The Yes and No of the Ethylene Involvement in Abscission
Abstract
1. Introduction
2. The Regulatory Role of Ethylene at the Abscission Zone
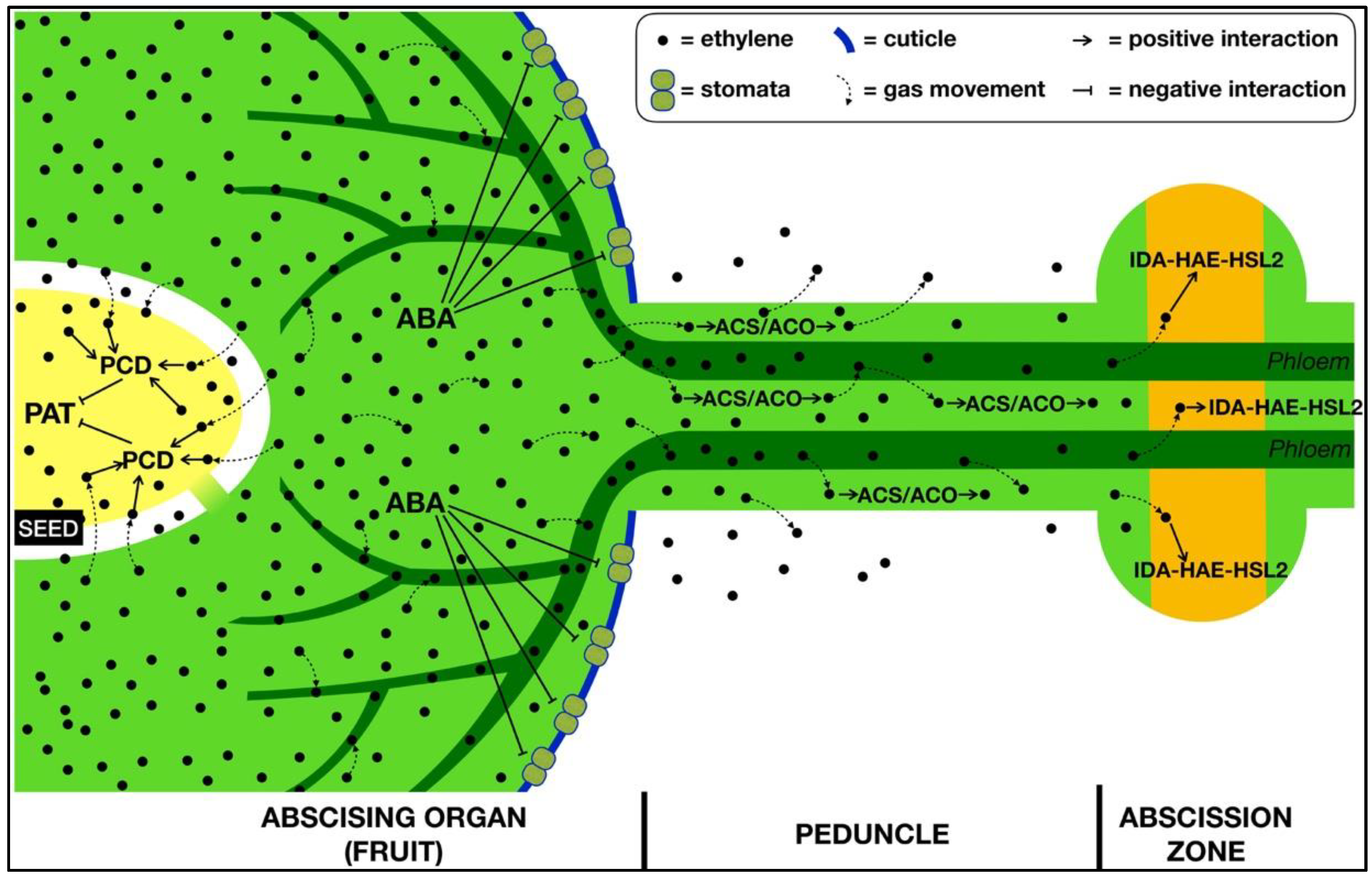
3. The Role of Ethylene within the Abscising Organ
4. The Transmission of the Signal from the Organ to the AZ
5. Conclusions and Perspectives
- Validation of the IDA-HAE-HSL2 pathway;
- Validation of the role of ethylene inside the AZs;
- Further investigations about the role of ethylene within different types of abscising organs/parts;
- Validation of the hypothesis of the propagation of the abscission signal from the organ to the AZ.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bergey, D.R.; Orozco-Cardenas, M.; de Moura, D.S.; Ryan, C.A. A wound- and systemin-inducible polygalacturonase in tomato leaves. Proc. Natl. Acad. Sci. USA 1999, 96, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Byers, R.E.; Carbaugh, D.H. Effect of chemical thinning sprays on apple fruit set. HortTechnology 1991, 1, 41–48. [Google Scholar] [CrossRef]
- Sawicki, M.; Barka, E.A.; Clement, C.; Vaillant-Gaveau, N.; Jacquard, C. Cross-talk between environmental stresses and plant metabolism during reproductive organ abscission. J. Exp. Bot. 2015, 66, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.E. Cutting loose. Abscission and dehiscence in Arabidopsis. Plant Physiol. 2001, 126, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.E.; Whitelaw, C.A. Signals in abscission. New Phytol. 2001, 151, 323–329. [Google Scholar] [CrossRef]
- Roberts, J.A.; Elliott, K.A.; Gonzalez-Carranza, Z.H. Abscission, dehiscence and other cell separation processes. Annu. Rev. Plant Biol. 2002, 53, 131–158. [Google Scholar] [CrossRef]
- Kim, J. Four shades of detachment: Regulation of floral organ abscission. Plant Signal. Behav. 2014, 9, e976154. [Google Scholar] [CrossRef] [PubMed]
- Patharkar, O.R.; Walker, J.C. Advances in abscission signaling. J. Exp. Bot. 2018, 69, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Meir, S.; Philosoph-Hadas, S.; Riov, J.; Tucker, M.L.; Patterson, S.E.; Roberts, J.A. Re-evaluation of the ethylene-dependent and -independent pathways in the regulation of floral and organ abscission. J. Exp. Bot. 2019, 70, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Begum, D.; Chuang, H.W.; Budiman, M.A.; Szymkowiak, E.J.; Irish, E.E.; Wing, R.A. JOINTLESS is a MADS-box gene controlling tomato flower abscission zone development. Nature 2000, 406, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.B.; Osborne, D.J. Ethylene, the natural regulator of leaf abscission. Nature 1970, 225, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Abeles, F.; Morgan, P.; Saltveit, M.J. Ethylene in Plant Biology, 2nd ed.; Academic Press: San Diego, CA, USA, 1992. [Google Scholar]
- Hall, W. Evidence on the auxin–ethylene balance hypothesis of foliar abscission. Bot. Gazette 1952, 113, 310–322. [Google Scholar] [CrossRef]
- Meir, S.; Sundaresan, S.; Riov, J.; Agarwal, I.; Philosoph-Hadas, S. Role of auxin depletion in abscission control. Stewart Postharvest Rev. 2015, 11, 1–15. [Google Scholar]
- Basu, M.M.; González-Carranza, Z.H.; Azam-Ali, S.; Tang, S.; Shahid, A.A.; Roberts, J.A. The manipulation of auxin in the abscission zone cells of Arabidopsis flowers reveals that indoleacetic acid signaling is a prerequisite for organ shedding. Plant. Physiol. 2013, 162, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Beyer, E.M.; Morgan, P.W. Abscission: The role of ethylene modification of auxin transport. Plant. Physiol. 1971, 48, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Riov, J.; Goren, R. Effect of ethylene on auxin transport and metabolism in midrib sections in relation to leaf abscission of woody plants. Plant Cell Environ. 1979, 2, 83–89. [Google Scholar] [CrossRef]
- Kuang, J.F.; Wu, J.Y.; Zhong, H.Y.; Li, C.Q.; Chen, J.Y.; Lu, W.J.; Li, J.G. Carbohydrate stress affecting fruitlet abscission and expression of genes related to auxin signal transduction pathway in litchi. Int. J. Mol. Sci. 2012, 13, 16084–16103. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Ge, T.; Zhang, J.; Pan, X.; Ma, Y.; Yi, S.; Zheng, Y. The molecular events of IAA inhibiting citrus fruitlet abscission revealed by digital gene expression profiling. Plant. Physiol. Biochem. 2018, 130, 192–204. [Google Scholar] [CrossRef]
- Abeles, F.B.; Rubinstein, B. Regulation of ethylene evolution and leaf abscission by auxin. Plant. Physiol. 1964, 39, 963–969. [Google Scholar] [CrossRef]
- Morgan, P.W.; Hall, W.C. Accelerated release of ethylene by cotton following application of indolyl-3-acetic acid. Nature 1964, 201, 99. [Google Scholar] [CrossRef]
- Kućko, A.; Wilmowicz, E.; Ostrowski, M. Spatio-temporal IAA gradient is determined by interactions with ET and governs flower abscission. J. Plant. Physiol. 2019, 236, 51–60. [Google Scholar]
- Bleecker, A.B.; Patterson, S.E. Last exit: Senescence, abscission, and meristem arrest in Arabidopsis. Plant Cell 1997, 9, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Butenko, M.A.; Patterson, S.E.; Grini, P.E.; Stenvik, G.E.; Amundsen, S.S.; Mandal, A.; Aalen, R.B. INFLORESCENCE DEFICIENT IN ABSCISSION controls floral organ abscission in Arabidopsis and identifies a novel family of putative ligands in plants. Plant Cell 2003, 15, 2296–2307. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.E.; Bleecker, A.B. Ethylene-dependent and -independent processes associated with floral organ abscission in Arabidopsis. Plant. Physiol. 2004, 134, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Jinn, T.L.; Stone, J.M.; Walker, J.C. HAESA, an Arabidopsis leucine-rich repeat receptor kinase, controls floral organ abscission. Genes Dev. 2000, 14, 108–117. [Google Scholar] [PubMed]
- Butenko, M.A.; Stenvik, G.E.; Alm, V.; Saether, B.; Patterson, S.E.; Aalen, R.B. Ethylene-dependent and -independent pathways controlling floral abscission are revealed to converge using promoter::reporter gene constructs in the ida abscission mutant. J. Exp. Bot. 2006, 57, 3627–3637. [Google Scholar] [CrossRef] [PubMed]
- Stenvik, G.E.; Butenko, M.A.; Urbanowicz, B.R.; Rose, J.K.; Aalen, R.B. Overexpression of Inflorescence Deficient in Abscission activates cell separation in vestigial abscission zones in Arabidopsis. Plant Cell 2006, 18, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Stø, I.M.; Orr, R.J.S.; Fooyontphanich, K.; Jin, X.; Knutsen, J.M.B.; Fischer, U.; Tranbarger, T.J.; Nordal, I.; Aalen, R.B. Conservation of the abscission signaling peptide IDA during Angiosperm evolution: Withstanding genome duplications and gain and loss of the receptors HAE/HSL2. Front. Plant Sci. 2015, 6, 931. [Google Scholar]
- Cho, S.K.; Larue, C.T.; Chevalier, D.; Wang, H.; Jinn, T.L.; Zhang, S.; Walker, J.C. Regulation of floral organ abscission in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2008, 105, 15629–15634. [Google Scholar] [CrossRef]
- Niederhuth, C.E.; Patharkar, O.R.; Walker, J.C. Transcriptional profiling of the Arabidopsis abscission mutant hae hsl2 by RNA-Seq. BMC Genom. 2013, 14, 37. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Tang, J.; Li, B.; de Oliveira, M.V.V.; Chai, J.; He, P.; Shan, L. Ligand-induced receptor-like kinase complex regulates floral organ abscission in Arabidopsis. Cell Rep. 2016, 14, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Ying, P.; Li, C.; Liu, X.; Xia, R.; Zhao, M.; Li, J. Identification and molecular characterization of an IDA-like gene from litchi, LcIDL1, whose ectopic expression promotes floral organ abscission in Arabidopsis. Sci. Rep. 2016, 6, 37135. [Google Scholar] [CrossRef] [PubMed]
- Wilmowicz, E.; Frankowski, K.; Kućko, A.; Świdziński, M.; de Dios Alché, J.; Nowakowska, A.; Kopcewicz, J. The influence of abscisic acid on the ethylene biosynthesis pathway in the functioning of the flower abscission zone in Lupinus luteus. J. Plant. Physiol. 2016, 206, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Dotson, B.; Rey, C.; Lindsey, J.; Bleecker, A.B.; Binder, B.M.; Patterson, S.E. New clothes for the jasmonic acid receptor COI1: Delayed abscission, meristem arrest and apical dominance. PLoS ONE 2013, 8, e60505. [Google Scholar] [CrossRef] [PubMed]
- Liljegren, S.J.; Leslie, M.E.; Darnielle, L.; Lewis, M.W.; Taylor, S.M.; Luo, R.; Geldner, N.; Chory, J.; Randazzo, P.A.; Yanofsky, M.F.; et al. Regulation of membrane trafficking and organ separation by the NEVERSHED ARF-GAP protein. Development 2009, 136, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Liljegren, S. Organ abscission: Exit strategies require signals and moving traffic. Curr. Opin. Plant. Biol. 2012, 15, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Gubert, C.M.; Liljegren, S.J. HAESA and HAESA-LIKE2 activate organ abscission downstream of NEVERSHED and EVERSHED in Arabidopsis flowers. Plant. Signal. Behav. 2014, 9, e29115. [Google Scholar] [CrossRef]
- Bar-Dror, T.; Dermastia, M.; Kladnik, A.; Znidaric, M.T.; Novak, M.P.; Meir, S.; Burd, S.; Philosoph-Hadas, S.; Ori, N.; Sonego, L.; et al. Programmed cell death occurs asymmetrically during abscission in tomato. Plant Cell 2011, 23, 4146–4163. [Google Scholar] [CrossRef]
- Lee, Y.; Yoon, T.H.; Lee, J.; Jeon, S.Y.; Lee, J.H.; Lee, M.K.; Chen, H.; Yun, J.; Oh, S.Y.; Wen, X.; et al. Lignin molecular brace controls precision processing of cell walls critical for surface integrity in Arabidopsis. Cell 2018, 173, 1468–1480. [Google Scholar] [CrossRef]
- Botton, A.; Eccher, G.; Forcato, C.; Ferrarini, A.; Begheldo, M.; Zermiani, M.; Moscatello, S.; Battistelli, A.; Velasco, R.; Ruperti, B. Signaling pathways mediating the induction of apple fruitlet abscission. Plant. Physiol. 2011, 155, 185–208. [Google Scholar] [CrossRef]
- Eccher, G.; Botton, A.; Dimauro, M.; Boschetti, A.; Ruperti, B.; Ramina, A. Early induction of apple fruitlet abscission is characterized by an increase of both isoprene emission and abscisic acid content. Plant. Physiol. 2013, 161, 1952–1969. [Google Scholar]
- Eccher, G.; Begheldo, M.; Boschetti, A.; Ruperti, B.; Botton, A. Role of ethylene production and ethylene-receptor expression in regulating apple fruitlet abscission. Plant. Physiol. 2015, 169, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Botton, A.; Vizzotto, G. Fruit Thinning: Advances and trends. Hortic. Rev. 2018, 46, 185–226. [Google Scholar]
- Dal Cin, V.; Danesin, M.; Boschetti, A.; Dorigoni, A.; Ramina, A. Ethylene biosynthesis and perception in apple fruitlet abscission (Malus domestica L. Borkh). J. Exp. Bot. 2005, 56, 2995–3005. [Google Scholar]
- Ruperti, B.; Bonghi, C.; Tonutti, P.; Ramina, A. Ethylene biosynthesis in peach fruitlet abscission. Plant Cell Environ. 1998, 21, 731–737. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botton, A.; Ruperti, B. The Yes and No of the Ethylene Involvement in Abscission. Plants 2019, 8, 187. https://doi.org/10.3390/plants8060187
Botton A, Ruperti B. The Yes and No of the Ethylene Involvement in Abscission. Plants. 2019; 8(6):187. https://doi.org/10.3390/plants8060187
Chicago/Turabian StyleBotton, Alessandro, and Benedetto Ruperti. 2019. "The Yes and No of the Ethylene Involvement in Abscission" Plants 8, no. 6: 187. https://doi.org/10.3390/plants8060187
APA StyleBotton, A., & Ruperti, B. (2019). The Yes and No of the Ethylene Involvement in Abscission. Plants, 8(6), 187. https://doi.org/10.3390/plants8060187





