In-Cold Exposure to Z-3-Hexenal Provides Protection Against Ongoing Cold Stress in Zea mays
Abstract
1. Introduction
2. Results
2.1. In-Cold Release of GLVs
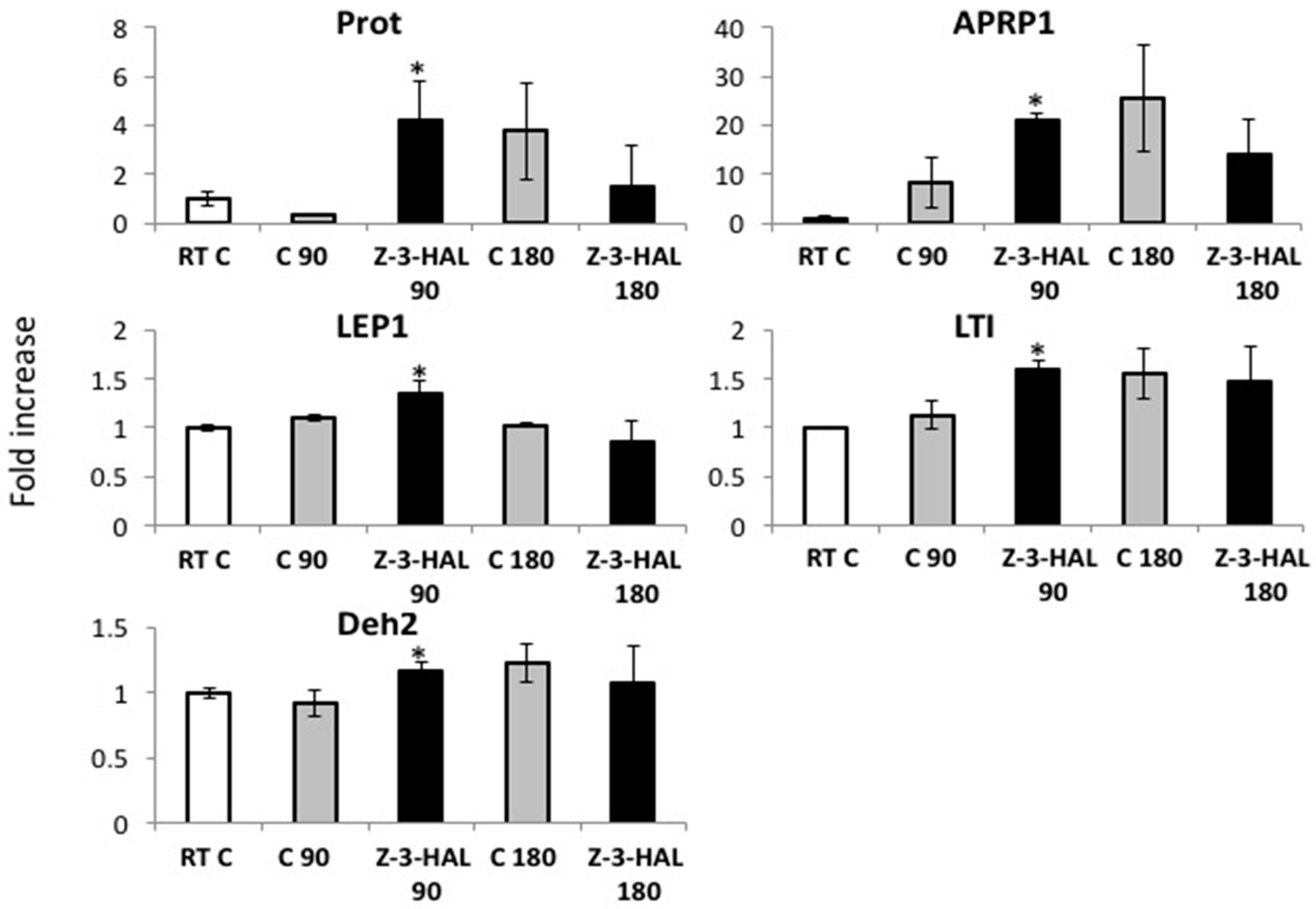
2.2. Z-3-HAL-Responsive Gene Expression during In-Cold Treatment
2.3. Conductivity Assays and Damage Assessment Revealed Significant Increase in Structural Integrity and Damage Protection during Cold Stress
2.4. Increased Growth Response of In-Cold Z-3-HAL Treated Maize Seedlings after Cold Stress
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Materials
4.3. GLV Release during Cold Stress
4.4. In-Cold Reatment with Z-3-HAL and Transcript Accumulation for Cold-Stress Related Genes
4.5. Primer Sequences
- APRP1: F 5′-CGAAATTTCCTCGCCGTAT, R 5′-CTTTTCCAAGGGTGGTTCAC
- DEH2: F 5′-GCCATCCCTAATTAAGCCAA, R 5′-CTGGGTGTTCCTCTCATCCT
- LEP1: F 5′-TGCTCGAGTACGAGATGTGG, R 5′-CTGATCATGTCCCAGACAGC
- LTI: F 5′-GCAGGTGGAGGATTCTCG, R 5′-GCTGGTCGTCGTAGAAGAGG
- PROT: F 5′-CTGGGGGTGTTCCTCAAGTA, R 5′-CGGTAGCAAAACACGACTGA
- CUL: F 5′- GAAGAGCCGCAAAGTTATGG, R 5′-ATGGTAGAAGTGGACGCACC
4.6. Conductivity Assays
4.7. Cold Stress Damage Protection by In-Cold Treatment with Z-3-HAL and Its Effect on Growth Response
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cofer, T.M.; Engelberth, M.J.; Engelberth, J. Green leaf volatiles protect maize (Zea mays) seedlings against damage from cold stress. Plant Cell Environ. 2018, 41, 1673–1682. [Google Scholar] [CrossRef]
- Hatanaka, A. The biogeneration of green odor by green leaves. Phytochemistry 1993, 34, 1201–1218. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef]
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green leaf volatiles: A plant’s multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17811. [Google Scholar] [CrossRef]
- Ameye, M.; Allmann, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C.; Audenaert, K. Green leaf volatile production by plants: A meta-analysis. New Phytol. 2017, 220, 666–683. [Google Scholar] [CrossRef]
- Matsui, K.; Sugimoto, K.; Mano, J.; Ozawa, R.; Takabayashi, J. Differential metabolism of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. PLoS ONE 2012, 7, e36433. [Google Scholar] [CrossRef]
- D’Auria, J.C.; Pichersky, E.; Schaub, A.; Hansel, A.; Gershenzon, J. Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J. 2007, 49, 194–207. [Google Scholar] [CrossRef]
- Kunishima, M.; Yamauchi, Y.; Mizutani, M.; Kuse, M.; Takikawa, H.; Sugimoto, Y. Identification of (Z)-3:(E)-2-hexenal isomerases essential to the production of the leaf aldehyde in plants. J. Biol. Chem. 2016, 291, 14023–14033. [Google Scholar] [CrossRef]
- Spyropoulou, E.A.; Dekker, H.L.; Steemers, L.; van Maarseveen, J.H.; de Koster, C.G.; Haring, M.A.; Schuurink, R.C.; Allmann, S. Identification and characterization of (3Z):(2E)-hexenal isomerases from cucumber. Front. Plant Sci. 2017, 8, 1342. [Google Scholar] [CrossRef]
- Chamberlain, K.; Khan, Z.R.; Pickett, J.A.; Toshova, T.; Wadhams, L.J. Diel periodicity in the production of green leaf volatiles by wild and cultivated host plants of stemborer moths, Chilo partellus and Busseola fusca. J. Chem. Ecol. 2006, 32, 565–577. [Google Scholar] [CrossRef]
- Engelberth, J.; Alborn, H.T.; Schmelz, E.A.; Tumlinson, J.H. Airborne signals prime plants against herbivore attack. Proc. Natl. Acad. Sci. USA 2004, 101, 1781–1785. [Google Scholar] [CrossRef]
- Kessler, A.; Halitschke, R.; Diezel, C.; Baldwin, I.T. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 2006, 148, 280–292. [Google Scholar] [CrossRef]
- Heil, M.; Bueno, J.C. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc. Natl. Acad. Sci. USA 2007, 104, 5467–5472. [Google Scholar] [CrossRef]
- Frost, C.J.; Mescher, M.C.; Dervinis, C.; Davis, J.M.; Carlson, J.E.; De Moraes, C.M. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytol. 2008, 180, 722–734. [Google Scholar] [CrossRef]
- Vallat, A.; Gu, H.; Dorn, S. How rainfall, relative humidity and temperature influence volatile emissions from apple trees in situ. Phytochem 2005, 66, 1540–1550. [Google Scholar] [CrossRef]
- Jardine, K.J.; Chambers, J.Q.; Holm, J.; Jardine, A.B.; Fontes, C.G.; Zorzanelli, R.F.; Meyers, K.T.; Fernandez de Souza, V.; Garcia, S.; Giminez, B.O.; et al. Green leaf volatile emissions during high temperature and drought stress in a Central Amazon rainforest. Plants 2015, 4, 678–690. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Kunishima, M.; Mizutani, M.; Sugimoto, Y. Reactive short-chain leaf volatiles act as powerful inducers of abiotic stress-related gene expression. Sci. Rep. 2015, 5, 8030. [Google Scholar] [CrossRef]
- Engelberth, J.; Contreras, C.F.; Dalvi, C.; Li, T.; Engelberth, M. Early Transcriptome Analyses of Z-3-Hexenol-Treated Zea mays Revealed Distinct Transcriptional Networks and Anti-Herbivore Defense Potential of Green Leaf Volatiles. PLoS ONE 2012, 8, e77465. [Google Scholar] [CrossRef]
- Dewey, R.E.; Schuster, A.M.; Levings, C.S., III; Timothy, D.H. Nucleotide sequence of F0-ATPase proteolipid (subunit 9) gene of maize mitochondria. Proc. Natl. Acad. Sci. USA 1985, 82, 1015–1019. [Google Scholar] [CrossRef]
- Welin, B.V.; Olson, A.; Nylander, M.; Palva, E.T. Characterisation and differential expression of DHN/LEA/RAB-like genes during cold-acclimation and drought stress in Arabidopsis thaliana. Plant Mol. Biol. 1994, 26, 131–144. [Google Scholar] [CrossRef]
- Hasenfratz, M.P.; Tsou, C.L.; Wilkins, T.A. Expression of two related vacuolar H(+)-ATPase 16-kilodalton proteolipid genes is differentially regulated in a tissue-specific manner. Plant Physiol. 1995, 108, 1395–1404. [Google Scholar] [CrossRef][Green Version]
- Borovskii, G.B.; Stupnikova, I.V.; Antipina, A.I.; Vladimirova, S.V.; Volnikov, V.K. Accumulation of dehydrin-like proteins in the mitochondria of cereals in response to cold, freezing, drought, and ABA treatment. BMC Plant Biol. 2002, 2, 5. [Google Scholar] [CrossRef]
- Rorat, T. Plant dehydrins—Tissue location, structure and function. Cell. Mol. Biol. Lett. 2006, 11, 536–556. [Google Scholar] [CrossRef]
- Tolleter, D.; Hincha, D.K.; Macherel, D. A mitochondrial late embryogenesis abundant protein stabilizes model membranes in the dry state. Biochem. Biophys. Acta 2010, 1798, 1926–1933. [Google Scholar] [CrossRef]
- Zhan, Z.; Wang, B.; Li, H.; Liu, R.; Kalia, R.K.; Zhu, J.K.; Chinnusamy, V. Arabidopsis proline-rich protein important for development and abiotic stress tolerance is involved in microRNA biogenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 18198–18203. [Google Scholar] [CrossRef]
- Tan, J.; Zhuo, C.; Guo, Z. Nitric oxide mediates cold- and dehydration-induced expression of a novel MfHyPRP that confers tolerance to abiotic stress. Physiol. Plant 2013, 149, 310–320. [Google Scholar]
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef]
- Bray, E.A.; Bailey-Serres, J.; Weretilnyk, E. Response to abiotic stresses. In Biochemistry and Molecular Biology of Plants; Gruissem, W., Buchanan, B.B., Jones, R., Eds.; American Society of Plant Physiologists: Rockville, MA, USA, 2000; pp. 1158–1249. [Google Scholar]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Koag, M.C.; Fenton, R.D.; Wilkens, S.; Close, T.J. The binding of maize DHN1 to lipid vesicles. Gain of structure and lipid specificity. Plant Physiol. 2003, 131, 309–316. [Google Scholar] [CrossRef]
- Szabala, B.M.; Fudali, S.; Rorat, T. Accumulation of acidic SK3 dehydrins in phloem cells of cold- and drought-stressed plants of the Solanaceae. Planta 2014, 239, 847–863. [Google Scholar] [CrossRef]
- Imamura, T.; Higuchi, A.; Takahashi, H. Dehydrins are highly expressed in overwintering buds and enhance drought and freezing tolerance in Gentiana triflora. Plant Sci. 2013, 213, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Vaseva, I.I.; Anders, I.; Feller, U. Identification and expression of different dehydrin subclasses involved in the drought response of Trifolium repens. Plant Physiol. 2013, 171, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cui, Y.C.; Ren, C.; Rocha, P.S.; Peng, M.; Xu, G.Y.; Wang, M.L.; Xia, X.J. Transgenic rice expressing a cassava (Manihot esculenta Crantz) plasma membrane gene MePMP3-2 exhibits enhanced tolerance to salt and drought stresses. Genet. Mol. Res. 2016, 15, 15017336. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, J.; Gadea, J.; Forment, J.; Perez-Valle, J.; Santiago, J.; Martinez-Godoy, M.A.; Yenush, L.; Belles, J.M.; Brumos, J.; Colmenero-Flors, J.M.; et al. Shared and novel molecular responses of mandarin to drought. Plant Mol. Biol. 2009, 70, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Cuming, A.C. LEA proteins. In Seed Proteins; Shewry, P.R., R. Casey, R., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 753–780. [Google Scholar]
- Roberts, J.K.; DeSimone, N.A.; Lingle, W.L.; Dure, L., III. Cellular concentrations and uniformity of cell-type accumulation of two LEA proteins in cotton embryos. Plant Cell 1993, 5, 769–780. [Google Scholar] [CrossRef]
- Bray, E.A. Molecular responses to water deficit. Plant Physiol. 1993, 103, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Park, S.W.; Chung, Y.S.; Chung, C.H.; Kim, J.I.; Lee, J.H. Molecular cloning of low-temperature-inducible ribosomal proteins from soybean. J. Exp. Bot. 2004, 55, 1153–1155. [Google Scholar] [CrossRef]
- Tseng, I.C.; Hong, C.Y.; Yu, S.M.; Ho, T.H.D. Abscisic acid- and stress-induced highly proline-rich glycoproteins regulate root growth in rice. Plant Physiol. 2013, 163, 118–134. [Google Scholar] [CrossRef]
- Recchia, G.H.; Caldas, D.G.; Beraldo, A.L.; da Silva, M.J.; Tsai, S.M. Transcriptional analysis of drought-induced genes in the roots of a tolerant genotype of the common bean (Phaseolus vulgaris L.). Int. J. Mol. Sci. 2013, 14, 7155–7179. [Google Scholar] [CrossRef]
- Kawarazaki, T.; Kimura, S.; Iizuka, A.; Hanamata, S.; Nibori, H.; Michikawa, M.; Imai, A.; Abe, M.; Kaya, H.; Kuchitsu, K. A low temperature-inducible protein AtSRC2 enhances the ROS-producing activity of NADPH oxidase AtRbohF. Biochim. Biophys. Acta 2013, 1833, 2775–2780. [Google Scholar] [CrossRef]
- Barrios, A.; Caminero, C.; Garcia, P.; Krezdorn, N.; Hoffmeier, K.; Winter, P.; Perez de la Vega, M. Deep Super-SAGE transcriptomic analysis of cold acclimation in lentil (Lens culinaris Medik.). BMC Plant Biol. 2017, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Seo, P.J. Airborne signals from salt-stressed Arabidopsis plants trigger salinity tolerance in neighboring plants. Plant Signal. Behav. 2014, 9, e28392. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Varkonyi, Z.; Szabo, M.; Maslenkova, L.; Nogues, I.; Kovac, L.; Peeva, V.; Busheva, M.; Garab, G.; Sharkey, T.D.; et al. Increased thermostability of thylakoid membranes in isoprene-emitting leaves with three biophysical methods. Plant Physiol. 2011, 157, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Copolovici, L.; Kannaste, A.; Pazouki, L.; Niinemets, U. Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. J. Plant Physiol. 2012, 169, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Harley, P.C.; Niinemets, Ü. Ozone-induced foliar damage and release of stress volatiles is highly dependent on stomatal openness and priming by low-level ozone exposure in Phaseolus vulgaris. Plant Cell Environ. 2017, 9, 1984–2003. [Google Scholar] [CrossRef]
- Ohlsson, A.B.; Segerfeld, P.; Lindstroem, A.; Borg-Karlson, A.K.; Berglund, T. UV-B exposure of indoor-grown Picea abies seedlings causes an epigenetic effect and selective emission of terpenes. Zeitschrift für Naturforschung C 2013, 68, 139–147. [Google Scholar]
- Centritto, M.; Haworth, M.; Marino, G.; Pallozzi, E.; Tsonev, T.; Velikova, V.; Nogues, I.; Loreto, F. Isoprene emission aids recovery of photosynthetic performance in transgenic Nicotiana tabacum following high intensity acute UV-B exposure. Plant Sci. 2014, 226, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Maja, M.M.; Kasurinen, A.; Holopainen, T.; Julkunen-Tiitto, R.; Holopainen, J.K. The effect or warming and enhanced ultraviolet radiation on gender-specific emissions of volatile compounds from European aspen. Sci. Total Environ. 2016, 15, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Manoli, A.; Sturaro, A.; Trevisian, S.; Quaggiotti, S.; Nonis, A. Evaluation of candidate reference genes for qPCR in maize. J. Plant Physiol. 2012, 169, 807–815. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engelberth, M.; Selman, S.M.; Engelberth, J. In-Cold Exposure to Z-3-Hexenal Provides Protection Against Ongoing Cold Stress in Zea mays. Plants 2019, 8, 165. https://doi.org/10.3390/plants8060165
Engelberth M, Selman SM, Engelberth J. In-Cold Exposure to Z-3-Hexenal Provides Protection Against Ongoing Cold Stress in Zea mays. Plants. 2019; 8(6):165. https://doi.org/10.3390/plants8060165
Chicago/Turabian StyleEngelberth, Marie, Samantha M. Selman, and Jurgen Engelberth. 2019. "In-Cold Exposure to Z-3-Hexenal Provides Protection Against Ongoing Cold Stress in Zea mays" Plants 8, no. 6: 165. https://doi.org/10.3390/plants8060165
APA StyleEngelberth, M., Selman, S. M., & Engelberth, J. (2019). In-Cold Exposure to Z-3-Hexenal Provides Protection Against Ongoing Cold Stress in Zea mays. Plants, 8(6), 165. https://doi.org/10.3390/plants8060165




