A Review of Plant Vacuoles: Formation, Located Proteins, and Functions
Abstract
1. Discovery History of the Vacuoles
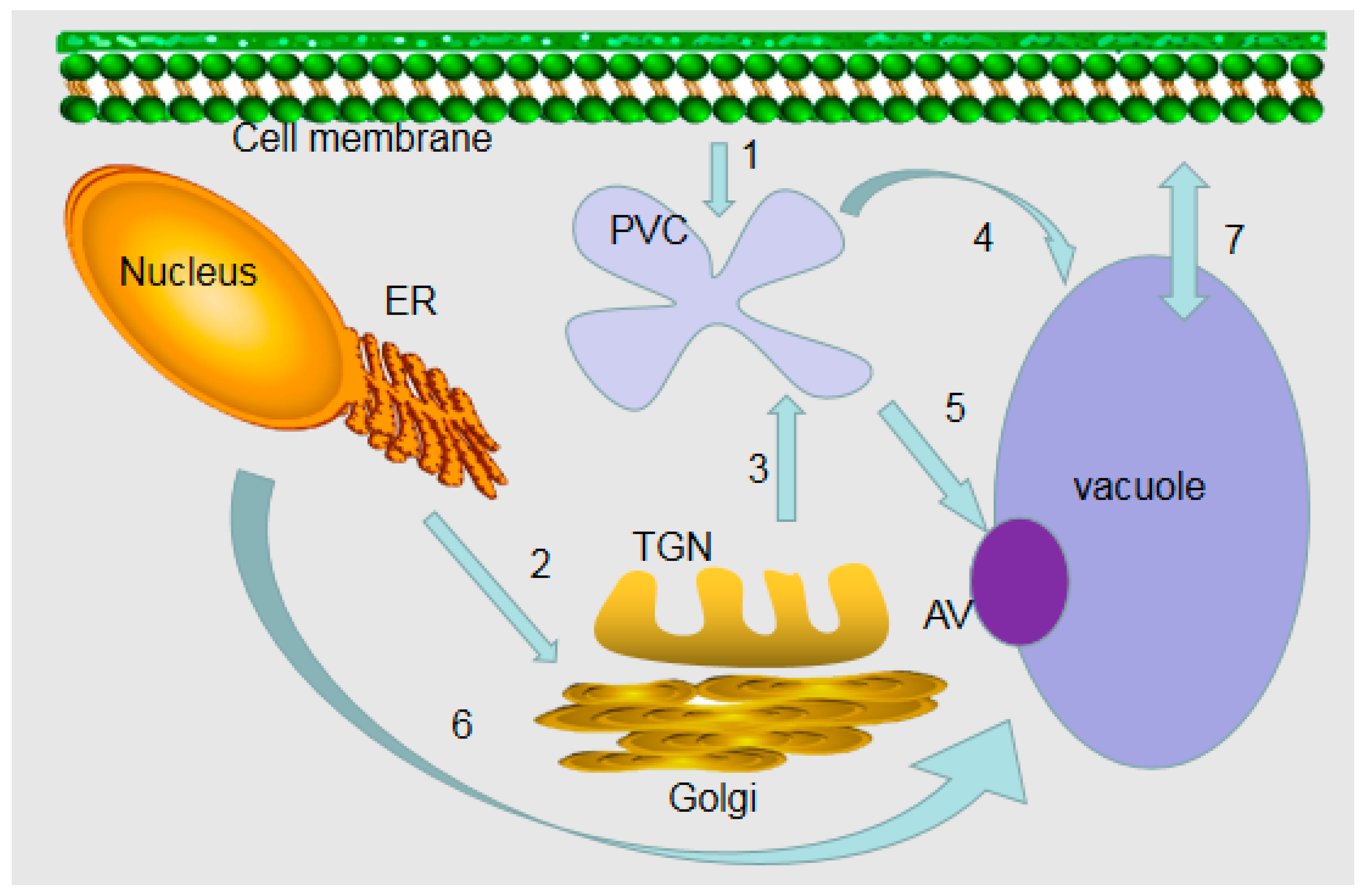
2. Formation Processes of Vacuoles
3. Different Types of the Vacuoles
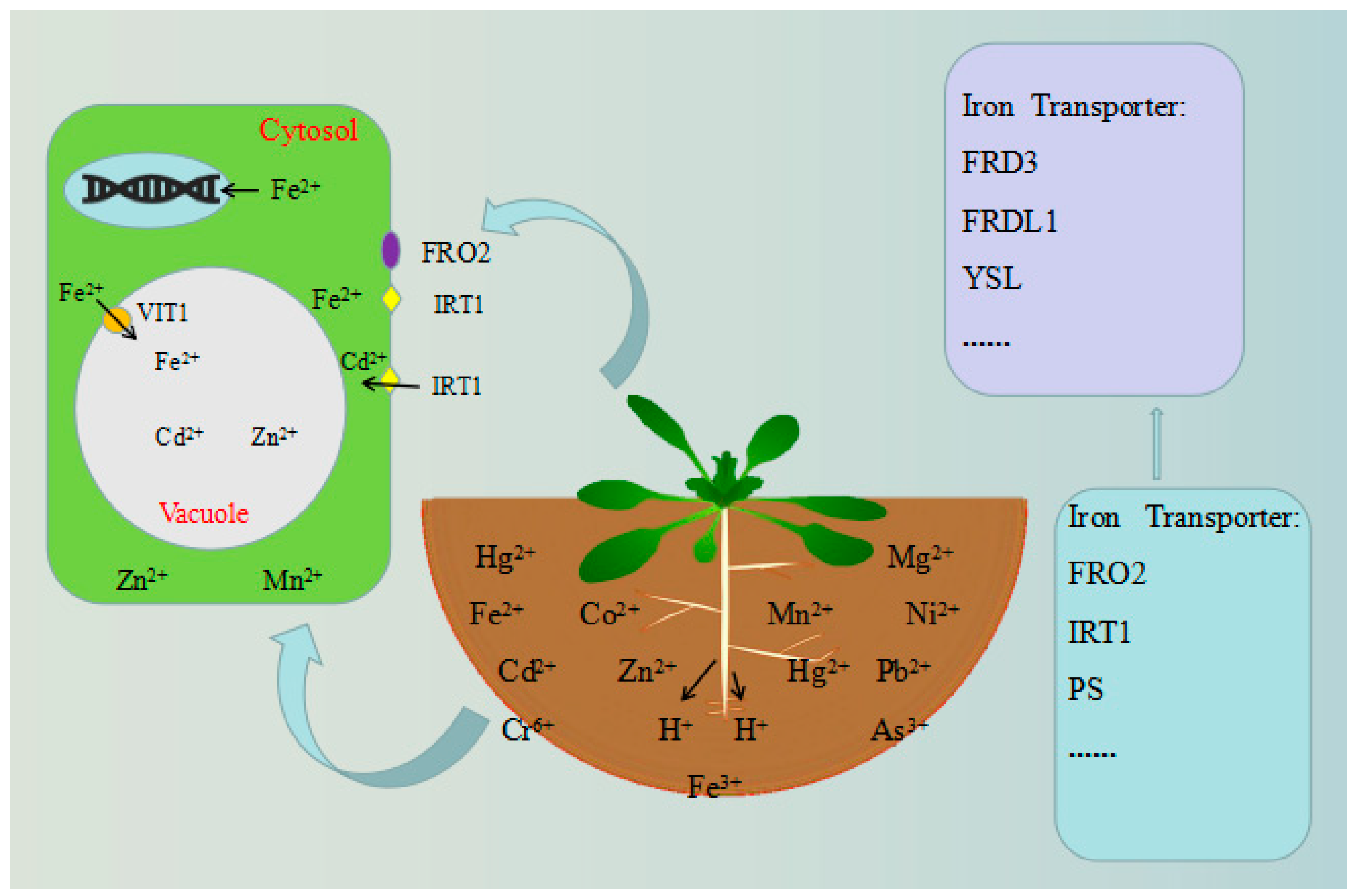
4. Vacuole-Localized Proteins
5. Multifaceted Roles of Plant Vacuoles
5.1. Vacuoles Can Be Used as Professional Repositories
5.2. The Roles of Plant Vacuoles in Protein Degradation
5.3. The Role of Vacuoles in Plant Metabolism
6. Our Prospect of Vacuole Research
Author Contributions
Funding
Conflicts of Interest
References
- Dujardin, F. Histoire Naturelle des Zoophytes: Infusoires; Librairie Encyclopédique de Roret: Paris, France, 1841. [Google Scholar]
- DeVries, H. Plasmolytische studien über die wand der vakuolen. Jahrb Bot. 1885, 16, 465–598. [Google Scholar]
- Blumwald, E.; Poole, R.J. Na+/H+ Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris. Plant Physiol. 1985, 78, 163–167. [Google Scholar] [CrossRef]
- Marty, F. Plant vacuoles. Plant Cell. 1999, 11, 587–600. [Google Scholar] [CrossRef]
- Xiang, L.; Etxeberria, E.; Van den Ende, W. Vacuolar protein sorting mechanisms in plants. FEBS J. 2013, 280, 979–993. [Google Scholar] [CrossRef]
- Viotti, C. ER to Golgi-Dependent Protein Secretion: The Conventional Pathway. Methods Mol. Biol. 2016, 1459, 3–29. [Google Scholar]
- Maîtrejean, M.; Wudick, M.M.; Voelker, C.; Prinsi, B.; Mueller-Roeber, B.; Czempinski, K.; Pedrazzini, E.; Vitale, A. Assembly and sorting of the tonoplast potassium channel AtTPK1 and its turnover by internalization into the vacuole. Plant Physiol. 2011, 156, 1783–1796. [Google Scholar] [CrossRef]
- Bellucci, M.; Marchis, F.D.; Pompa, A. The endoplasmic reticulum is a hub to sort proteins toward unconventional traffic pathways and endosymbiotic organelles. J. Exp. Bot. 2017, 69, 7–20. [Google Scholar] [CrossRef]
- Sansebastiano, G.P.D.; Barozzi, F.; Piro, G.; Denecke, J.; Lousa, C.D.M. Trafficking routes to the plant vacuole: Connecting alternative and classical pathways. J. Exp. Bot. 2017, 69, 79–90. [Google Scholar] [CrossRef]
- Viotti, C.; Krüger, F.; Krebs, M.; Neubert, C.; Fink, F.; Lupanga, U.; Scheuring, D.; Boutté, Y.; Frescatada-Rosa, M.; Wolfenstetter, S.; et al. The endoplasmic reticulum is the main membrane source for biogenesis of the lytic vacuole in Arabidopsis. Plant cell 2013, 25, 3434–3449. [Google Scholar] [CrossRef]
- Sanderfoot, A.A.; Raikhel, N.V. The specificity of vesicle trafficking: Coat proteins and SNAREs. Plant Cell 1999, 11, 629–642. [Google Scholar] [CrossRef]
- Goring, D.R.; Sansebastiano, G.P.D. Protein and membrane trafficking routes in plants: Conventional or unconventional? J. Exp. Bot. 2018, 69, 1–5. [Google Scholar] [CrossRef]
- Wang, X.; Chung, K.P.; Lin, W.; Jiang, L. Protein secretion in plants: Conventional and unconventional pathways and new techniques. J. Exp. Bot. 2017, 69, 21–37. [Google Scholar] [CrossRef]
- Stigliano, E.; Faraco, M.; Neuhaus, J.M.; Montefusco, A.; Dalessandro, G.; Piro, G.; Di Sansebastiano, G.P. Two glycosylated vacuolar GFPs are new markers for ER-to-vacuole sorting. Plant Physiol. Biochem. 2013, 73, 337–343. [Google Scholar] [CrossRef]
- Jauh, G.; Phillips, T.; Rogers, J. Tonoplast intrinsic protein isoforms as markers for vacuolar functions. Plant Cell 1999, 11, 1867–1882. [Google Scholar] [CrossRef]
- Zouhar, J.; Rojo, E. Plant vacuoles: Where did they come from and where are they heading? Curr. Opin. Plant Biol. 2009, 12, 677–684. [Google Scholar] [CrossRef]
- Paris, N.; Stanley, C.M.; Jones, R.L.; Rogers, J.C. Plant cells contain two functionally distinct vacuolar compartments. Cell 1996, 85, 563–572. [Google Scholar] [CrossRef]
- Shimada, T.; Koumoto, Y.; Li, L.; Yamazaki, M.; Kondo, M.; Nishimura, M.; Hara-Nishimura, I. AtVPS29, a putative component of a retromer complex, is required for the efficient sorting of seed storage proteins. Plant Cell Physiol. 2006, 47, 1187–1194. [Google Scholar] [CrossRef]
- Yamazaki, M.; Shimada, T.; Takahashi, H.; Tamura, K.; Kondo, M.; Nishimura, M.; Hara-Nishimura, I. Arabidopsis VPS35, a retromer component, is required for vacuolar protein sorting and involved in plant growth and leaf senescence. Plant Cell Physiol. 2008, 49, 142–156. [Google Scholar] [CrossRef]
- Kruger, F.; Schumacher, K. Pumping up the volume-vacuole biogenesis in Arabidopsis thaliana. Semin. Cell Dev. Biol. 2018, 80, 106–112. [Google Scholar] [CrossRef]
- Hara-Nishimura, I.; Inoue, K.; Nishimura, M. A unique vacuolar processing enzyme responsible for conversion of several proprotein precursors into the mature forms. FEBS Lett. 1991, 294, 89–93. [Google Scholar] [CrossRef]
- Chrispeels, M.J.; Schaewen, A.V. Sorting of proteins in the secretory system of plant cells. Antonie Van Leeuwenhoek. 1992, 61, 161–165. [Google Scholar] [CrossRef]
- Herman, E.M.; Larkins, B.A. Protein storage bodies and vacuoles. Plant Cell 1999, 11, 601–614. [Google Scholar] [CrossRef]
- Frigerio, L.; Hinz, G.; Robinson, D.G. Multiple vacuoles in plant cells: Rule or exception? Traffic 2008, 9, 1564–1570. [Google Scholar] [CrossRef]
- Feeney, M.; Kittelmann, M.; Menassa, R.; Hawes, C.; Frigerio, L. Protein Storage Vacuoles Originate from Remodeled Preexisting Vacuoles in Arabidopsis thaliana. Plant Physiol. 2018, 177, 241–254. [Google Scholar]
- Viotti, C. ER and vacuoles: Never been closer. Front Plant Sci. 2014, 5, 20. [Google Scholar] [CrossRef]
- Vitale, A.; Galili, G. The endomembrane system and the problem of protein sorting. Plant Physiol. 2001, 125, 115–118. [Google Scholar] [CrossRef]
- Martinoia, E. Vacuolar Transporters-Companions on a Longtime Journey. Plant Physiol. 2018, 176, 1384–1407. [Google Scholar] [CrossRef]
- Robert, S.; Zouhar, J.; Carter, C.; Raikhel, N. Isolation of intact vacuoles from Arabidopsis rosette leaf-derived protoplasts. Nat. Protoc. 2007, 2, 259–262. [Google Scholar] [CrossRef]
- Cocking, E.C. A Method for the Isolation of Plant Protoplasts and Vacuoles. Nature 1960, 187, 962–963. [Google Scholar] [CrossRef]
- Buser-Suter, C.; Wiemken, A.; Matile, P. A malic Acid permease in isolated vacuoles of a crassulacean Acid metabolism plant. Plant Physiol. 1982, 69, 456–459. [Google Scholar] [CrossRef]
- Schmidt, U.G.; Endler, A.; Schelbert, S.; Brunner, A.; Schnell, M.; Neuhaus, H.E.; Marty-Mazars, D.; Marty, F.; Baginsky, S.; Martinoia, E. Novel tonoplast transporters identified using a proteomic approach with vacuoles isolated from cauliflower buds. Plant Physiol. 2007, 145, 216–229. [Google Scholar] [CrossRef]
- Schroeder, J.I.; Hedrich, R.; Fernandez, J.M. Potassium-selective single channels in guard cell protoplasts of Vicia faba. Nature 1984, 312, 361–362. [Google Scholar] [CrossRef]
- Hedrich, R.; Kurkdjian, A.; Guern, J.; Flugge, U.I. Comparative studies on the electrical properties of the H+ translocating ATPase and pyrophosphatase of the vacuolar-lysosomal compartment. EMBO J. 1989, 8, 2835–2841. [Google Scholar] [CrossRef]
- Shimaoka, T.; Ohnishi, M.; Sazuka, T.; Mitsuhashi, N.; Hara-Nishimura, I.; Shimazaki, K.; Maeshima, M.; Yokota, A.; Tomizawa, K.; Mimura, T. Isolation of intact vacuoles and proteomic analysis of tonoplast from suspension-cultured cells of Arabidopsis thaliana. Plant Cell Physiol. 2004, 45, 672–683. [Google Scholar] [CrossRef]
- Jaquinod, M.; Villiers, F.; Kieffer-Jaquinod, S.; Hugouvieux, V.; Bruley, C.; Garin, J.; Bourguignon, J. A proteomics dissection of Arabidopsis thaliana vacuoles isolated from cell culture. Mol. Cell. Proteom. 2007, 6, 394–412. [Google Scholar] [CrossRef]
- Melroy, D.L.; Herman, E.M. TIP, an integral membrane protein of the protein-storage vacuoles of the soybean cotyledon undergoes developmentally regulated membrane accumulation and removal. Planta 1991, 184, 113–122. [Google Scholar] [CrossRef]
- Maeshima, M. Characterization of the major integral protein of vacuolar membrane. Plant Physiol. 1992, 98, 1248–1254. [Google Scholar] [CrossRef]
- Hedrich, R.; Schroeder, J.I. The Physiology of ION Channels and Electrogenic Pumps in Higher Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 539–569. [Google Scholar] [CrossRef]
- Sze, H.; Li, X.; Palmgren, M.G. Energization of plant cell membranes by H+-pumping ATPases. Regulation and biosynthesis. Plant Cell 1999, 11, 677–690. [Google Scholar]
- Li, J.; Yang, H.; Peer, W.A.; Richter, G.; Blakeslee, J.; Bandyopadhyay, A.; Titapiwantakun, B.; Undurraga, S.; Khodakovskaya, M.; Richards, E.L.; et al. Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science 2005, 310, 121–125. [Google Scholar] [CrossRef]
- Yoshida, K.; Kawachi, M.; Mori, M.; Maeshima, M.; Kondo, M.; Nishimura, M.; Kondo, T. The involvement of tonoplast proton pumps and Na+(K+)/H+ exchangers in the change of petal color during flower opening of Morning Glory, Ipomoea tricolor cv. Heavenly Blue. Plant Cell Physiol. 2005, 46, 407–415. [Google Scholar] [CrossRef]
- Blumwald, E. Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 2000, 12, 431–434. [Google Scholar] [CrossRef]
- Cheng, N.H.; Pittman, J.K.; Barkla, B.J.; Shigaki, T.; Hirschi, K.D. The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. Plant Cell 2003, 15, 347–364. [Google Scholar] [CrossRef]
- Klaumann, S.; Nickolaus, S.D.; Furst, S.H.; Starck, S.; Schneider, S.; Ekkehard Neuhaus, H.; Trentmann, O. The tonoplast copper transporter COPT5 acts as an exporter and is required for interorgan allocation of copper in Arabidopsis thaliana. New Phytol. 2011, 192, 393–404. [Google Scholar] [CrossRef]
- Wege, S.; De Angeli, A.; Droillard, M.J.; Kroniewicz, L.; Merlot, S.; Cornu, D.; Gambale, F.; Martinoia, E.; Barbier-Brygoo, H.; Thomine, S.; et al. Phosphorylation of the vacuolar anion exchanger AtCLCa is required for the stomatal response to abscisic acid. Sci. Signal. 2014, 7, ra65. [Google Scholar] [CrossRef]
- De Angeli, A.; Zhang, J.B.; Meyer, S.; Martinoia, E. AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat. Commun. 2013, 4, 1804. [Google Scholar] [CrossRef]
- Tommasini, R.; Vogt, E.; Fromenteau, M.; Hortensteiner, S.; Matile, P.; Amrhein, N.; Martinoia, E. An ABC-transporter of Arabidopsis thaliana has both glutathione-conjugate and chlorophyll catabolite transport activity. Plant J. 1998, 13, 773–780. [Google Scholar] [CrossRef]
- Takanashi, K.; Yamada, Y.; Sasaki, T.; Yamamoto, Y.; Sato, F.; Yazaki, K. A multidrug and toxic compound extrusion transporter mediates berberine accumulation into vacuoles in, coptis japonica. Phytochemistry 2017, 138, 76–82. [Google Scholar] [CrossRef]
- Shitan, N.; Yazaki, K. New insights into the transport mechanisms in plant vacuoles. Int. Rev. Cell Mol. Biol. 2013, 305, 383–433. [Google Scholar]
- Darbani, B.; Motawia, M.S.; Olsen, C.E.; Nour-Eldin, H.H.; Moller, B.L.; Rook, F. The biosynthetic gene cluster for the cyanogenic glucoside dhurrin in Sorghum bicolor contains its co-expressed vacuolar MATE transporter. Sci. Rep. 2016, 6, 37079. [Google Scholar] [CrossRef]
- Momonoi, K.; Tsuji, T.; Kazuma, K.; Yoshida, K. Specific expression of the vacuolar iron transporter, TgVit, causes iron accumulation in blue-colored inner bottom segments of various tulip petals. Biosci. Biotechnol. Biochem. 2012, 76, 319–325. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Yi, H.; Gong, J. Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J. 2012, 72, 400–410. [Google Scholar] [CrossRef]
- Zhu, W.; Zuo, R.; Zhou, R.; Huang, J.; Tang, M.; Cheng, X.; Liu, Y.; Tong, C.; Xiang, Y.; Dong, C.; et al. Vacuolar Iron Transporter BnMEB2 Is Involved in Enhancing Iron Tolerance of Brassica napus. Front. Plant Sci. 2016, 7, 1353. [Google Scholar] [CrossRef]
- Sinclair, S.A.; Kramer, U. The zinc homeostasis network of land plants. Biochim. Biophys. Acta 2012, 1823, 1553–1567. [Google Scholar] [CrossRef]
- Delhaize, E.; Kataoka, T.; Hebb, D.M.; White, R.G.; Ryan, P.R. Genes encoding proteins of the cation diffusion facilitator family that confer manganese tolerance. Plant Cell 2003, 15, 1131–1142. [Google Scholar] [CrossRef]
- Endler, A.; Meyer, S.; Schelbert, S.; Schneider, T.; Weschke, W.; Peters, S.W.; Keller, F.; Baginsky, S.; Martinoia, E.; Schmidt, U.G. Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiol. 2006, 141, 196–207. [Google Scholar] [CrossRef]
- Wink, M. The Plant Vacuole: A Multifunctional Compartment. J. Exp. Bot. 1993, 44, 231–246. [Google Scholar]
- Boller, T.; Kende, H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979, 63, 1123–1132. [Google Scholar] [CrossRef]
- Pedrazzini, E.; Caprera, A.; Fojadelli, I.; Stella, A.; Rocchetti, A.; Bassin, B.; Martinoia, E.; Vitale, A. The Arabidopsis tonoplast is almost devoid of glycoproteins with complex N-glycans, unlike the rat lysosomal membrane. J. Exp. Bot. 2016, 67, 1769–1781. [Google Scholar] [CrossRef]
- Müntz, K. Deposition of storage proteins. Plant Mol. Biol. 1998, 38, 77–99. [Google Scholar] [CrossRef]
- Staswick, P.E. Storage Proteins of Vegetative Plant Tissues. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1994, 45, 303–322. [Google Scholar] [CrossRef]
- Mainieri, D.; Morandini, F.; Maîtrejean, M.; Saccani, A.; Pedrazzini, E.; Alessandro, V. Protein body formation in the endoplasmic reticulum as an evolution of storage protein sorting to vacuoles: Insights from maize γ-zein. Front Plant Sci. 2014, 5, 331. [Google Scholar] [CrossRef][Green Version]
- Vitale, A.; Hinz, G. Sorting of proteins to storage vacuoles: how many mechanisms? Trends Plant Sci. 2015, 10, 316–323. [Google Scholar] [CrossRef]
- Bartrons, M.; Catalan, J.; Penuelas, J. Spatial and Temporal Trends of Organic Pollutants In Vegetation From Remote And Rural Areas. Sci. Rep. 2016, 6, 25446. [Google Scholar] [CrossRef]
- Fasani, E.; Manara, A.; Martini, F.; Furini, A.; DalCorso, G. The potential of genetic engineering of plants for the remediation of soils contaminated with heavy metals. Plant Cell Environ. 2018, 41, 1201–1232. [Google Scholar] [CrossRef]
- Zhang, J.; Martinoia, E.; Lee, Y. Vacuolar transporters for cadmium and arsenic in plants and their applications in phytoremediation and crop development. Plant Cell Physiol. 2018, 59, 1317–1325. [Google Scholar] [CrossRef]
- Curie, C.; Briat, J. Iron transport and signaling in plants. Ann. Rev. Plant Biol. 2003, 54, 183–206. [Google Scholar] [CrossRef]
- Conte, S.; Walker, E. Transporters contributing to iron trafficking in plants. Mol. Plant. 2011, 4, 464–476. [Google Scholar] [CrossRef]
- Wu, H.; Ji, Y.; Du, J.; Kong, D.; Liang, H.; Ling, H. ClpC1, an ATP-dependent Clp protease in plastids, is involved in iron homeostasis in Arabidopsis leaves. Ann. Bot. 2010, 105, 823–833. [Google Scholar] [CrossRef]
- Haddad, L.; Ross, J.; Oshaug, A.; Torheim, L.E.; Cogill, B. 5th Report on the World Nutrition Situation: Nutrition for Improved Development Outcomes; SCN: Geneva, Switzerland, 2004; Volume 39, pp. 257–258. [Google Scholar]
- Briat, J.; Ravet, K.; Arnaud, N.; Duc, C.; Boucherez, J.; Touraine, B.; Cellier, F.; Gaymard, F. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann. Bot. 2010, 105, 811–822. [Google Scholar] [CrossRef]
- Nestel, P.; Bouis, H.; Meenakshi, J.; Pfeiffer, W. Biofortification of staple food crops. J. Nutr. 2006, 136, 1064–1067. [Google Scholar] [CrossRef]
- Kim, S.; Punshon, T.; Lanzirotti, A.; Li, L.; Alonso, J.; Ecker, J.R.; Kaplan, J.; Guerinot, M.L. Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 2006, 314, 1295–1298. [Google Scholar] [CrossRef]
- Briat, J.; Curie, C.; Gaymard, F. Iron utilization and metabolism in plants. Curr. Opin. Plant Biol. 2007, 10, 276–282. [Google Scholar] [CrossRef]
- Curie, C.; Mari, S. New routes for plant iron mining. New Phytol. 2017, 214, 521–525. [Google Scholar] [CrossRef]
- Guerinot, M.; Yi, Y. Iron: Nutritious, Noxious, and Not Readily Available. Plant Physiol. 1994, 104, 815–820. [Google Scholar] [CrossRef]
- Robinson, N.; Procter, C.; Connolly, E.; Guerinot, M. A ferric-chelate reductase for iron uptake from soils. Nature. 1999, 397, 694–697. [Google Scholar] [CrossRef]
- Rodríguez-Celma, J.; Schmidt, W. Reduction-based iron uptake revisited: On the role of secreted iron-binding compounds. Plant Signal. Behav. 2013, 8, 1473–1485. [Google Scholar] [CrossRef]
- Miethke, M.; Marahiel, M. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef]
- Ma, J.F.; Nomoto, K. Effective regulation of iron acquisition in graminaceous plants. The role of mugineic acids as phytosiderophores. Physiol. Plant 2010, 97, 609–617. [Google Scholar] [CrossRef]
- Takagi, S.; Nomoto, K.; Takemoto, T. Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants. J. Plant Nutr. 2008, 7, 469–477. [Google Scholar] [CrossRef]
- Curie, C.; Panaviene, Z.; Loulergue, C.; Dellaporta, S.; Briat, J.; Walker, E. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 2001, 409, 346–349. [Google Scholar] [CrossRef]
- Liu, Y.; Bassham, D.C. Autophagy: Pathways for self-eating in plant cells. Annu. Rev. Plant Biol. 2012, 63, 215–237. [Google Scholar] [CrossRef]
- Michaeli, S.; Avin-Wittenberg, T.; Galili, G. Involvement of autophagy in the direct ER to vacuole protein trafficking route in plants. Front Plant Sci. 2014, 5, 134. [Google Scholar] [CrossRef]
- Chanoca, A.; Kovinich, N.; Burkel, B.; Stecha, S.; Bohorquez-Restrepo, A.; Ueda, T.; Eliceiri, K.W.; Grotewold, E.; Otegui, M.S. Anthocyanin Vacuolar Inclusions Form by a Microautophagy Mechanism. Plant Cell 2015, 27, 2545–2559. [Google Scholar] [CrossRef]
- Herman, E.; Schmidt, M. Endoplasmic reticulum to vacuole trafficking of endoplasmic reticulum bodies provides an alternate pathway for protein transfer to the vacuole. Plant Physiol. 2004, 136, 3440–3446. [Google Scholar] [CrossRef]
- Martinoia, E.; Meyer, S.; De Angeli, A.; Nagy, R. Vacuolar Transporters in Their Physiological Context. Ann. Rev. Plant Biol. 2012, 63, 183–213. [Google Scholar] [CrossRef]
- Ludevid, D.; Hofte, H.; Himelblau, E.; Chrispeels, M.J. The Expression Pattern of the Tonoplast Intrinsic Protein gamma-TIP in Arabidopsis thaliana Is Correlated with Cell Enlargement. Plant Physiol. 1992, 100, 1633–1639. [Google Scholar] [CrossRef]
- Maurel, C. Aquaporins and Water Permeability of Plant Membranes. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 399–429. [Google Scholar] [CrossRef]
- Aubert, S.; Gout, E.; Bligny, R.; Marty-Mazars, D.; Barrieu, F.; Alabouvette, J.; Marty, F.; Douce, R. Ultrastructural and biochemical characterization of autophagy in higher plant cells subjected to carbon deprivation: Control by the supply of mitochondria with respiratory substrates. J. Cell Biol. 1996, 133, 1251–1263. [Google Scholar] [CrossRef]
- Moriyasu, Y.; Ohsumi, Y. Autophagy in Tobacco Suspension-Cultured Cells in Response to Sucrose Starvation. Plant Physiol. 1996, 111, 1233–1241. [Google Scholar] [CrossRef]
- Francisco, R.D.B.; Martinoia, E. The Vacuolar Transportome of Plant Specialized Metabolites. Plant Cell Physiol. 2018, 59, 1326–1336. [Google Scholar]
- Martinoia, E.; Maeshima, M.; Neuhaus, H.E. Vacuolar transporters and their essential role in plant metabolism. J. Exp. Bot. 2007, 58, 83–102. [Google Scholar] [CrossRef]
- Shitan, N. Secondary metabolites in plants: Transport and self-tolerance mechanisms. Biosci. Biotechnol. Biochem. 2016, 80, 1283–1293. [Google Scholar] [CrossRef]
- Zhao, J. Flavonoid transport mechanisms: How to go, and with whom. Trends Plant Sci. 2015, 20, 576–585. [Google Scholar] [CrossRef]
- Aufschnaiter, A.; Büttner, S. The vacuolar shapes of ageing: From function to morphology. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 957–970. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kutsuna, N.; Kanazawa, Y.; Kondo, N.; Hasezawa, S.; Sano, T. Intra-vacuolar reserves of membranes during stomatal closure: The possible role of guard cell vacuoles estimated by 3-D reconstruction. Plant Cell Physiol. 2007, 48, 1159–1169. [Google Scholar] [CrossRef]
- Zheng, H.; Staehelin, L. Protein storage vacuoles are transformed into lytic vacuoles in root meristematic cells of germinating seedlings by multiple, cell type-specific mechanisms. Plant Physiol. 2011, 155, 2023–2035. [Google Scholar] [CrossRef]
- Shimada, T.; Takagi, J.; Ichino, T.; Shirakawa, M.; Hara-Nishimura, I. Plant Vacuoles. Annu Rev. Plant Biol. 2018, 69, 123–145. [Google Scholar] [CrossRef]
- Martinoia, E.; Mimura, T.; Hara-Nishimura, I.; Shiratake, K. The Multifaceted Roles of Plant Vacuoles. Plant Cell Physiol. 2018, 59, 1285–1287. [Google Scholar] [CrossRef]
- Pedrazzini, E.; Komarova, N.Y.; Rentsch, D.; Vitale, A. Traffic routes and signals for the tonoplast. Traffic 2013, 14, 622–628. [Google Scholar] [CrossRef]
| Classification | Name | Functions | References |
|---|---|---|---|
| Proton Pumps | Vacuolar-type H+-pumping ATP hydrolase (H+-ATPase, VHA) | For the acidification of the vacuole. | [39,40] |
| H+-pumping pyrophosphatase (H+-PPase, AVP1) | For the acidification of vacuoles and the control of auxin transport. | [39,40,41] | |
| Proton antiporters | Cation (Na+/K+) proton antiporters (NHXs) | To change the color of flowers. | [42] |
| Na+/H+ antiporter (AtNHX1) | To mediate Na+ isolation in vacuoles and improve plant salt tolerance. | [43] | |
| Ca2+/H+ antiporters | To regulate plant processes, including ionic homeostasis and development. | [44] | |
| The characterization of the copper transporter COPT5 | To export copper in vacuoles. | [45] | |
| Vacuolar anion exchanger AtCLCa, AtALMT9 | Stomatal regulation and vacuole delivery of their anions. | [46,47] | |
| ATP-binding cassette (ABC) transporters | MRPs, AtTAP2 | For transporting glutathione conjugates and glucosidic acid conjugates. | [35,48] |
| Multidrug and toxic compound extrusion (MATE) transporters | SbMATE2 | To transport secondary compounds such as alkaloids, cyano glucoside, and some flavonoids. | [49,50,51] |
| Heavy Metal Transporters | Vacuole iron transporter (VIT) | To regulate the synthesis of anthocyanins; resistance to heavy metal ions; to regulate cytosolic iron homeostasis. | [52,53] |
| BnMEB2 | Resistance to heavy metal ions | [54] | |
| Mn2+ transporters | Resistance to heavy metal ions | [55,56] | |
| Vacuolar Sugar Transporters | AtSuc4 | Resistance to heavy metal ions | [57] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, X.; Li, K.; Wang, Z.; Zhu, K.; Tan, X.; Cao, J. A Review of Plant Vacuoles: Formation, Located Proteins, and Functions. Plants 2019, 8, 327. https://doi.org/10.3390/plants8090327
Tan X, Li K, Wang Z, Zhu K, Tan X, Cao J. A Review of Plant Vacuoles: Formation, Located Proteins, and Functions. Plants. 2019; 8(9):327. https://doi.org/10.3390/plants8090327
Chicago/Turabian StyleTan, Xiaona, Kaixia Li, Zheng Wang, Keming Zhu, Xiaoli Tan, and Jun Cao. 2019. "A Review of Plant Vacuoles: Formation, Located Proteins, and Functions" Plants 8, no. 9: 327. https://doi.org/10.3390/plants8090327
APA StyleTan, X., Li, K., Wang, Z., Zhu, K., Tan, X., & Cao, J. (2019). A Review of Plant Vacuoles: Formation, Located Proteins, and Functions. Plants, 8(9), 327. https://doi.org/10.3390/plants8090327





