WL, McCullough C, Iversen M, Hazlett M. Leaf Age and Position Effects on Quantum Yield and Photosynthetic Capacity in Hemp Crowns. Plants. 2020; 9(2):271. https://doi.org/10.3390/plants9020271
Chicago/Turabian StyleBauerle, William L., Cole McCullough, Megan Iversen, and Michael Hazlett. 2020. "Leaf Age and Position Effects on Quantum Yield and Photosynthetic Capacity in Hemp Crowns" Plants 9, no. 2: 271. https://doi.org/10.3390/plants9020271
APA StyleBauerle, W. L., McCullough, C., Iversen, M., & Hazlett, M. (2020). Leaf Age and Position Effects on Quantum Yield and Photosynthetic Capacity in Hemp Crowns. Plants, 9(2), 271. https://doi.org/10.3390/plants9020271





