Antioxidant, Antidiabetic, and Anticholinesterase Activities and Phytochemical Profile of Azorella glabra Wedd
Abstract
:1. Introduction
2. Results and Discussion
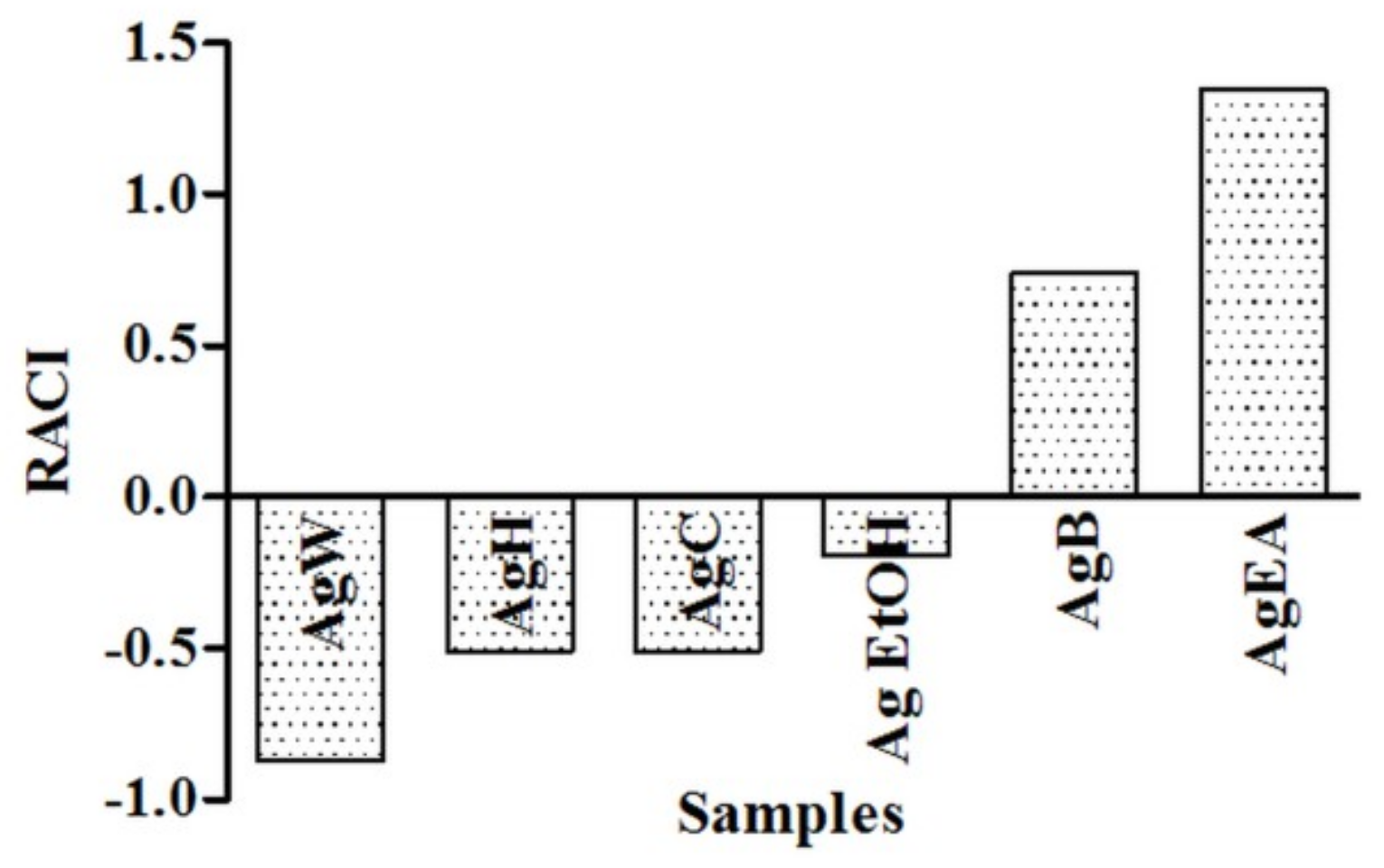
2.1. Antioxidant Activity
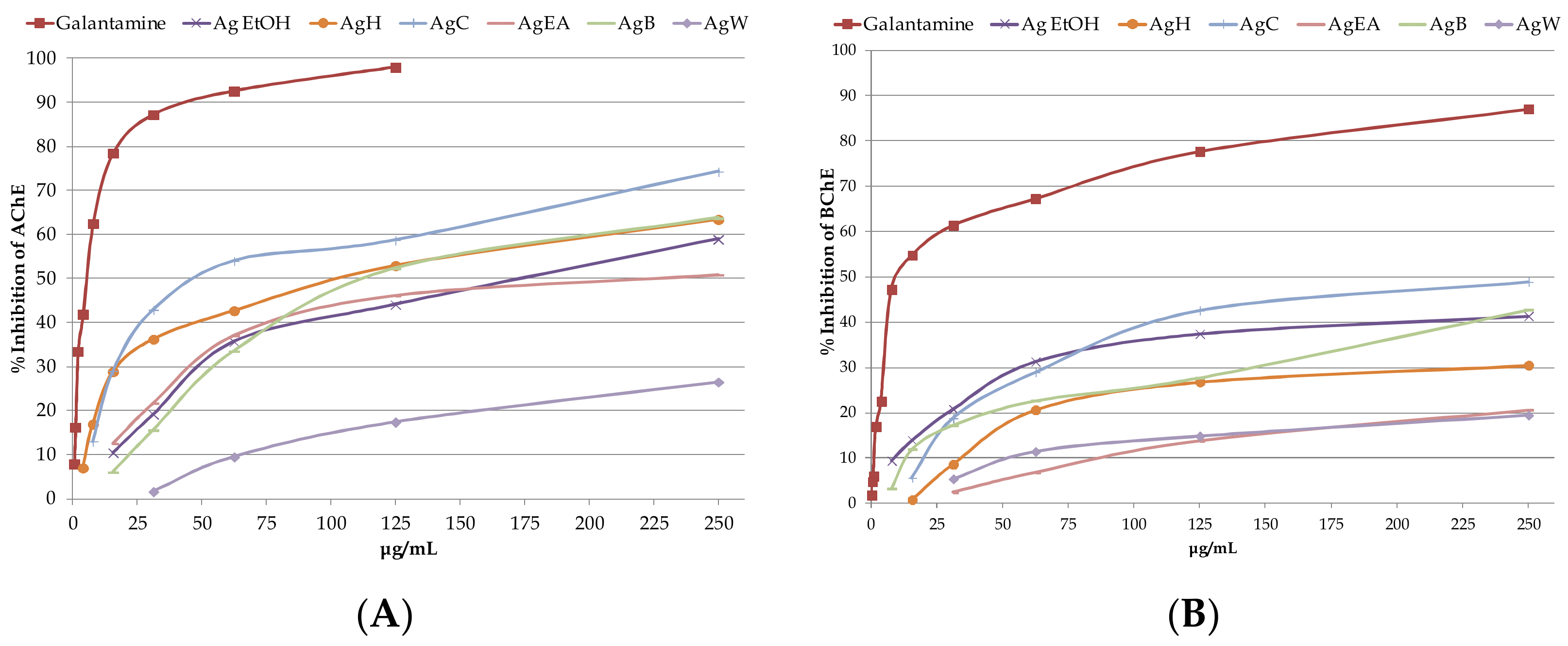
2.2. Determination of Anticholinesterase Activity of A. glabra Samples
2.3. Potential Antidiabetic Activity of A. glabra Samples
2.4. Identification and Quantification of Phytochemicals
3. Materials and Methods
3.1. Chemicals, Reagents, and Equipment
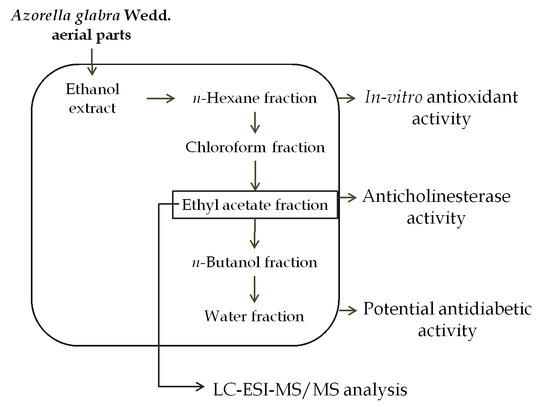
3.2. Plant Material and Samples Preparation
3.3. Antioxidant Activity
3.3.1. Radical Scavenging Activity
3.3.2. Ferric Reducing Antioxidant Power Assay (FRAP)
3.3.3. β-Carotene Bleaching Assay (BCB)
3.4. Determination of Anticholinesterase Activity
3.5. Antidiabetic Activity
3.5.1. α-Amylase Inhibition
3.5.2. α-Glucosidase Inhibition
3.6. Identification and Quantification by Liquid Chromatography Mass Spectrometry
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data Availability
References
- Sung, M.H.; Kwon, O.-K.; Oh, S.-R.; Lee, J.; Park, S.-H.; Han, S.B.; Ahn, K.-S. Azorella compacta methanolic extract induces apoptosis via activation of mitogen-activated protein kinase. Mol. Med. Rep. 2015, 12, 6821–6828. [Google Scholar] [CrossRef] [PubMed]
- Tůmová, L.; Dučaiová, Z.; Cheel, J.; Vokřál, I.; Sepúlveda, B.; Vokurková, D. Azorella compacta infusion activates human immune cells and scavenges free radicals in vitro. Pharmacogn. Mag. 2017, 13, 260. [Google Scholar] [PubMed]
- Lamorte, D.; Faraone, I.; Laurenzana, I.; Milella, L.; Trino, S.; De Luca, L.; Del Vecchio, L.; Armentano, M.; Sinisgalli, C.; Chiummiento, L. Future in the Past: Azorella glabra Wedd. as a source of new natural compounds with antiproliferative and cytotoxic activity on multiple myeloma cells. Int. J. Mol. Sci. 2018, 19, 3348. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Valentão, P.; Andrade, P.B.; Fernandez, E.C.; Milella, L. Evaluation of antioxidant, antidiabetic and anticholinesterase activities of Smallanthus sonchifolius landraces and correlation with their phytochemical profiles. Int. J. Mol. Sci. 2015, 16, 17696–17718. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of Wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Saltos, M.B.V.; Puente, B.F.N.; Faraone, I.; Milella, L.; De Tommasi, N.; Braca, A. Inhibitors of α-amylase and α-glucosidase from Andromachia igniaria Humb. & Bonpl. Phytochem. Lett. 2015, 14, 45–50. [Google Scholar]
- Faraone, I.; Rai, D.; Chiummiento, L.; Fernandez, E.; Choudhary, A.; Prinzo, F.; Milella, L. Antioxidant activity and phytochemical characterization of Senecio clivicolus Wedd. Molecules 2018, 23, 2497. [Google Scholar] [CrossRef]
- Areche, C.; Cejas, P.; Thomas, P.; San-Martín, A.; Astudillo, L.; Gutiérrez, M.; Loyola, L.A. Triterpenoids from Azorella trifurcata (Gaertn.) Pers and their effect against the enzyme acetylcholinesterase. Quim. Nova 2009, 32, 2023–2025. [Google Scholar] [CrossRef]
- Loyola, L.A.; Bórquez, J.; Morales, G.; San-Martín, A.; Darias, J.; Flores, N.; Giménez, A. Mulinane-type diterpenoids from Azorella compacta display antiplasmodial activity. Phytochemistry 2004, 65, 1931–1935. [Google Scholar] [CrossRef]
- Bórquez, J.; Kennelly, E.J.; Simirgiotis, M.J. Activity guided isolation of isoflavones and hyphenated HPLC-PDA-ESI-ToF-MS metabolome profiling of Azorella madreporica Clos. from northern Chile. Food Res. Int. 2013, 52, 288–297. [Google Scholar] [CrossRef]
- Nakamura, M.; Ra, J.-H.; Jee, Y.; Kim, J.-S. Impact of different partitioned solvents on chemical composition and bioavailability of Sasa quelpaertensis Nakai leaf extract. J. Food Drug Anal. 2017, 25, 316–326. [Google Scholar] [CrossRef]
- Fuentes, N.L.; Sagua, H.; Morales, G.; Borquez, J.; Martin, A.S.; Soto, J.; Loyola, L.A. Experimental antihyperglycemic effect of diterpenoids of llareta Azorella compacta (Umbelliferae) Phil in rats. Phytother. Res. 2005, 19, 713–716. [Google Scholar] [CrossRef]
- Prabhakar, P.; Doble, M. Mechanism of action of medicinal plants towards diabetes mellitus-a review. Rec. Prog. Med. Plants 2008, 22, 181–204. [Google Scholar]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Booren, A.M.; Gray, J.I. Novel antioxidant compounds from Tart cherries (Prunus cerasus). J. Nat. Prod. 1999, 62, 86–88. [Google Scholar] [CrossRef]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Hung, T.M.; Na, M.; Thuong, P.T.; Su, N.D.; Sok, D.; Song, K.S.; Seong, Y.H.; Bae, K. Antioxidant activity of caffeoyl quinic acid derivatives from the roots of Dipsacus asper Wall. J. Ethnopharmacol. 2006, 108, 188–192. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT-Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Razavi, S.M.; Zahri, S.; Zarrini, G.; Nazemiyeh, H.; Mohammadi, S. Biological activity of quercetin-3-O-glucoside, a known plant flavonoid. Russ. J. Bioorg. Chem. 2009, 35, 376–378. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Kovalyov, V.; Goryacha, O.; Ivanauskas, L.; Georgiyants, V. Biologically active compounds and pharmacological activities of species of the genus Crocus: A review. Phytochemistry 2019, 162, 56–89. [Google Scholar] [CrossRef]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini-Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Uma Devi, P.; Ganasoundari, A.; Vrinda, B.; Srinivasan, K.; Unnikrishnan, M. Radiation protection by the Ocimum flavonoids orientin and vicenin: Mechanisms of action. Radiat. Res. 2000, 154, 455–460. [Google Scholar] [CrossRef]
- Han, J.; Ye, M.; Qiao, X.; Xu, M.; Wang, B.-R.; Guo, D.-A. Characterization of phenolic compounds in the Chinese herbal drug Artemisia annua by liquid chromatography coupled to electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2008, 47, 516–525. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef]
- Araujo, N.; Mü Ller, R.; Nowicki, C.; Ibisch, P. Análisis de Vacíos de Representatividad del Sistema Nacional de Áreas Protegidas; FAN: Santa Cruz, CA, USA, 2005. [Google Scholar]
- Krzyzanowska-Kowalczyk, J.; Pecio, Ł.; Mołdoch, J.; Ludwiczuk, A.; Kowalczyk, M. Novel phenolic constituents of Pulmonaria officinalis L. LC-MS/MS comparison of Spring and Autumn metabolite profiles. Molecules 2018, 23, 2277. [Google Scholar] [CrossRef]
- Iswaldi, I.; Arráez-Román, D.; Rodríguez-Medina, I.; Beltrán-Debón, R.; Joven, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification of phenolic compounds in aqueous and ethanolic rooibos extracts (Aspalathus linearis) by HPLC-ESI-MS (TOF/IT). Anal. Bioanal. Chem. 2011, 400, 3643–3654. [Google Scholar] [CrossRef]
- Ferracane, R.; Graziani, G.; Gallo, M.; Fogliano, V.; Ritieni, A. Metabolic profile of the bioactive compounds of burdock (Arctium lappa) seeds, roots and leaves. J. Pharm. Biomed. Anal. 2010, 51, 399–404. [Google Scholar] [CrossRef]
- Ibrahim, R.M.; El-Halawany, A.M.; Saleh, D.O.; El Naggar, E.M.B.; El-Shabrawy, A.E.-R.O.; El-Hawary, S.S. HPLC-DAD-MS/MS profiling of phenolics from Securigera securidaca flowers and its anti-hyperglycemic and anti-hyperlipidemic activities. Rev. Bras. Farmacogn. 2015, 25, 134–141. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef]
- Cid-Pérez, T.S.; Ávila-Sosa, R.; Ochoa-Velasco, C.E.; Rivera-Chavira, B.E.; Nevárez-Moorillón, G.V. Antioxidant and antimicrobial activity of Mexican Oregano (Poliomintha longiflora) essential oil, hydrosol and extracts from waste solid residues. Plants 2019, 8, 22. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue light added with Red LEDs enhance growth characteristics, pigments content, and antioxidant capacity in lettuce, spinach, kale, basil, and sweet pepper in a controlled environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef]
- Milella, L.; Milazzo, S.; De Leo, M.; Vera Saltos, M.B.; Faraone, I.; Tuccinardi, T.; Lapillo, M.; De Tommasi, N.; Braca, A. α-Glucosidase and α-amylase inhibitors from Arcytophyllum thymifolium. J. Nat. Prod. 2016, 79, 2104–2112. [Google Scholar] [CrossRef]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Amato, J.; D’Alonzo, D.; Piccialli, V.; Mayol, L.; Piccialli, G. A facile synthesis of 5′-Fluoro-5′-deoxyacadesine (5′-F-AICAR): A novel non-phosphorylable AICAR Analogue. Molecules 2012, 17, 13036–13044. [Google Scholar] [CrossRef]


| Samples | Extraction Yield (%) |
|---|---|
| Ag EtOH | 9.01 |
| AgH | 31.52 |
| AgC | 44.50 |
| AgEA | 2.23 |
| AgB | 5.66 |
| AgW | 16.10 |
| Samples | DPPH (mgTE/g) | FRAP (mgTE/g) | BCB %AA |
|---|---|---|---|
| Ag EtOH | 28.17 ± 2.32 a | 73.58 ± 0.71 a | 26.70 ± 0.61 a |
| AgH | nc | 18.79 ± 0.66 b | 22.50 ± 0.65 b |
| AgC | 5.94 ± 0.27 b | 15.45 ± 0.44 b | 22.18 ± 1.54 b |
| AgEA | 240.33 ± 10.73 c | 410.29 ± 5.69 c | 34.93 ± 1.37 c |
| AgB | 224.91 ± 4.84 d | 318.57 ± 2.77 d | 21.68 ± 0.57 b |
| AgW | 43.12 ± 1.23 e | 95.33 ± 3.86 e | nc |
| Samples | AChE Inhibition (IC50) | BChE Inhibition (IC50) |
|---|---|---|
| Galantamine | 4.68 ± 0.31 a | 16.07 ± 1.04 a |
| Ag EtOH | 193.81 ± 13.32 b | 421.50 ± 39.38 b |
| AgH | 99.19 ± 6.18 c | nc |
| AgC | 30.75 ± 0.67 d | 240.28 ± 8.91 c |
| AgEA | 215.29 ± 17.10 b | nc |
| AgB | 113.08 ± 5.18 c | 362.06 ± 28.60 b |
| AgW | nc | nc |
| Samples | α-Amylase Inhibition (IC50) | α-Glucosidase Inhibition (IC50) |
|---|---|---|
| Acarbose | 22.78 ± 0.29 a | 401.15 ± 25.94 a |
| Ag EtOH | 172.25 ± 7.25 b | 207.70 ± 2.56 b |
| AgH | nc | 159.91 ± 5.59 b |
| AgC | nc | nc |
| AgEA | nc | 163.54 ± 9.72 b |
| AgB | nc | 373.77 ± 29.84 a |
| AgW | nc | nc |
| Peak No. | RT (min) | [M-H]− Observed m/z | [M-H]−Calculated m/z | Molecular Formula | MS/MS | Tentative Identity | mg/g DW | References |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.17 | 353.0888 | 353.0873 | C16H18O9 | 191, 173, 135, 127, 93, 85 | Chlorogenic acid | 7.12 ± 0.83 | [25] |
| 2 | 6.48 | 427.1980 | 427.1968 | C21H32O9 | 367, 327, 297, 285, 179, 161, 135, 101, 73, 61, 59 | Methyl chlorogenate derivative | nq | [26] |
| 3 | 6.64 | 463.0859 | 463.0877 | C21H20O12 | 300, 271, 255, 179, 151 | Quercetin-3-O-glucoside | 0.07 ± 0.00 | [26,27] |
| 4 | 6.68 | 447.0918 | 447.0927 | C21H20O11 | 357, 339, 327, 311, 299, 297, 285, 269, 253, 191, 175, 149, 133, 109 | Iso-orientin | 13.22 ± 1.43 | [28] |
| 5 | 6.84 | 447.0910 | 447.0927 | C21H20O11 | 357, 339, 327, 311, 299, 297, 285, 269, 253, 191, 175, 149, 133, 109 | Orientin | 104.22 ± 4.01 | [28] |
| 6 | 6.92 | 367.1038 | 367.1029 | C17H20O9 | 191, 179, 161, 107 | Chlorogenic acid methyl ester | 12.33 ± 0.04 | [26] |
| 7 | 7.14 | 515.1180 | 515.1190 | C25H24O12 | 353, 179 | Cynarin isomer | 0.15 ± 0.03 | [29] |
| 8 | 7.41 | 447.0921 | 447.0927 | C21H20O11 | 285, 151 | Luteolin-7-O-glucoside | 0.65 ± 0.10 | [30] |
| 9 | 7.67 | 515.1411 | 515.1401 | C22H28O14 | 353, 191, 179, 173, 161, 135 | Chlorogenic acid glucoside | nq | |
| 10 | 7.83 | 515.1197 | 515.1190 | C25H24O12 | 353, 335, 191, 179, 161, 135 | 3,5-di-O-caffeoyl quinic acid | 44.70 ± 4.14 | [26] |
| 11 | 8.07 | 515.1209 | 515.1190 | C25H24O12 | 353, 179, 173, 135, 93 | 3,4-di-O-caffeoyl quinic acid | 23.12 ± 1.64 | [26] |
| 12, 13, 14, 16 | 8.49, 8.86, 9.12, 9.56 | 529.1365 | 529.1346 | C26H26O12 | 367, 349, 191, 179, 161, 135 | Feruloyl-caffeoylquinic acid isomers | nq | [23] |
| 15 | 9.32 | 285.0380 | 285.0399 | C15H10O6 | 151, 133 | Luteolin | 0.39 ± 0.01 | [25] |
| 17 | 9.98 | 325.1651 | 325.1651 | C17H26O6 | 281, 263, 235, 219, 203, 191, 151, 111, 83, 59 | Unknown | nq | |
| 18 | 10.55 | 853.4720 | 853.4738853.4679 | C48H70O13C55H66O8 | 584, 513, 191, 179, 161, 135, 119, 113, 101, 89, 85, 71, 59 | Caffeoylquinic acid derivative | nq | |
| 19 | 10.97 | 649.3929 | 649.3952 | C36H58O10 | 407, 191, 129, 113, 85, 75 | Unknown | nq | |
| 20 | 11.37 | 691.4073 | 691.4057 | C38H60O11 | 631, 191, 113, 85, 95 | Unknown | nq | |
| 21 | 11.87 | 867.4739 | 867.4742 | C45H72O16 | 513, 408, 333, 285, 191, 179, 173, 153, 139, 89 | Unknown | nq | |
| 22 | 14.14 | 391.1744 | 391.1757 | C21H28O7 | 391, 347, 305, 287, 259, 245, 217, 165 | Unknown | nq | |
| 23 | 15.17 | 677.3729 | 677.3748 | C33H58O14 | 415, 397, 279, 179, 161, 119, 101 | Unknown | nq | |
| 24 | 15.46 | 504.3098 | 504.3087 | C29H45O7 | 279, 242, 224, 168, 153, 79, 59 | Unknown | nq | |
| 25 | 15.76 | 426.9764 | 426.9785 | C15H8O15 | 407, 387, 293, 283, 255, 217, 81 | Unknown | nq | |
| 26 | 16.22 | 480.3083 | 480.3087 | C27H45O7 | 255, 242, 224, 168, 153, 79 | Unknown | nq | |
| 27 | 16.95 | 579.3354 | 579.3381 | C28H52O12 | 269, 255, 89 | Unknown | nq | |
| 28 | 18.10 | 553.3193 | 553.3165 | C33H46O7 | 523, 345, 97, 84, 73 | Unknown | nq | |
| 29 | 21.16 | 455.3539 | 455.3525 | C30H48O3 | 407, 377 | Oleanolic acid | 0.23 ± 0.05 | [31] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faraone, I.; Rai, D.K.; Russo, D.; Chiummiento, L.; Fernandez, E.; Choudhary, A.; Milella, L. Antioxidant, Antidiabetic, and Anticholinesterase Activities and Phytochemical Profile of Azorella glabra Wedd. Plants 2019, 8, 265. https://doi.org/10.3390/plants8080265
Faraone I, Rai DK, Russo D, Chiummiento L, Fernandez E, Choudhary A, Milella L. Antioxidant, Antidiabetic, and Anticholinesterase Activities and Phytochemical Profile of Azorella glabra Wedd. Plants. 2019; 8(8):265. https://doi.org/10.3390/plants8080265
Chicago/Turabian StyleFaraone, Immacolata, Dilip K. Rai, Daniela Russo, Lucia Chiummiento, Eloy Fernandez, Alka Choudhary, and Luigi Milella. 2019. "Antioxidant, Antidiabetic, and Anticholinesterase Activities and Phytochemical Profile of Azorella glabra Wedd" Plants 8, no. 8: 265. https://doi.org/10.3390/plants8080265
APA StyleFaraone, I., Rai, D. K., Russo, D., Chiummiento, L., Fernandez, E., Choudhary, A., & Milella, L. (2019). Antioxidant, Antidiabetic, and Anticholinesterase Activities and Phytochemical Profile of Azorella glabra Wedd. Plants, 8(8), 265. https://doi.org/10.3390/plants8080265