Assessment of Primary Cell Wall Nanomechanical Properties in Internal Cells of Non-Fixed Maize Roots
Abstract
1. Introduction
2. Results and Discussion
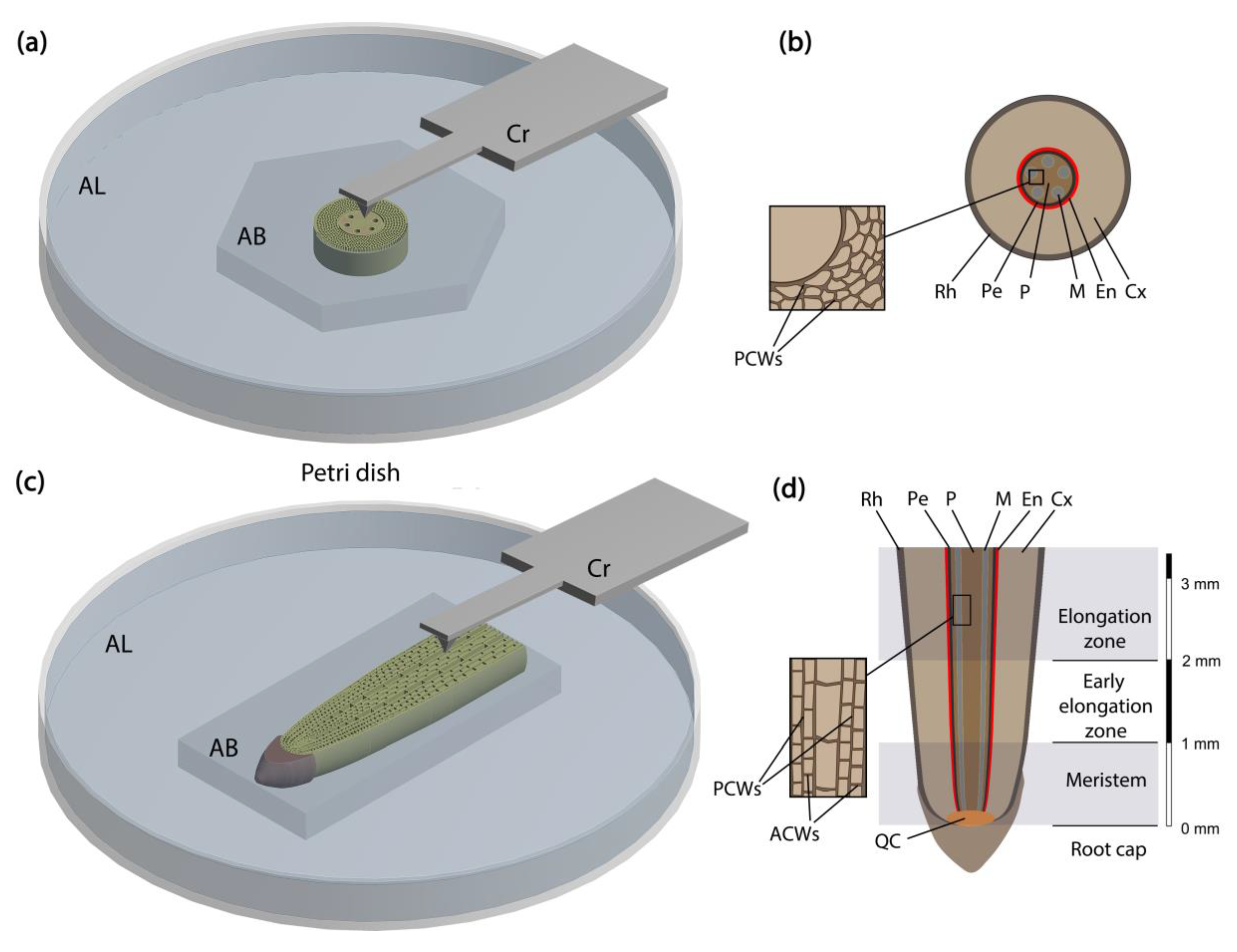
2.1. Plant Section Preparation to be Studied by AFM
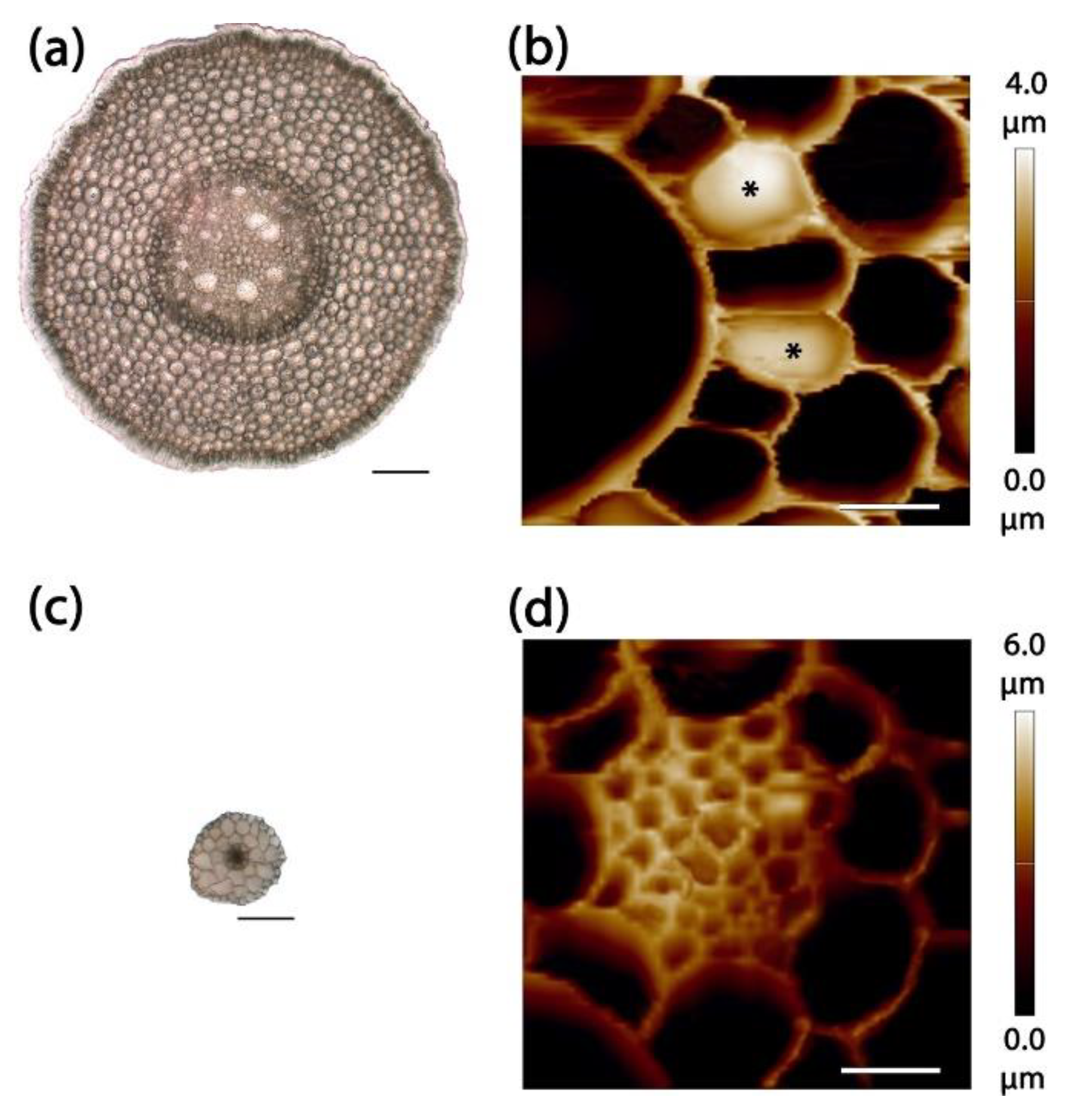
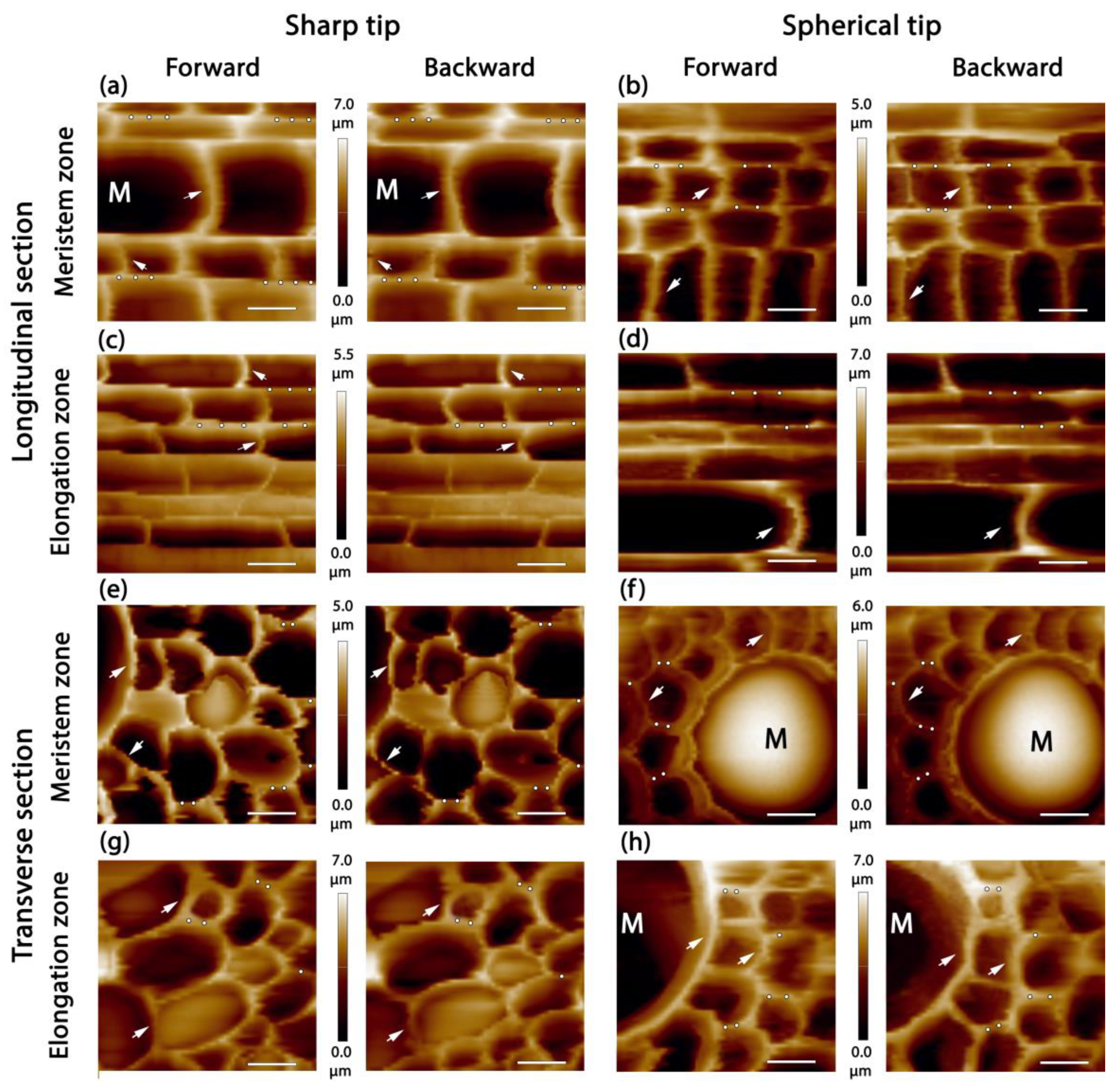
2.2. Stele Tissue Topography and Force Spectroscopy
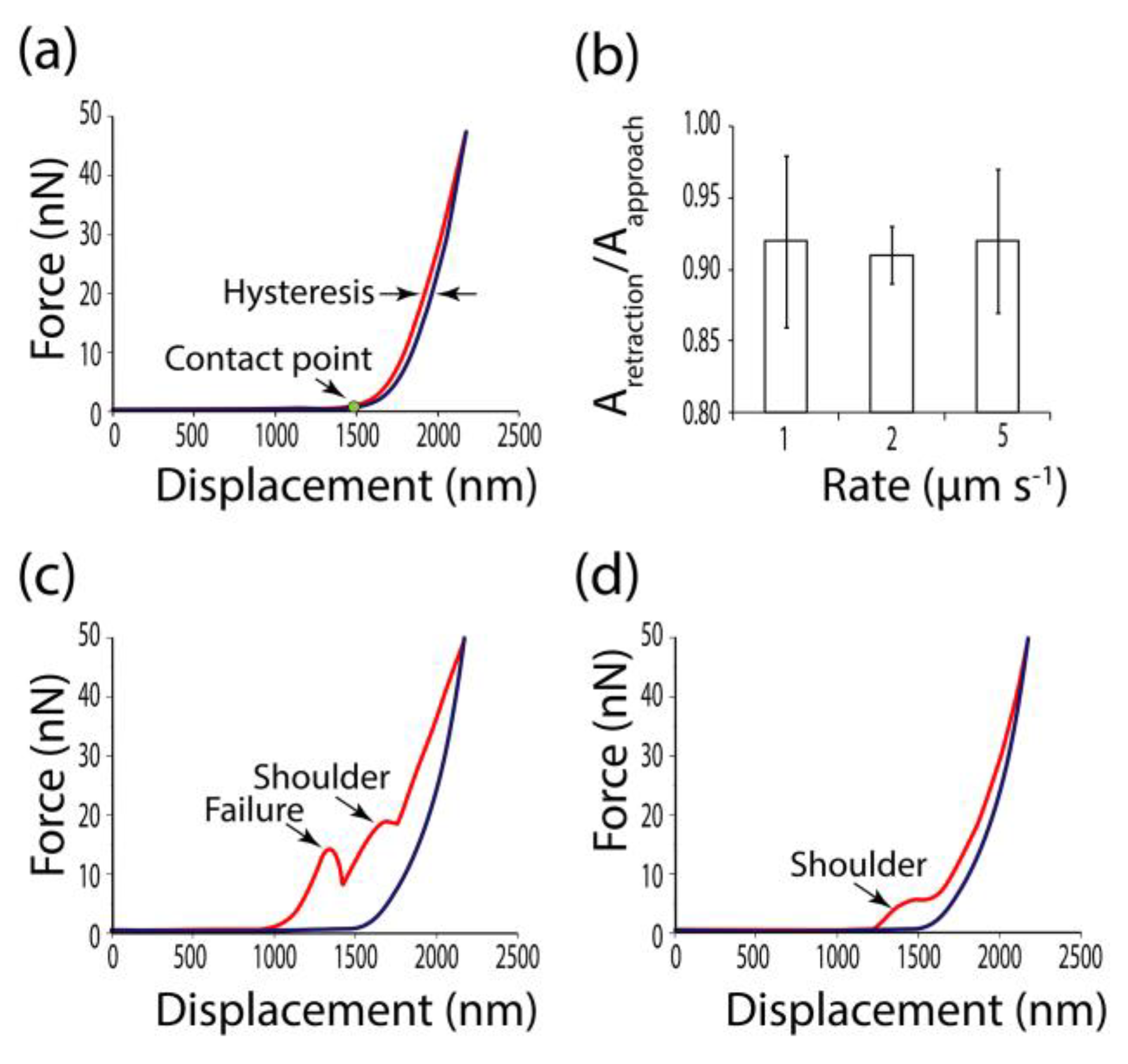
2.3. Stiffness and Elasticity of Maize Root Central Cylinder Cell Walls
2.4. Potential Limitations of the Proposed Method
3. Materials and Methods
3.1. Plant Material and Sample Preparation
3.2. Atomic Force Microscopy
3.3. Calculations
3.4. Statistics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Höfte, H.; Voxeur, A. Plant cell walls. Curr. Biol. 2017, 27, R853–R909. [Google Scholar] [CrossRef] [PubMed]
- Bidhendi, A.J.; Geitmann, A. Relating the mechanics of the primary plant cell wall to morphogenesis. J. Exp. Bot. 2016, 67, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Routier-Kierzkowska, A.L.; Smith, R.S. Measuring the mechanics of morphogenesis. Curr. Opin. Plant Biol. 2013, 16, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Peaucelle, A. AFM-based mapping of the elastic properties of cell walls: At tissue, cellular, and subcellular resolutions. J. Vis. Exp. 2014, 89, e51317. [Google Scholar] [CrossRef] [PubMed]
- Milani, P.; Gholamirad, M.; Traas, J.; Arnéodo, A.; Boudaoud, A.; Argoul, F.; Hamant, O. In vivo analysis of local wall stiffness at the shoot apical meristem in Arabidopsis using atomic force microscopy. Plant J. 2011, 67, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.N.; Chen, X.; Scotchford, C.A.; Walker, J.; Wells, D.M.; Roberts, C.J.; Everitt, N.M. Mechanical properties of epidermal cells of whole living roots of Arabidopsis thaliana: An atomic force microscopy study. Phys. Rev. E Stat. Nonlin Soft. Matter Phys. 2012, 85, 021916. [Google Scholar] [CrossRef] [PubMed]
- Digiuni, S.; Berne-Dedieu, A.; Martinez-Torres, C.; Szecsi, J.; Bendahmane, M.; Arneodo, A.; Argoul, F. Single cell wall nonlinear mechanics revealed by a multiscale analysis of AFM force-indentation curves. Biophys. J. 2015, 108, 2235–2248. [Google Scholar] [CrossRef]
- Majda, M.; Grones, P.; Sintorn, I.M.; Vain, T.; Milani, P.; Krupinski, P.; Zagórska-Marek, B.; Viotti, C.; Jönsson, H.; Mellerowicz, E.J.; et al. Mechanochemical polarization of contiguous cell walls shapes plant pavement cells. Dev. Cell 2017, 43, 290–304. [Google Scholar] [CrossRef]
- Beauzamy, L.; Derr, J.; Boudaoud, A. Quantifying hydrostatic pressure in plant cells by using indentation with an atomic force microscope. Biophys. J. 2015, 108, 2448–2456. [Google Scholar] [CrossRef]
- Xi, X.; Kim, S.H.; Tittmann, B. Atomic force microscopy based nanoindentation study of onion abaxial epidermis walls in aqueous environment. J. Appl. Phys. 2015, 117, 024703. [Google Scholar] [CrossRef]
- Radotić, K.; Roduit, C.; Simonović, J.; Hornitschek, P.; Fankhauser, C.; Mutavdžić, D.; Steinbach, G.; Dietler, G.; Kasas, S. Atomic force microscopy stiffness tomography on living Arabidopsis thaliana cells reveals the mechanical properties of surface and deep cell-wall layers during growth. Biophys. J. 2012, 103, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Yakubov, G.E.; Bonilla, M.R.; Chen, H.; Doblin, M.S.; Bacic, A.; Gidley, M.J.; Stokes, J.R. Mapping nano-scale mechanical heterogeneity of primary plant cell walls. J. Exp. Bot. 2016, 67, 2799–2816. [Google Scholar] [CrossRef] [PubMed]
- Zdunek, A.; Kurenda, A. Determination of the elastic properties of tomato fruit cells with an atomic force microscope. Sensors 2013, 13, 12175–12191. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Wang, Y.; Qiu, S.; Kang, Z.; Che, S.; Wang, G.; Huang, J. Overexpression of OsEXPA8, a root-specific gene, improves rice growth and root system architecture by facilitating cell extension. PLoS ONE 2013, 8, e75997. [Google Scholar] [CrossRef] [PubMed]
- Arnould, O.; Siniscalco, D.; Bourmaud, A.; Le Duigou, A.; Baley, C. Better insight into the nano-mechanical properties of flax fibre cell walls. Ind. Crop. Prod. 2017, 97, 224–228. [Google Scholar] [CrossRef]
- Liang, K.; Du, G.; Hosseinaei, O.; Wang, S.; Wang, H. Mechanical properties of secondary wall and compound corner middle lamella near the phenol-formaldehyde (PF) adhesive bond line measured by nanoindentation. Adv. Mat. Res. 2011, 236, 1746–1751. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, S.; Cai, Z.; Young, T.M.; Du, G.; Li, Y. A novel sample preparation method to avoid influence of embedding medium during nano-indentation. Appl. Phys. A 2013, 110, 361–396. [Google Scholar] [CrossRef]
- Eisenberg, B.R.; Mobley, B.A. Size changes in single muscle fibers during fixation and embedding. Tissue Cell 1975, 7, 383–387. [Google Scholar] [CrossRef]
- Evered, C.; Majevadia, B.; Thompson, D.S. Cell wall water content has a direct effect on extensibility in growing hypocotyls of sunflower (Helianthus annuus L.). J. Exp. Bot. 2007, 58, 3361–3371. [Google Scholar] [CrossRef][Green Version]
- Cosgrove, D.J. Re-constructing our models of cellulose and primary cell wall assembly. Curr. Opin. Plant Biol. 2014, 22, 122–131. [Google Scholar] [CrossRef]
- Braybrook, S.A. Measuring the elasticity of plant cells with atomic force microscopy. In Biophysical Methods in Cell Biology, 1st ed.; Paluch, E.K., Ed.; Academic Press (Elsevier): London, UK, 2015; Volume 125, pp. 237–254. [Google Scholar] [CrossRef]
- Kozlova, L.V.; Snegireva, A.V.; Gorshkova, T.A. Distribution and structure of mixed linkage glucan at different stages of elongation of maize root cells. Russ. J. Plant Physiol. 2012, 59, 339–347. [Google Scholar] [CrossRef]
- Baluška, F.; Kubica, S.; Hauskrecht, M. Postmitotic ‘isodiametric’ cell growth in the maize root apex. Planta 1990, 181, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Daher, F.B.; Chen, Y.; Bozorg, B.; Clough, J.; Jönsson, H.; Braybrook, S.A. Anisotropic growth is achieved through the additive mechanical effect of material anisotropy and elastic asymmetry. Elife 2018, 7, e38161. [Google Scholar] [CrossRef] [PubMed]
- Krieg, M.; Fläschner, G.; Alsteens, D.; Gaub, B.M.; Roos, W.H.; Wuite, G.J.L.; Gaub, H.E.; Gerber, C.; Dufrêne, Y.F.; Müller, D.J. Atomic force microscopy-based mechanobiology. Nat. Rev. Phys. 2019, 1, 41–57. [Google Scholar] [CrossRef]
- Schillers, H. Measuring the elastic properties of living cells. In Atomic Force Microscopy: Methods and Protocols, Methods in Molecular Biology; Santos, N.C., Carvalho, F.A., Eds.; Humana Press (Springer): New York, NY, USA, 2019; Volume 1886, pp. 291–313. [Google Scholar] [CrossRef]
- Baclayon, M.; Roos, W.H.; Wuite, G.J.L. Sampling protein form and function with the atomic force microscope. Mol. Cell Proteom. 2010, 9, 1678–1688. [Google Scholar] [CrossRef] [PubMed]
- Attwood, S.J.; Choi, Y.; Leonenko, Z. Preparation of DOPC and DPPC supported planar lipid bilayers for atomic force microscopy and atomic force spectroscopy. Int. J. Mol. Sci. 2013, 14, 3514–3539. [Google Scholar] [CrossRef]
- Guo, Y.; Roos, W.H. AFM nanoindentation experiments on protein shells: A protocol. In Atomic Force Microscopy: Methods and Protocols, Methods in Molecular Biology; Santos, N.C., Carvalho, F.A., Eds.; Humana Press (Springer): New York, NY, USA, 2019; Volume 1886, pp. 243–257. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, Y.; Li, J.; Wang, H.; Wen, S. Investigation on the nanomechanics of liposome adsorption on titanium alloys: Temperature and loading effects. Polymers 2018, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Braunsmann, C.; Schäffer, T.E. Note: Artificial neural networks for the automated analysis of force map data in atomic force microscopy. Rev. Sci. Instrum. 2014, 85, 056104. [Google Scholar] [CrossRef]
- Zerzour, R.; Kroeger, J.; Geitmann, A. Polar growth in pollen tubes is associated with spatially confined dynamic changes in cell mechanical properties. Dev. Biol. 2009, 334, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Felekis, D.; Muntwyler, S.; Beyeler, F.; Nelson, B.J. The Cellular Force Microscope (CFM): A microrobotic system for quantitating the growth mechanics of living, growing plant cells in situ. In Proceedings of the 2011 IEEE/RSJ International Conference on Intelligent Robots and Systems, San Francisco, CA, USA, 25–30 September 2011; pp. 919–924. [Google Scholar] [CrossRef]
- Erickson, R.O.; Sax, K.B. Elemental growth rate of the primary root of Zea mays. Proc. Am. Philos. Soc. 1956, 100, 487–498. [Google Scholar]
- Silk, W.K.; Walker, R.C.; Labavitch, J. Uronide deposition rates in the primary root of Zea mays. Plant Physiol. 1984, 74, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Peaucelle, A.; Braybrook, S.A.; Le Guillou, L.; Bron, E.; Kuhlemeier, C.; Höfte, H. Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr. Biol. 2011, 21, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Peaucelle, A.; Wightman, R.; Höfte, H. The control of growth symmetry breaking in the Arabidopsis hypocotyl. Curr. Biol. 2015, 25, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Boudon, F.; Chopard, J.; Ali, O.; Gilles, B.; Hamant, O.; Boudaoud, A.; Traas, J.; Godin, C. A computational framework for 3D mechanical modelling of plant morphogenesis with cellular resolution. PLoS Comput. Biol. 2015, 11, e1003950. [Google Scholar] [CrossRef] [PubMed]
- Fayant, P.; Girlanda, O.; Chebli, Y.; Aubin, C.E.; Villemure, I.; Geitmann, A. Finite element model of polar growth in pollen tubes. Plant Cell. 2010, 22, 2579–2593. [Google Scholar] [CrossRef] [PubMed]
- Ryden, P.; Sugimoto-Shirasu, K.; Smith, A.C.; Findlay, K.; Reiter, W.-D.; McCann, M.C. Tensile properties of Arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes. Plant Physiol. 2003, 132, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Carpita, N.C. Structure and biogenesis of the cell walls of grasses. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 445–476. [Google Scholar] [CrossRef] [PubMed]
- Kärkönen, A.; Kuchitsu, K. Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 2015, 112, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Hongo, S.; Sato, K.; Yokoyama, R.; Nishitani, K. Demethylesterification of the primary wall by PECTIN METHYLESTERASE35 provides mechanical support to the Arabidopsis stem. Plant Cell. 2012, 24, 2624–2634. [Google Scholar] [CrossRef]
- Johnsen, H.R.; Striberny, B.; Olsen, S.; Vidal-Melgosa, S.; Fangel, J.U.; Willats, W.G.; Rose, J.K.; Krause, K. Cell wall composition profiling of parasitic giant dodder (Cuscutareflexa) and its hosts: A priori differences and induced changes. New Phytol. 2015, 207, 805–816. [Google Scholar] [CrossRef]
- Hamant, O.; Heisler, M.G.; Jönsson, H.; Krupinski, P.; Uyttewaal, M.; Bokov, P.; Corson, F.; Sahlin, P.; Boudaoud, A.; Meyerowitz, E.M.; et al. Developmental patterning by mechanical signals in Arabidopsis. Science 2008, 322, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Sampathkumar, A.; Krupinski, P.; Wightman, R.; Milani, P.; Berquand, A.; Boudaoud, A.; Hamant, O.; Jönsson, H.; Meyerowitz, E.M. Subcellular and supracellular mechanical stress prescribes cytoskeleton behavior in Arabidopsis cotyledon pavement cells. ELife 2014, 3, e01967. [Google Scholar] [CrossRef] [PubMed]
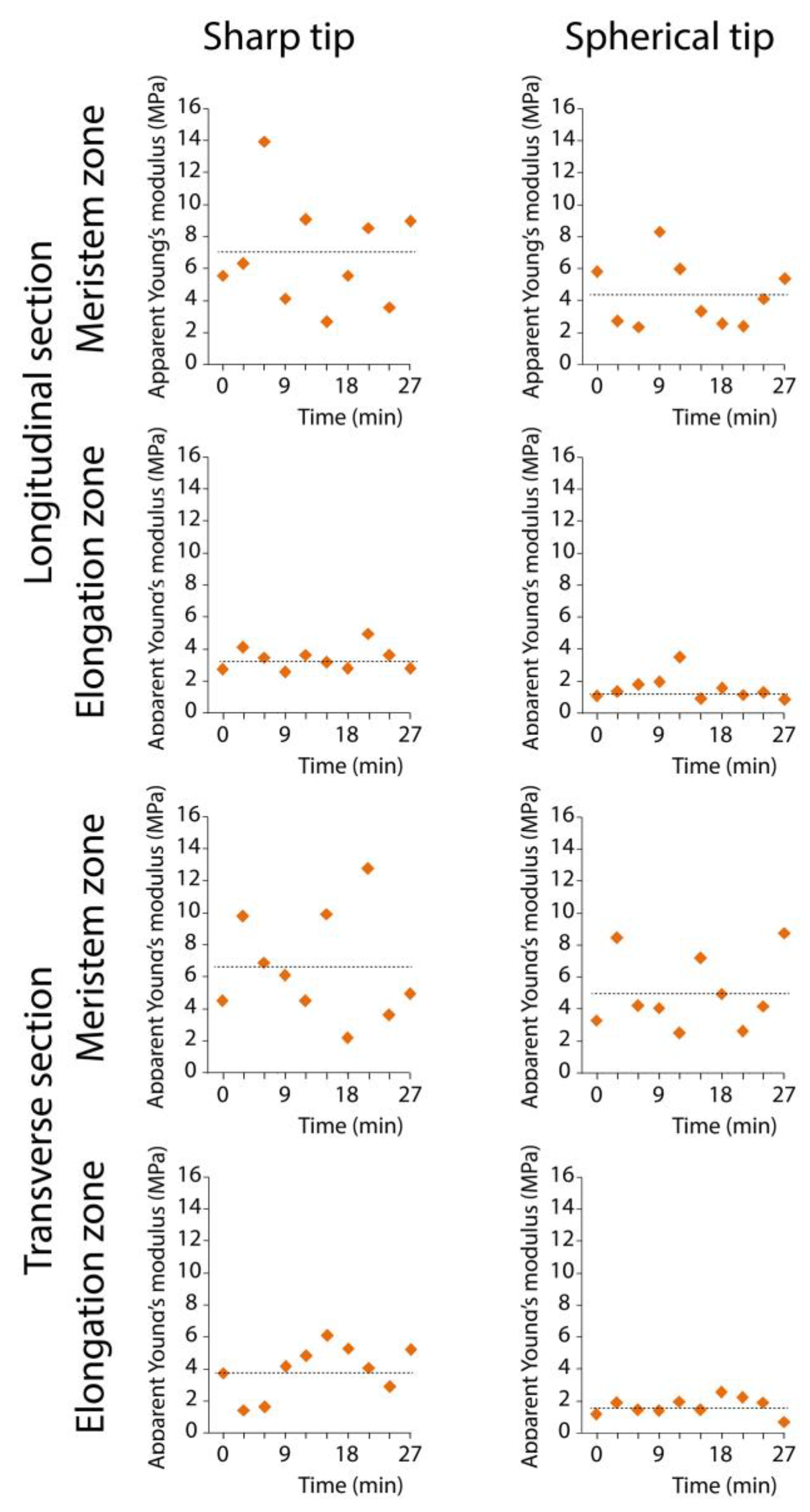
| Meristem Zone, Longitudinal Section | Elongation Zone, Longitudinal Section | Meristem Zone, Transverse Section | Elongation Zone, Transverse Section | ||
|---|---|---|---|---|---|
| Apparent stiffness (N m−1) | CSG10 | 0.07±0.01a | 0.05±0.01b | 0.07±0.01a | 0.05±0.01b |
| B100 | 0.56±0.13a | 0.27±0.04b | 0.58±0.07a | 0.25±0.04b | |
| Apparent Young’s modulus (MPa) | CSG10 | 6.46±1.02a | 3.75±0.43b | 6.73±1.59a | 3.47±0.51b |
| B100 | 4.26±0.97a | 1.65±0.34b | 4.54±0.83a | 1.44±0.29b | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlova, L.; Petrova, A.; Ananchenko, B.; Gorshkova, T. Assessment of Primary Cell Wall Nanomechanical Properties in Internal Cells of Non-Fixed Maize Roots. Plants 2019, 8, 172. https://doi.org/10.3390/plants8060172
Kozlova L, Petrova A, Ananchenko B, Gorshkova T. Assessment of Primary Cell Wall Nanomechanical Properties in Internal Cells of Non-Fixed Maize Roots. Plants. 2019; 8(6):172. https://doi.org/10.3390/plants8060172
Chicago/Turabian StyleKozlova, Liudmila, Anna Petrova, Boris Ananchenko, and Tatyana Gorshkova. 2019. "Assessment of Primary Cell Wall Nanomechanical Properties in Internal Cells of Non-Fixed Maize Roots" Plants 8, no. 6: 172. https://doi.org/10.3390/plants8060172
APA StyleKozlova, L., Petrova, A., Ananchenko, B., & Gorshkova, T. (2019). Assessment of Primary Cell Wall Nanomechanical Properties in Internal Cells of Non-Fixed Maize Roots. Plants, 8(6), 172. https://doi.org/10.3390/plants8060172





