Polysaccharides from New Zealand Native Plants: A Review of Their Structure, Properties, and Potential Applications
Abstract
1. Introduction
2. Exudate Gums and Mucilages from NZ Plants
2.1. Puka (Meryta sinclairii)
2.2. Mamaku (Cyathea medullaris)
2.3. Houhere (Hoheria populnea)
2.4. Harakeke (Phormium tenax and P. cookianum)
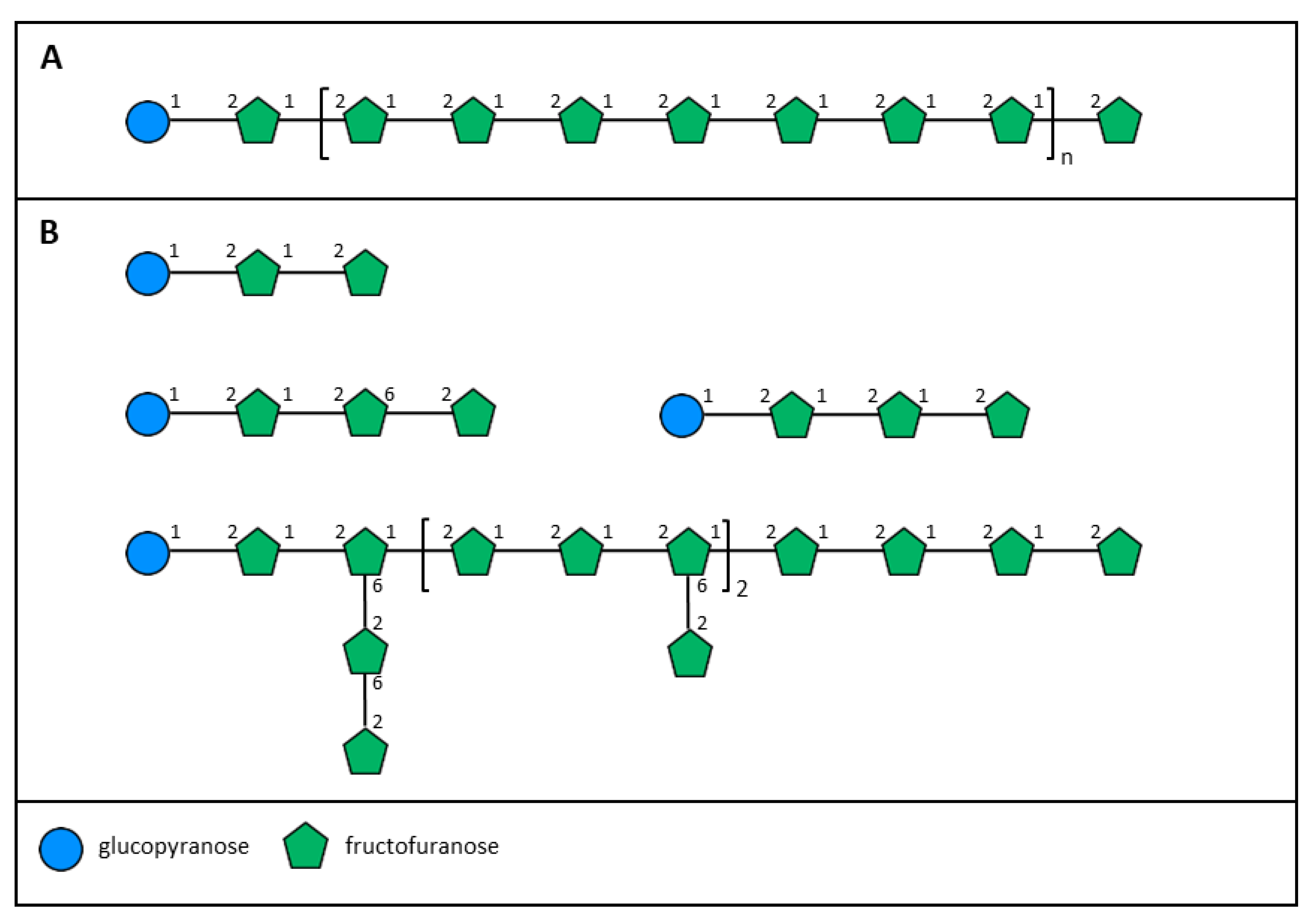
3. Fructans
4. Potential Utilization of New Zealand Polysaccharides
Author Contributions
Funding
Conflicts of Interest
References
- Brooker, S.G.; Cambie, R.C.; Cooper, R.C. New Zealand Medicinal Plants; Heinenmann Publishers: Auckland, New Zealand, 1987. [Google Scholar]
- Pilkington, L.I.; Yang, X.; Liu, M.-W.; Hemar, Y.; Brimble, M.A.; Reynisson, J. A Chemometric analysis of compounds from native New Zealand medicinal flora. Chem. Asian J. 2019, 14, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Bloor, S.J. A survey of extracts of New Zealand indigenous plants for selected biological activities. N. Zeal. J. Bot. 1995, 33, 523–540. [Google Scholar] [CrossRef]
- Hurd, C.L.; Nelson, W.A.; Falshaw, R.; Neill, K.F. History, current status and future of marine macroalgal research in New Zealand: Taxonomy, ecology, physiology and human uses. Phycol. Res. 2004, 52, 80–106. [Google Scholar] [CrossRef]
- Miller, I.J. The chemical structure of galactans from some New Zealand red algae. Bot. Mar. 2003, 46, 572–577. [Google Scholar] [CrossRef]
- Falshaw, R.; Bixler, H.J.; Johndro, K. Structure and performance of commercial κ-2 carrageenan extracts. Part III. Structure analysis and performance in two dairy applications of extracts from the New Zealand red seaweed, Gigartina atropurpurea. Food Hydrocolloids 2003, 17, 129–139. [Google Scholar] [CrossRef]
- Ren, L.; Hemar, Y.; Perera, C.O.; Lewis, G.; Krissansen, G.W.; Buchanan, P.K. Antibacterial and antioxidant activities of aqueous extracts of eight edible mushrooms. Bioact. Carbohydr. Diet. Fibre 2014, 3, 41–51. [Google Scholar] [CrossRef]
- Ren, L.; Edwards, P.J.B.; Perera, C.O.; Hemar, Y. Structural features of a novel polysaccharide isolated from a New Zealand Maori mushroom Iliodiction cibarium. Carbohyd. Res. 2015, 406, 19–26. [Google Scholar] [CrossRef]
- Choudhary, P.D.; Pawar, H.A. Recently investigated natural gums and mucilages as pharmaceutical excipients: An overview. J. Pharm. 2014, 2014, 204849. [Google Scholar] [CrossRef]
- BeMiller, J.N. Gums and related polysaccharides. In Glycoscience; Fraser-Reid, B., Tatsuta, K., Thiem, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1513–1533. [Google Scholar]
- Verbeken, D.; Dierckx, S.; Dewettinck, K. Exudate gums: Occurrence, production, and applications. Appl. Microbiol. Biotechnol. 2003, 63, 10–21. [Google Scholar] [CrossRef]
- Kelliher, F.M.; Kirkham, M.B.; Hunt, J.E. Photosynthesis and stomatal conductance of the New Zealand tree, Meryta sinclairii, grown under two watering regimes. N. Z. J. Bot. 2000, 38, 515–519. [Google Scholar] [CrossRef]
- Yariv, J.; Lis, H.; Katchalski, E. Precipitation of arabic acid and some seed polysaccharides by glycosylphenylazo dyes. Biochem. J. 1967, 105, 1c–2c. [Google Scholar] [CrossRef] [PubMed]
- Sims, I.M.; Furneaux, R.H. Structure of the exudate gum from Meryta sinclairii. Carbohyd. Polym. 2003, 52, 423–431. [Google Scholar] [CrossRef]
- Centanni, M.; Hutchison, J.C.; Carnachan, S.M.; Daines, A.M.; Kelly, W.J.; Tannock, G.W.; Sims, I.M. Differential growth of bowel commensal Bacteroides species on plant xylans of differing structural complexity. Carbohyd. Polym. 2017, 157, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Wee, M.S.M.; Matia-Merino, L.; Carnachan, S.M.; Sims, I.M.; Goh, K.K.T. Structure of a shear-thickening polysaccharide extracted from the New Zealand black tree fern, Cyathea medullaris. Int. J. Biol. Macromol. 2014, 70, 86–91. [Google Scholar] [CrossRef]
- Sims, I.M.; Smith, A.M.; Morris, G.A.; Ghori, M.U.; Carnachan, S.M. Structural and rheological studies of a polysaccharide mucilage from lacebark leaves (Hoheria populnea A. Cunn.). Int. J. Biol. Macromol. 2018, 111, 839–847. [Google Scholar] [CrossRef]
- Breckenridge, J.; Holthaus, D. (TIC Gums, White Marsh, MD, USA). Personal Communication, 2019. [Google Scholar]
- Wee, M.S.M.; Nurhazwani, S.; Tan, K.W.J.; Goh, K.K.T.; Sims, I.M.; Matia-Merino, L. Complex coacervation of an arabinogalactan-protein extracted from the Meryta sinclarii tree (puka gum) and whey protein isolate. Food Hydrocolloids 2014, 42, 130–138. [Google Scholar] [CrossRef]
- Wee, M.S.M.; Sims, I.M.; Goh, K.K.T.; Matia-Merino, L. Molecular, rheological and physicochemical characterisation of puka gum, an arabinogalactan-protein extracted from the Meryta sinclairii tree. Carbohyd. Polym. 2019, 220, 247–255. [Google Scholar] [CrossRef]
- Cooper, R.; Cambie, R.C. New Zealand’s Economic Native Plants; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Goh, K.K.T.; Matia-Merino, L.; Hall, C.E.; Moughan, P.J.; Singh, H. Complex rheological properties of a water-soluble extract from the fronds of the black tree fern, Cyathea medullaris. Biomacromolecules 2007, 8, 3414–3421. [Google Scholar] [CrossRef]
- Goh, K.K.T.; Matia-Merino, L.; Pinder, D.N.; Saavedra, C.; Singh, H. Molecular characteristics of a novel water-soluble polysaccharide from the New Zealand black tree fern (Cyathea medullaris). Food Hydrocolloids 2011, 25, 286–292. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Setty, C.M.; Badiger, A.M.; Muralikrishna, K.S. Gum ghatti: A promising polysaccharide for pharmaceutical applications. Carbohyd. Polym. 2012, 87, 980–986. [Google Scholar] [CrossRef]
- Jaishankar, A.; Wee, M.; Matia-Merino, L.; Goh, K.K.T.; McKinley, G.H. Probing hydrogen bond interactions in a shear thickening polysaccharide using nonlinear shear and extensional rheology. Carbohyd. Polym. 2015, 123, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Singh, J.; Singh, H. Characterization of gum ghatti (Anogeissus latifolia): A structural and rheological approach. J. Food Sci. 2009, 74, E328–E332. [Google Scholar] [CrossRef] [PubMed]
- Nep, E.I.; Carnachan, S.M.; Ngwuluka, N.C.; Kontogiorgos, V.; Morris, G.A.; Sims, I.M.; Smith, A.M. Structural characterisation and rheological properties of a polysaccharide from sesame leaves (Sesamum radiatum Schumach. & Thonn.). Carbohyd. Polym. 2016, 152, 541–547. [Google Scholar] [CrossRef]
- Wee, M.S.M.; Matia-Merino, L.; Goh, K.K.T. The cation-controlled and hydrogen bond-mediated shear-thickening behaviour of a tree-fern isolated polysaccharide. Carbohyd. Polym. 2015, 130, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Matia-Merino, L.; Goh, K.K.T.; Singh, H. A natural shear-thickening water-soluble polymer from the fronds of the black tree fern, Cyathea medullaris: Influence of salt, pH and temperature. Carbohyd. Polym. 2012, 87, 131–138. [Google Scholar] [CrossRef]
- Sengkhamparn, N.; Bakx, E.J.; Verhoef, R.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G.J. Okra pectin contains an unusual substitution of its rhamnosyl residues with acetyl and alpha-linked galactosyl groups. Carbohyd. Res. 2009, 344, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Capek, P.; Rosik, J.; Kardosova, A.; Toman, R. Polysaccharides from the roots of the marsh mallow (Althaea officinalis L., var. Rhobusta): Structural features of an acidic polysaccharide. Carbohyd. Res. 1987, 164, 443–452. [Google Scholar] [CrossRef]
- Tomoda, M.; Shimada, K.; Shimizu, N.; Kanari, M.; Kaneko, E. The carbohydrate structure of a mucilage from the roots of Hibiscus moscheutos L. Carbohyd. Res. 1986, 151, 29–35. [Google Scholar] [CrossRef]
- Austarheim, I.; Mahamane, H.; Sanogo, R.; Togola, A.; Khaledabadi, M.; Vestrheim, A.C.; Inngjerdingen, K.T.; Michaelsen, T.E.; Diallo, D.; Paulsen, B.S. Anti-ulcer polysaccharides from Cola cordifolia bark and leaves. J. Ethnopharmacol. 2012, 143, 221–227. [Google Scholar] [CrossRef]
- Oku New Zealand Native Herbal Products. Available online: https://www.oku.co.nz (accessed on 13 May 2019).
- Nep, E.I.; Sims, I.M.; Morris, G.A.; Kontogiorgos, V.; Smith, A.M. Evaluation of some important physicochemical properties of starch free grewia gum. Food Hydrocolloids 2016, 53, 134–140. [Google Scholar] [CrossRef][Green Version]
- Yuan, B.; Ritzoulis, C.; Chen, J. Extensional and shear rheology of a food hydrocolloid. Food Hydrocolloids 2018, 74, 296–306. [Google Scholar] [CrossRef]
- Tauwhare, S.E.K.; Newman, R.H.; Scheele, S.; Te Kanawa, R. Chemotaxonomy of Phormium based on sugar-residue analyses of the leaf exudates. N. Z. J. Bot. 2006, 44, 129–133. [Google Scholar] [CrossRef]
- Sims, I.M.; Newman, R.H. Structural studies of acidic xylans exuded from leaves of the monocotyledonous plants Phormium tenax and Phormium cookianum. Carbohyd. Polym. 2006, 63, 379–384. [Google Scholar] [CrossRef]
- Benaoun, F.; Delattre, C.; Boual, Z.; Ursu, A.V.; Vial, C.; Gardarin, C.; Wadouachi, A.; Le Cerf, D.; Varacavoudin, T.; El-Hadj, M.D.O.; et al. Structural characterization and rheological behavior of a heteroxylan extracted from Plantago notata Lagasca (Plantaginaceae) seeds. Carbohydr. Polym. 2017, 175, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.-Y.; Chen, H.-H.; Lin, H.-X.; Xie, M.-Y.; Nie, S.-P. Structural features of alkaline extracted polysaccharide from the seeds of Plantago asiatica L. and its rheological properties. Molecules 2016, 21, 1181. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.H.; Yu, N.; Gray, G.R.; Ralph, J.; Anderson, L.; Marlett, J.A. The gel-forming polysaccharide of psyllium husk (Plantago ovata Forsk). Carbohydr. Res. 2004, 339, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Richardson, R.K.; Morris, E.R.; Dea, I.C.M. Xanthan-like ‘weak gel’ rheology from dispersions of ispaghula seed husk. Carbohydr. Polym. 1993, 22, 223–232. [Google Scholar] [CrossRef]
- Yadav, M.P.; Johnston, D.B.; Hicks, K.B. Structural characterization of corn fiber gums from coarse and fine fiber and a study of their emulsifying properties. J. Agric. Food Chem. 2007, 55, 6366–6371. [Google Scholar] [CrossRef]
- Farahnaky, A.; Askari, H.; Majzoobi, M.; Mesbahi, G. The impact of concentration, temperature and pH on dynamic rheology of psyllium gels. J. Food. Eng. 2010, 100, 294–301. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Razavi, S.H.; Mousavi, S.M. Psyllium husk gum: An attractive carbohydrate biopolymer for the production of stable canthaxanthin emulsions. Carbohydr. Polym. 2013, 92, 2002–2011. [Google Scholar] [CrossRef]
- Housley, T.L.; Pollock, C.J. The metabolism of frucatn in higher plants. In Science and Technology of Fructans; Suzuki, M., Chatterton, N.J., Eds.; CRC Press: Boca Raton, FL, USA, 1993; pp. 191–225. [Google Scholar]
- Hendry, G.A.F.; Wallace, R.K. The origin, distribution, and evolutionary significance of fructans. In Science and Technology of Fructans; Suzuki, M., Chatterton, N.J., Eds.; CRC Press: Boca Raton, FL, USA, 1993; pp. 119–129. [Google Scholar]
- Brasch, D.J.; Fankhauser, B.L.; McDonald, A.G. A study of the glucofructofuranan from the New Zealand cabbage tree Cordyline australis. Carbohyd. Res. 1988, 180, 315–324. [Google Scholar] [CrossRef]
- Sims, I.M.; Cairns, A.J.; Furneaux, R.H. Structure of fructans from excised leaves of New Zealand flax. Phytochemistry 2001, 57, 661–668. [Google Scholar] [CrossRef]
- Sims, I.M. Structural diversity of fructans from members of the order Asparagales in New Zealand. Phytochemistry 2003, 63, 351–359. [Google Scholar] [CrossRef]
- Snowberry New Zealand Limited. Available online: https://snowberry.co.nz/about#Snowberry-Gardens (accessed on 13 May 2019).
- Fiszman, S.; Varela, P. The role of gums in satiety/satiation. A review. Food Hydrocolloids 2013, 32, 147–154. [Google Scholar] [CrossRef]
- Wee, M.S.M.; Lentle, R.G.; Goh, K.K.T.; Matia-Merino, L. The first of the viscoceuticals? A shear thickening gum induces gastric satiety in rats. Food Funct. 2017, 8, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Khangwal, I.; Shukla, P. Potential prebiotics and their transmission mechanisms: Recent approaches. J. Food Drug Anal 2019, in press. [Google Scholar] [CrossRef]
- Singh, R.S.; Singh, T.; Larroche, C. Biotechnological application of inulin-rich feedstocks. Bioresour. Technol. 2019, 273, 641–653. [Google Scholar] [CrossRef]
- Harris, G. The significance of rengarenga Arthropodium cirratum to Maori. N. Z. Garden J. 1996, 1, 19–21. [Google Scholar]
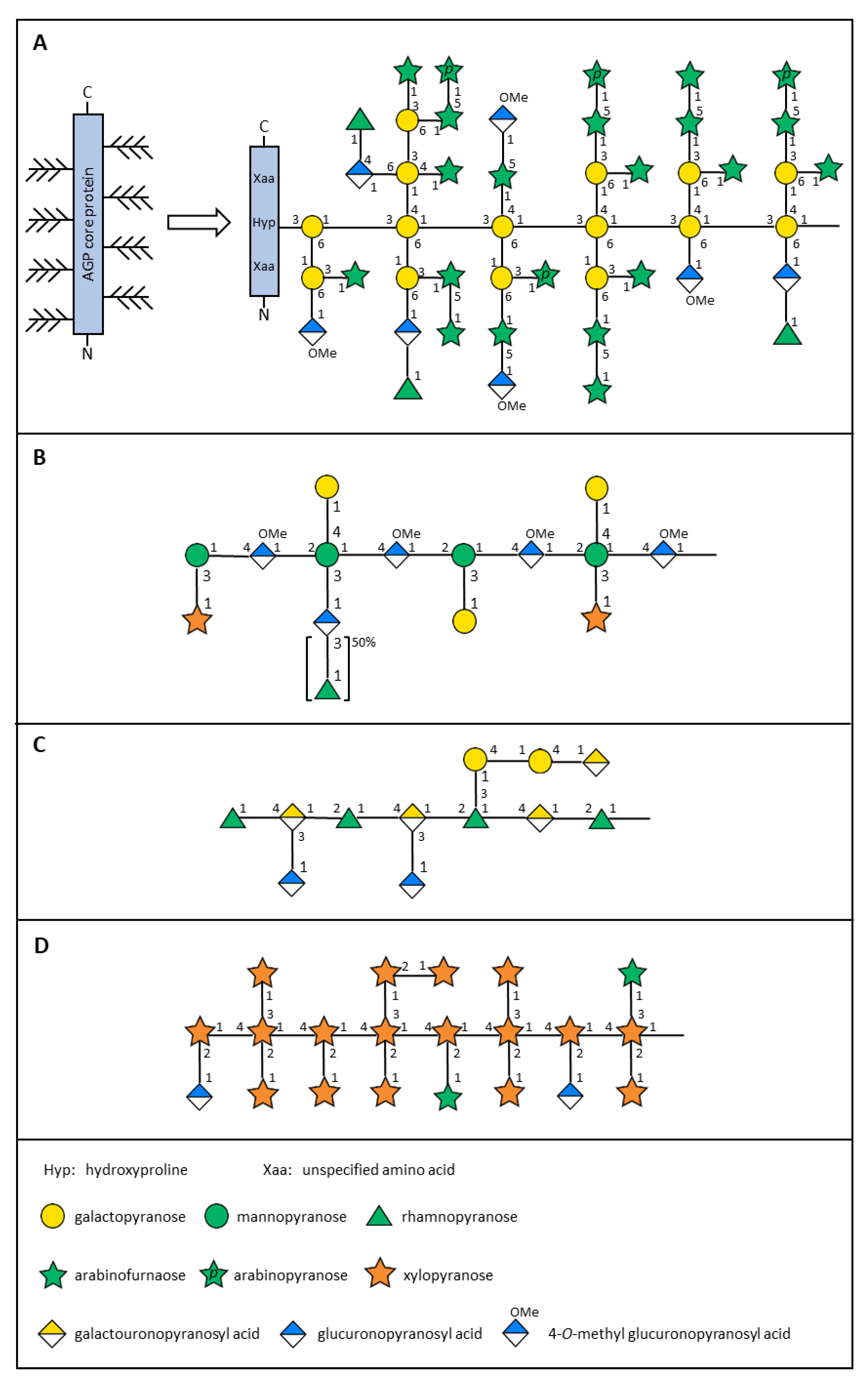
| Composition (mol%)a | |||||
|---|---|---|---|---|---|
| Sugar | Linkage | Puka [14] | NZ flax [15] | Mamaku [16] | Houhere [17] |
| Rhamnose | terminal-p | 9 | -b | 3 | - |
| 2-p | - | - | - | 22 | |
| 2,3-p | 3 | ||||
| 2,4-p | - | - | - | 8 | |
| Arabinose | terminal-p | 8 | - | 2 | - |
| terminal-f | 23 | 9 | 1 | - | |
| 3-f | 7 | - | - | - | |
| 5-f | 9 | - | - | - | |
| Xylose | terminal-p | - | 33 | 9 | - |
| 2-p | - | 4 | 2 | - | |
| 4-p | - | 2 | 3 | - | |
| 2,4-p | - | 17 | - | - | |
| 2,3,4-p | - | 16 | - | - | |
| Galactose | terminal-p | - | - | 15 | 15 |
| 3,6-p | 19 | - | 1 | - | |
| 3,4,6-p | 9 | - | - | - | |
| Mannose | 2,3-p | - | - | 9 | - |
| 2,3,4-p | - | - | 11 | - | |
| Galacturonic acid | terminal-p | - | - | - | 12 |
| 4-p | - | - | - | 7 | |
| 3,4-p | - | - | - | 14 | |
| Glucuronic acid | terminal-p | 8 | 15 | 2 | 16 |
| 3-p | - | - | 6 | - | |
| 4-p | 6 | - | 28 | - | |
| Other minor linkage | 2 | 4 | 8 | 3 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnachan, S.M.; Bell, T.J.; Hinkley, S.F.R.; Sims, I.M. Polysaccharides from New Zealand Native Plants: A Review of Their Structure, Properties, and Potential Applications. Plants 2019, 8, 163. https://doi.org/10.3390/plants8060163
Carnachan SM, Bell TJ, Hinkley SFR, Sims IM. Polysaccharides from New Zealand Native Plants: A Review of Their Structure, Properties, and Potential Applications. Plants. 2019; 8(6):163. https://doi.org/10.3390/plants8060163
Chicago/Turabian StyleCarnachan, Susan M., Tracey J. Bell, Simon F. R. Hinkley, and Ian M. Sims. 2019. "Polysaccharides from New Zealand Native Plants: A Review of Their Structure, Properties, and Potential Applications" Plants 8, no. 6: 163. https://doi.org/10.3390/plants8060163
APA StyleCarnachan, S. M., Bell, T. J., Hinkley, S. F. R., & Sims, I. M. (2019). Polysaccharides from New Zealand Native Plants: A Review of Their Structure, Properties, and Potential Applications. Plants, 8(6), 163. https://doi.org/10.3390/plants8060163




