Chenopodium album L. and Sisymbrium officinale (L.) Scop.: Phytochemical Content and In Vitro Antioxidant and Anti-Inflammatory Potential
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Profile
2.2. Antioxidant Activity
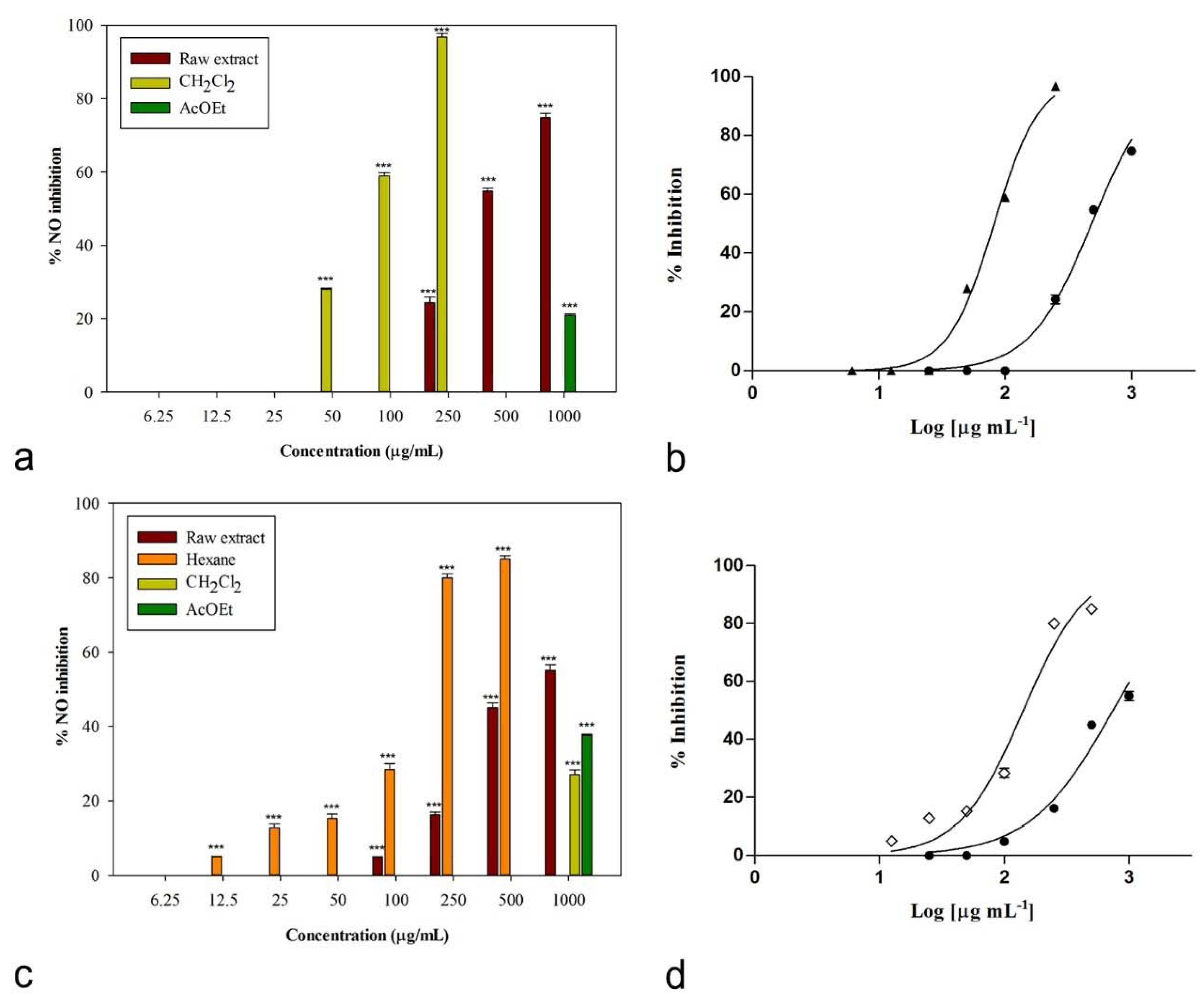
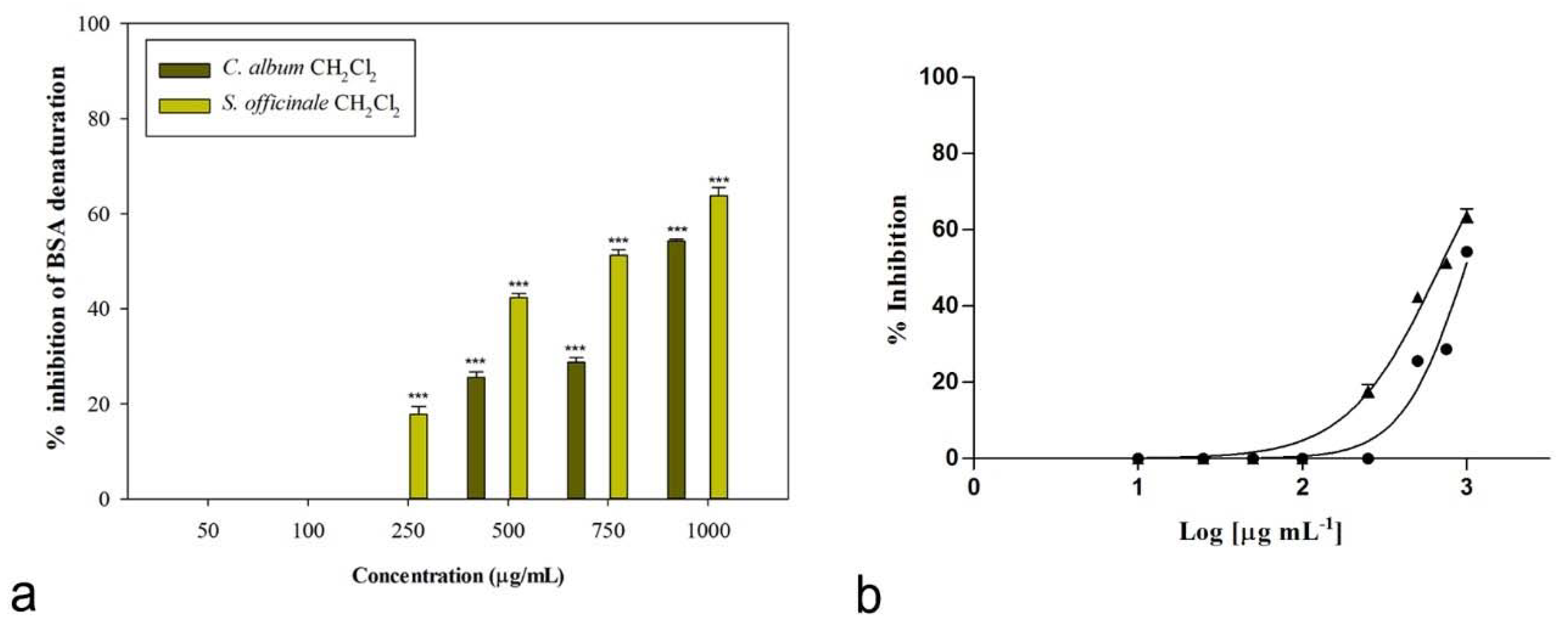
2.3. Anti-Inflammatory and Anti-Arthritic Potential
3. Materials and Methods
3.1. Chemicals
3.2. Extraction Procedure
3.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analyses
3.4. Total Phenolic Content and Flavonoid Content
3.5. High-Performance Thin Layer Chromatography (HPTLC) Analyses
3.6. Free Radical Scavenging Activity (FRSA) Assay
3.7. β-Carotene Bleaching Test
3.8. Microsomal Suspensions
3.8.1. Addition of Extracts to Microsomes
3.8.2. Malondialdehyde Formation
3.9. Nitric Oxide Production Inhibition
3.10. Anti-Arthritic Potential
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nishimura, E.; Suzaki, E.; Irie, M.; Nagashima, H.; Hirose, T. Architecture and growth of an annual plant Chenopodium album in different light climates. Ecol. Res. 2010, 25, 383–393. [Google Scholar] [CrossRef]
- Pérez, S.G.; Zavala, M.S.; Arias, L.G.; Ramos, M.L. Anti-inflammatory activity of some essential oils. J. Essent. Oil Res. 2011, 23, 38–44. [Google Scholar] [CrossRef]
- Nelly, A.; Annick, D.D.; Frederic, D. Plants used as remedies antirheumatic and antineuralgic in the traditional medicine of Lebanon. J. Ethnopharmacol. 2008, 120, 315–334. [Google Scholar]
- Bianco, V.V.; Santamaria, P.; Elia, A. Nutritional value and nitrate content in edible wild species used in southern Italy. III International Symposium Diversification of Vegetable Crops. Acta Hortic. 1996, 467, 71–90. [Google Scholar]
- Calcinoni, O. Sisymbrium “Singers’ Plant” Efficacy in Reducing Perceived Vocal Tract Disability. J. Otolaryngol. ENT Res. 2017, 8, 00243. [Google Scholar] [CrossRef]
- Guarise, M.; Borgonovo, G.; Bassoli, A.; Ferrante, A. Evaluation of two wild populations of Hedge Mustard (Sisymbrium officinale (L.) Scop.) as a potential leafy vegetable. Horticulturae 2019, 5, 13. [Google Scholar] [CrossRef]
- Di Sotto, A.; Vitalone, A.; Nicoletti, M.; Piccin, A.; Mazzanti, G. Pharmacological and phytochemical study on a Sisymbrium officinale Scop. extract. J. Ethnopharmacol. 2010, 127, 731–736. [Google Scholar] [CrossRef]
- Dogan, Y.; Baslar, S.; Ay, G.; Mert, H.H. The use of wild edible plants in western and central Anatolia (Turkey). Econ. Bot. 2004, 58, 684–690. [Google Scholar] [CrossRef]
- Asfaw, Z.; Tadesse, M. Prospects for sustainable use and development of wild food plants in Ethiopia. Econ. Bot. 2001, 55, 47–62. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Jamous, R.M.; Al-Shafie’, J.H.; Elgharabah, W.A.; Kherfan, F.A.; Qarariah, K.H.; Khdair, I.S.; Soos, I.M.; Musleh, A.A.; Isa, B.A.; et al. Traditional knowledge of wild edible plants used in Palestine (Northern West Bank): A comparative study. J. Ethnobiol. Ethnomed. 2008, 4, 13. [Google Scholar] [CrossRef]
- Garnatje, T.; Peñuelas, J.; Vallès, J. Ethnobotany, phylogeny, and ‘omics’ for human health and food security. Trends Plant Sci. 2017, 22, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Vallès, J.; Garnatje, T. A vindication of ethnobotany: Between natural and social science. Mètode Sci. Stud. J. 2016, 6, 22–27. [Google Scholar] [CrossRef]
- Arora, S.K.; Itankar, P.R.; Verma, P.R.; Bharne, A.P.; Kokare, D.M. Involvement of NFκB in the antirheumatic potential of Chenopodium album L. aerial parts extracts. J. Ethnopharmacol. 2014, 155, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Politi, M.; Braca, A.; Altinier, G.; Sosa, S.; Ndjoko, K.; Wolfender, J.L.; Hostettmann, K.; Jimenez-Barbero, J. Different approaches to study the traditional remedy of ‘hierba del canto’, Sisymbrium officinale (L.) Scop. Bol. Latinoam. Caribe Plantas Med. Aromát. 2008, 7, 30–37. [Google Scholar]
- Marrelli, M.; Cristaldi, B.; Menichini, F.; Conforti, F. Inhibitory effects of wild dietary plants on lipid peroxidation and on the proliferation of human cancer cells. Food Chem. Toxicol. 2015, 86, 16–24. [Google Scholar] [CrossRef]
- Marrelli, M.; Conforti, F.; Araniti, F.; Casacchia, T.; Statti, G. Seasonal and environmental variability of non-cultivated edible Cichorioideae (Asteraceae). Plant Biosyst. 2018, 152, 759–766. [Google Scholar] [CrossRef]
- Marrelli, M.; La Grotteria, S.; Araniti, F.; Conforti, F. Investigation of the potential health benefits as lipase inhibitor and antioxidant of Leopoldia comosa (L.) Parl.: Variability of chemical composition of wild and cultivated bulbs. Plant Foods Hum. Nutr. 2017, 72, 274–279. [Google Scholar] [CrossRef]
- Conforti, F.; Perri, V.; Menichini, F.; Marrelli, M.; Uzunov, D.; Statti, G.A.; Menichini, F. Wild Mediterranean dietary plants as inhibitors of pancreatic lipase. Phytother. Res. 2012, 26, 600–604. [Google Scholar] [CrossRef]
- Nicoletti, M.; Petitto, V.; Gallo, F.R.; Multari, G.; Federici, E.; Palazzino, G. The modern analytical determination of botanicals and similar novel natural products by the HPTLC fingerprint approach. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Oxford, UK, 2012; pp. 217–258. [Google Scholar]
- Trombino, S.; Serini, S.; Di Nicuolo, F.; Celleno, L.; Andò, S.; Picci, N.; Palozza, P. Antioxidant effect of ferulic acid in isolated membranes and intact cells: Synergistic interactions with alpha-tocopherol, beta-carotene, and ascorbic acid. J. Agric. Food Chem. 2004, 52, 2411–2420. [Google Scholar] [CrossRef]
- Serini, S.; Cassano, R.; Corsetto, P.A.; Rizzo, A.M.; Calviello, G.; Trombino, S. Omega-3 PUFA loaded in resveratrol-based solid lipid nanoparticles: Physicochemical properties and antineoplastic activities in human colorectal cancer cells in vitro. Int. J. Mol. Sci. 2018, 19, 586. [Google Scholar] [CrossRef]
- Trombino, S.; Serini, S.; Cassano, R.; Calviello, G. Xanthan gum-based materials for omega-3 PUFA delivery: Preparation, characterization and antineoplastic activity evaluation. Carb. Polym. 2019, 208, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Gupta, R.K. Screening of nutritional, phytochemical, antioxidant and antibacterial activity of Chenopodium album (Bathua). J. Pharmacogn. Phytochem. 2014, 3, 1–9. [Google Scholar]
- Chludil, H.D.; Corbino, G.B.; Leicach, S.R. Soil quality effects on Chenopodium album flavonoid content and antioxidant potential. J. Agric. Food Chem. 2008, 56, 5050–5056. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, A.; Janiak, V.; Dürr, C.M.; Lüdeke, S.; Trachsel, E.; Heinrich, M. In vitro antioxidant activity of non-cultivated vegetables of ethnic Albanians in southern Italy. Phytother. Res. 2002, 16, 467–473. [Google Scholar] [CrossRef]
- Chandra, S.; Chatterjee, P.; Dey, P.; Bhattacharya, S. Evaluation of in vitro anti-inflammatory activity of coffee against the denaturation of protein. Asian Pac. J. Trop. Biomed. 2012, 2, S178–S180. [Google Scholar] [CrossRef]
- Usman, L.A.; Hamid, A.A.; Muhammad, N.O.; Olawore, N.O.; Edewor, T.I.; Saliu, B.K. Chemical constituents and anti-inflammatory activity of leaf essential oil of Nigerian grown Chenopodium album L. EXCLI J. 2010, 9, 181. [Google Scholar]
- Conforti, F.; Marrelli, M.; Statti, G.; Menichini, F.; Uzunov, D.; Solimene, U.; Menichini, F. Comparative chemical composition and antioxidant activity of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) Nyman and Calamintha grandiflora (L.) Moench (Labiatae). Nat. Prod. Res. 2012, 26, 91–97. [Google Scholar]
- Menichini, G.; Alfano, C.; Provenzano, E.; Marrelli, M.; Statti, G.A.; Somma, F.; Menichini, F.; Conforti, F. Fig latex (Ficus carica L. cultivar Dottato) in combination with UV irradiation decreases the viability of A375 melanoma cells in vitro. Anticancer Agents Med. Chem. 2012, 12, 959–965. [Google Scholar] [CrossRef]
- Marrelli, M.; Menichini, F.; Conforti, F. A comparative study of Zingiber officinale Roscoe pulp and peel: Phytochemical composition and evaluation of antitumour activity. Nat. Prod. Res. 2015, 29, 2045–2049. [Google Scholar] [CrossRef]
- Menichini, G.; Alfano, C.; Marrelli, M.; Toniolo, C.; Provenzano, E.; Statti, G.A.; Nicoletti, M.; Menichini, F.; Conforti, F. Hypericum perforatum L. subsp. perforatum induces inhibition of free radicals and enhanced phototoxicity in human melanoma cells under ultraviolet light. Cell Prolif. 2013, 46, 193–202. [Google Scholar]
- Conforti, F.; Marrelli, M.; Statti, G.; Menichini, F. Antioxidant and cytotoxic activities of methanolic extract and fractions from Senecio gibbosus subsp. gibbosus (GUSS) DC. Nat. Prod. Res. 2006, 20, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Loizzo, M.R.; Marrelli, M.; Menichini, F.; Statti, G.A.; Uzunov, D.; Menichini, F. Quantitative determination of Amaryllidaceae alkaloids from Galanthus reginae-olgae subsp. vernalis and in vitro activities relevant for neurodegenerative diseases. Pharm. Biol. 2010, 48, 2–9. [Google Scholar] [PubMed]
- Tatum, V.L.; Changoit, C.; Chow, C.K. Measurement of malondialdehyde by HPLC with fluorescence detection. Lipids 1990, 25, 226–229. [Google Scholar] [CrossRef]
- Ohta, T.; Nakano, T.; Egashira, Y.; Sanada, H. Antioxidant activity of ferulic acid beta-glucuronide in the LDL oxidation system. Biosci. Biotechnol. Biochem. 1997, 61, 1942–1943. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Conforti, F.; Marrelli, M.; Menichini, F.; Tundis, R.; Statti, G.A.; Solimene, U.; Menichini, F. Chemical composition and protective effect of oregano (Origanum heracleoticum L.) ethanolic extract on oxidative damage and on inhibition of NO in LPS-stimulated RAW 264.7 macrophages. J. Enzyme Inhib. Med. Chem. 2011, 26, 404–411. [Google Scholar] [CrossRef]
- Marrelli, M.; Cachet, X.; Conforti, F.; Sirianni, R.; Chimento, A.; Pezzi, V.; Michel, S.; Statti, G.A.; Menichini, F. Synthesis of a new bis (indolyl) methane that inhibits growth and induces apoptosis in human prostate cancer cells. Nat. Prod. Res. 2013, 27, 2039–2045. [Google Scholar] [CrossRef]
- Palit, P.; Mukherjee, D.; Mahanta, P.; Shadab, M.; Ali, N.; Roychoudhury, S.; Asad, M.; Mandal, S.C. Attenuation of nociceptive pain and inflammatory disorders by total steroid and terpenoid fraction of Euphorbia tirucalli Linn root in experimental in vitro and in vivo model. Inflammopharmacology 2018, 26, 235–250. [Google Scholar] [CrossRef]
- Kumar, S.; Bajwa, B.S.; Kuldeep, S.; Kalia, A.N. Anti-inflammatory activity of herbal plants: A review. Int. J. Adv. Pharm. Biol. Chem. 2013, 2, 272–281. [Google Scholar]
- Shah, B.N.; Seth, A.K.; Maheshwari, K.M. A review on medicinal plants as a source of anti-inflammatory agents. Res. J. Med. Plant 2011, 5, 101–115. [Google Scholar] [CrossRef]
- Kushagra, N.; Singh, M.K.; Dhansay, D.; Verma, V.K.; Tripathi, D.K. Anti-inflammatory activity and chemo profile of plants used in traditional medicine: A review. J. Chem. Pharm. Res. 2010, 2, 122–130. [Google Scholar]
- Rout, S.P.; Choudary, K.A.; Kar, D.M.; Das, L.; Jain, A. Plants in traditional medicinal system-future source of new drugs. Int. J. Pharm. Pharm. Sci. 2009, 1, 1–23. [Google Scholar]
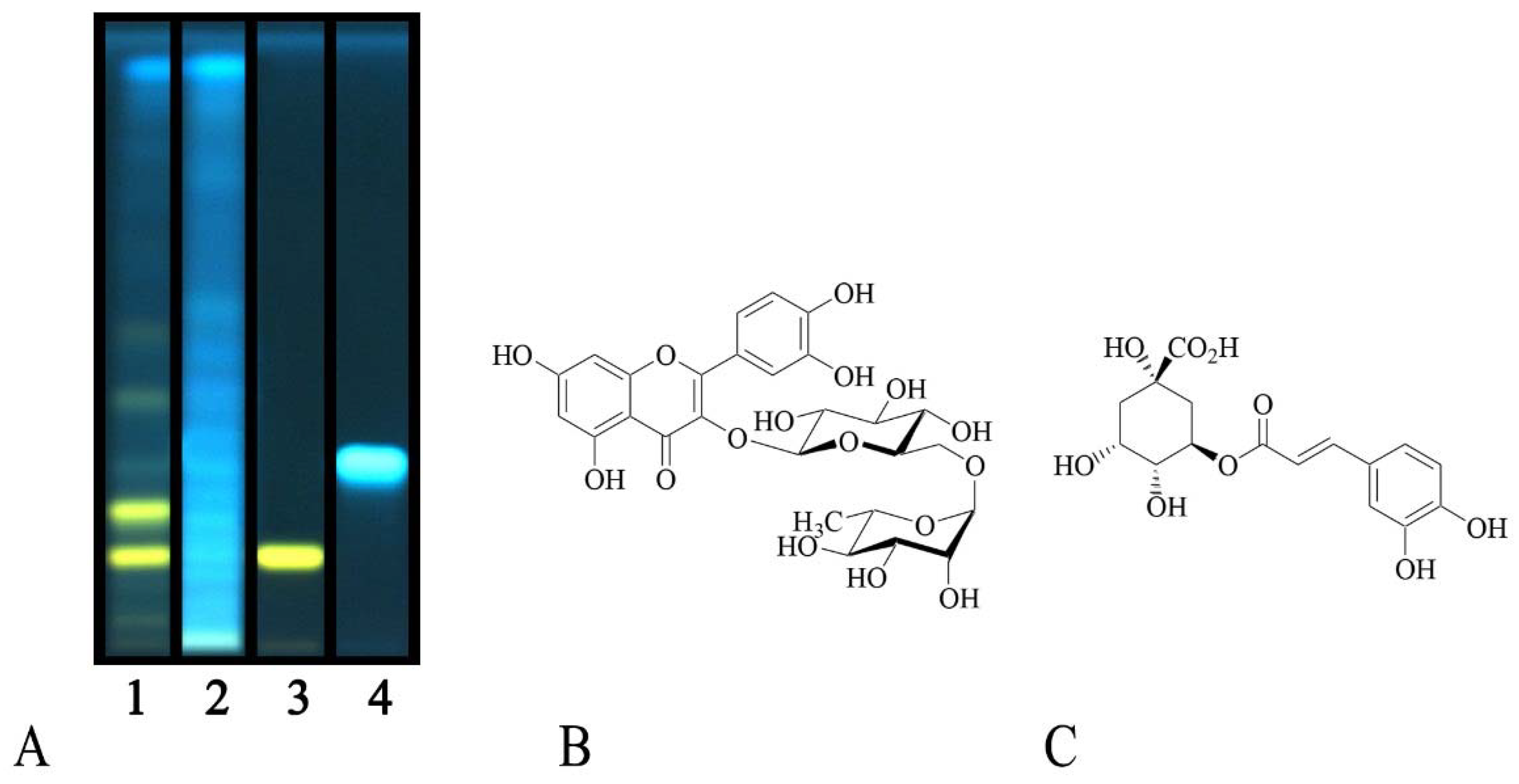


| Botanical Name | Family | Voucher Number | Yield (%) | TP 1 | TF 2 |
|---|---|---|---|---|---|
| Chenopodium album L. | Amaranthaceae | 26247 | 23.2 | 12.8 ± 1.6 | 0.77 ± 0.01 |
| Sisymbrium officinale (L.) Scop. | Brassicaceae | 26236 | 10.6 | 8.1 ± 0.1 | 0.50 ± 0.01 |
| Compound 1 | Rt 2 | RAP 3 | |
|---|---|---|---|
| C. album L. | S. officinale (L.) Scop. | ||
| Fatty Acids | |||
| Caprylic acid | 10.106 | Tr 4 | Tr |
| Pelargonic acid | 12.00 | - | 0.1 |
| Lauric acid | 15.044 | Tr | 0.1 |
| Myristic acid | 16.799 | 0.7 | 0.3 |
| 4,8,12-Trimethyltridecanoic acid | 16.930 | - | 0.1 |
| Pentadecanoic acid | 17.616 | Tr | 0.2 |
| Tetradecanoic acid, 5,9,13-trimethyl- | 17.708 | Tr | - |
| Palmitelaidic acid | 18.096 | Tr | - |
| Palmitic acid | 18.159 | 15.7 | 10.0 |
| Oleic acid | 18.531 | Tr | - |
| Margaric acid | 18.902 | 0.3 | - |
| Isooleic acid | 19.456 | 0.8 | - |
| Stearic acid | 19.634 | 1.3 | 1.0 |
| Arachidic acid | 20.988 | 0.8 | 1.0 |
| Linoleic acid | 21.051 | - | 0.5 |
| Behenic acid | 22.274 | 0.9 | 0.8 |
| Tricosylic acid | 22.966 | Tr | 0.4 |
| Lignoceric acid | 23.731 | - | 0.6 |
| Pentacosylic acid | 24.696 | - | 0.3 |
| Cerotic acid | 25.829 | - | 0.9 |
| Montanic acid | 28.847 | - | tr |
| Terpenes | |||
| Dihydroactinidiolide | 14.987 | 0.9 | 0.4 |
| Neophytadiene | 17.473 | 0.7 | - |
| Phytosterols | |||
| β-Sitosterol | 33.882 | 3.2 | - |
| Compound 1 | Rt 2 | RAP 3 | |
|---|---|---|---|
| C. album L. | S. officinale (L.) Scop. | ||
| Phenol | 7.677 | - | Tr 4 |
| Benzoic acid | 10.758 | 1.0 | - |
| Methylethylmaleimide | 11.575 | 1.4 | 0.3 |
| 2,4-Di-tert-butylphenol | 14.621 | - | Tr |
| Loliolide | 17.073 | 1.7 | - |
| Coniferyl alcohol | 17.250 | - | 1.2 |
| Ferulic acid | 17.565 | - | 0.7 |
| Species | Sample | IC50 (µg/mL) | ||
|---|---|---|---|---|
| DPPH Test | β-carotene Bleaching Test | |||
| 30 min | 60 min | |||
| C. album L. | Raw extract | 172.70 ± 2.18 d | 60.51 ± 2.34 e | ˃100 |
| n-Hexane | ˃1000 | ˃100 | ˃100 | |
| CH2Cl2 | 435.60 ± 12.97 f | ˃100 | ˃100 | |
| EtOAc | 140.40 ± 4.36 c | 12.07 ± 0.04 b | 38.03 ± 1.88 d | |
| H2O | ˃1000 | ˃100 | ˃100 | |
| S. officinale (L.) Scop. | Raw extract | 143.00 ± 2.61 c | 2.61 ± 0.06 a,b | 8.53 ± 0.27 b |
| n-Hexane | ˃1000 | ˃100 | ˃100 | |
| CH2Cl2 | ˃1000 | 61.02 ± 2.31 e | ˃100 | |
| EtOAc | 60.11 ± 1.79 b | 12.62 ± 0.75 b | 30.49 ± 1.17 c | |
| H2O | 262.9 ± 0.93 e | ˃100 | ˃100 | |
| Ascorbic acid 1 | 2.00 ± 0.01 a | - | - | |
| Propyl gallate 1 | - | 1.00 ± 0.02 a | 1.00 ± 0.02 a | |
| Species | Sample | IC50 (µg/mL) | |
|---|---|---|---|
| NO Inhibition | BSA Denaturation Inhibition | ||
| C. album L. | Raw extract | 483.2 ± 6.4 c | n.a. |
| n-Hexane | n.a. | n.a. | |
| CH2Cl2 | 81.7 ± 0.9 a | 975.6 ± 5.5 c | |
| EtOAc | n.a. | n.a. | |
| H2O | n.a. | n.a. | |
| S. officinale (L.) Scop. | Raw extract | 734.4 ± 21.2 d | n.a. |
| n-Hexane | 142.0 ± 5.5 b | n.a. | |
| CH2Cl2 | n.a. | 680.9 ± 13.2 b | |
| EtOAc | n.a. | n.a. | |
| H2O | n.a. | n.a. | |
| Indomethacin 1 | 58.0 ± 0.9 a | - | |
| L-NAME 1 | 45.9 ± 0.5 a | - | |
| Diclofenac 1 | - | 15.73 ± 0.2 a | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amodeo, V.; Marrelli, M.; Pontieri, V.; Cassano, R.; Trombino, S.; Conforti, F.; Statti, G. Chenopodium album L. and Sisymbrium officinale (L.) Scop.: Phytochemical Content and In Vitro Antioxidant and Anti-Inflammatory Potential. Plants 2019, 8, 505. https://doi.org/10.3390/plants8110505
Amodeo V, Marrelli M, Pontieri V, Cassano R, Trombino S, Conforti F, Statti G. Chenopodium album L. and Sisymbrium officinale (L.) Scop.: Phytochemical Content and In Vitro Antioxidant and Anti-Inflammatory Potential. Plants. 2019; 8(11):505. https://doi.org/10.3390/plants8110505
Chicago/Turabian StyleAmodeo, Valentina, Mariangela Marrelli, Veronica Pontieri, Roberta Cassano, Sonia Trombino, Filomena Conforti, and Giancarlo Statti. 2019. "Chenopodium album L. and Sisymbrium officinale (L.) Scop.: Phytochemical Content and In Vitro Antioxidant and Anti-Inflammatory Potential" Plants 8, no. 11: 505. https://doi.org/10.3390/plants8110505
APA StyleAmodeo, V., Marrelli, M., Pontieri, V., Cassano, R., Trombino, S., Conforti, F., & Statti, G. (2019). Chenopodium album L. and Sisymbrium officinale (L.) Scop.: Phytochemical Content and In Vitro Antioxidant and Anti-Inflammatory Potential. Plants, 8(11), 505. https://doi.org/10.3390/plants8110505









