Signaling in Pollen Tube Growth: Beyond the Tip of the Polarity Iceberg
Abstract
1. Introduction
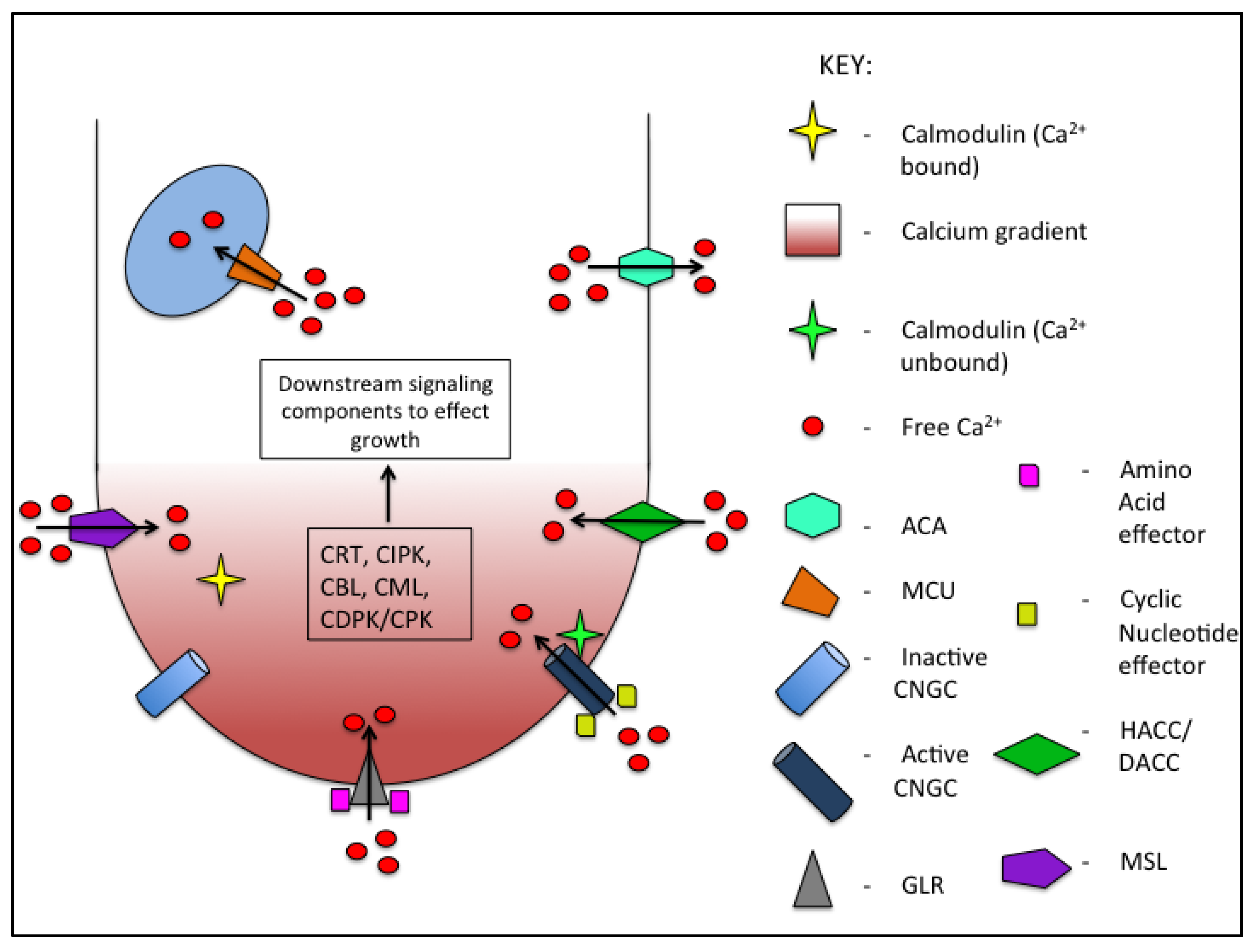
2. Calcium
2.1. Calcium Influx into Pollen Tubes
2.2. Calcium Sequestration and Efflux from Pollen Tubes
2.3. Calcium Signaling—Deciphering the Code
2.4. Interaction Between Calcium Signaling and Other Signaling Pathways
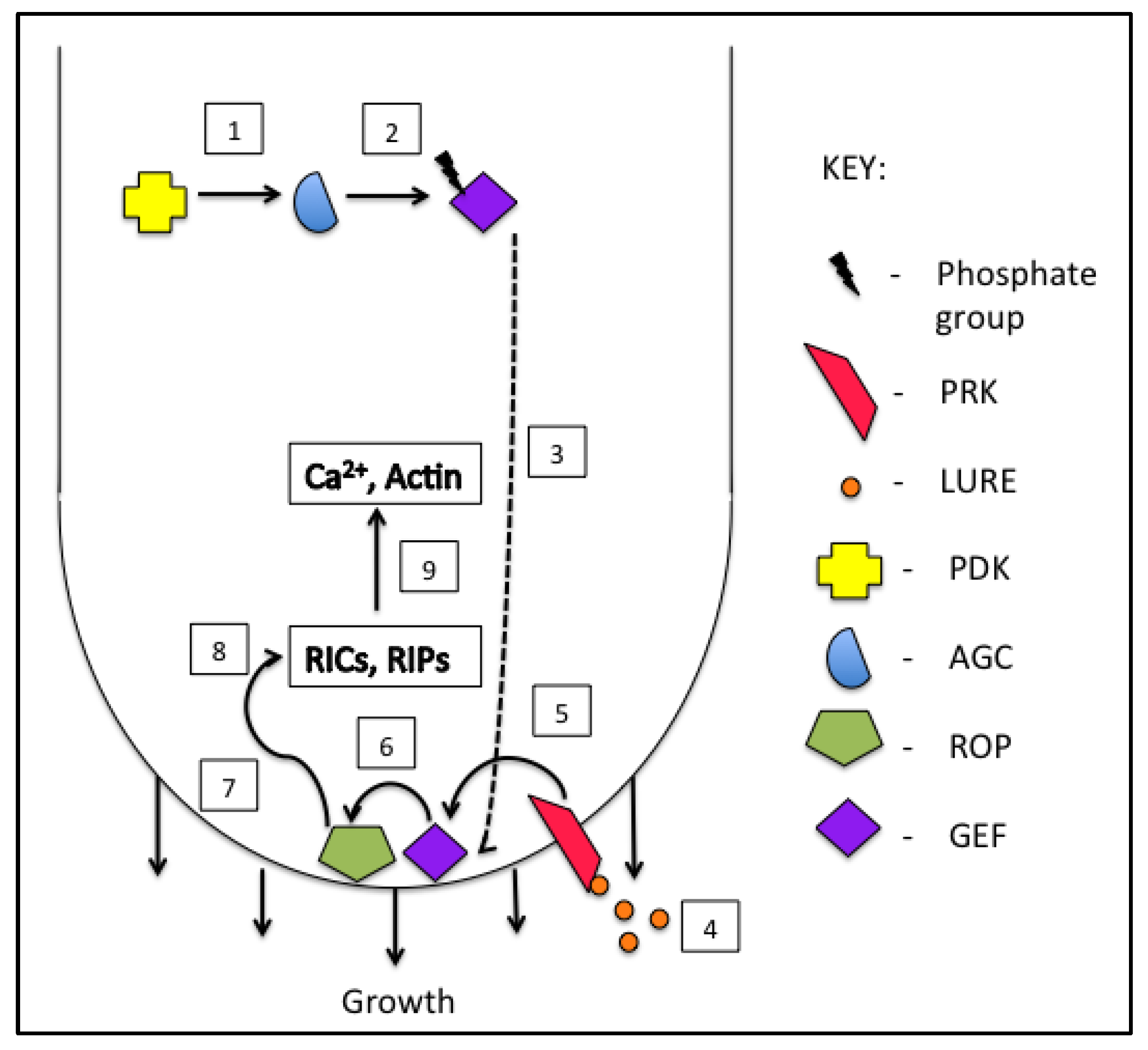
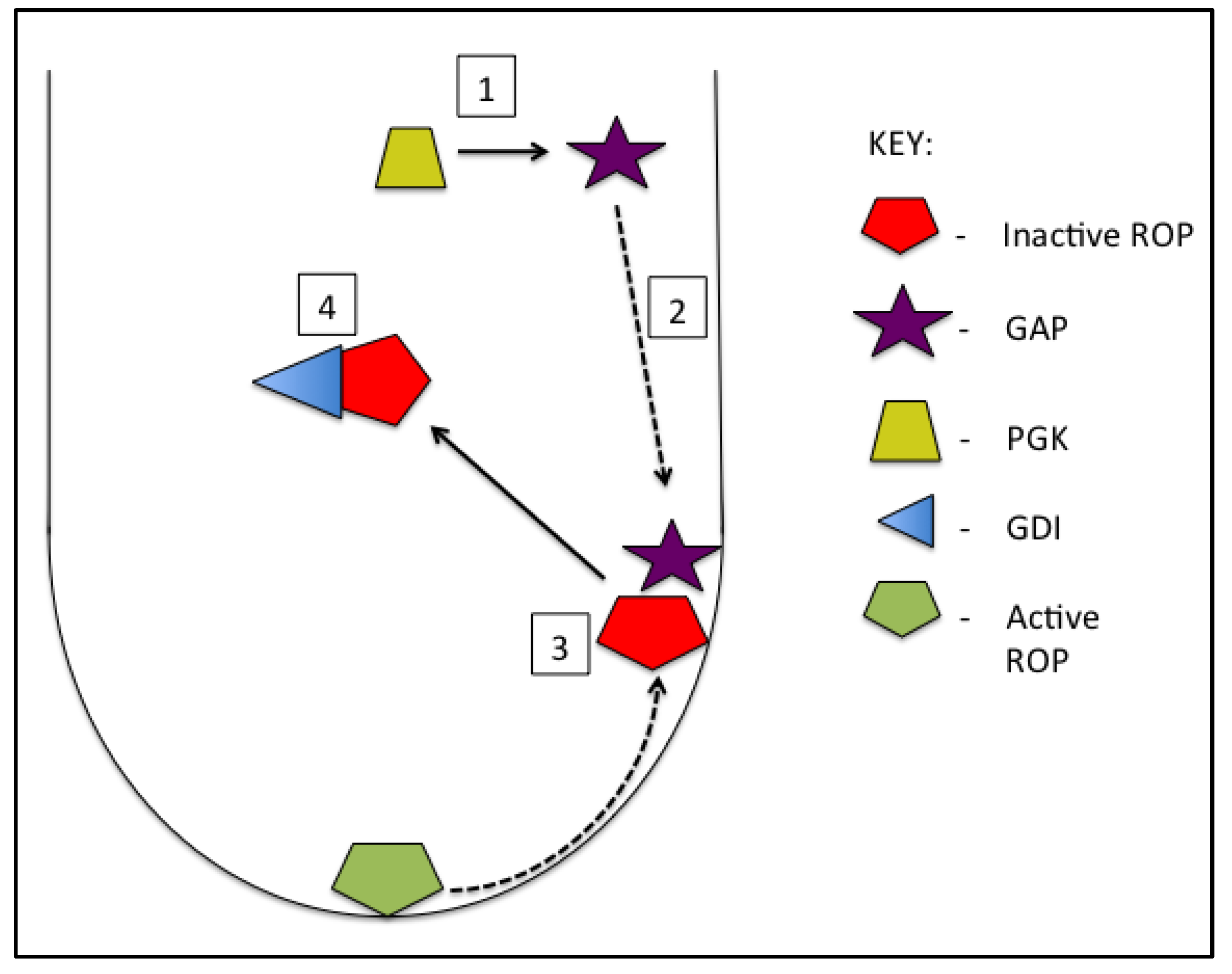
3. GTPase Signaling
3.1. GTPase Regulatory Proteins
3.2. GTPase Signaling—Effectors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lord, E.M. Adhesion and guidance in compatible pollination. J. Exp. Bot. 2003, 54, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, J.P. The male gametophyte of flowering plants. Plant Cell. 1989, 1, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, Y.; Higashiyama, T. Chemical signaling for pollen tube guidance at a glance. J. Cell Sci. 2018, 131, jcs208447. [Google Scholar] [CrossRef] [PubMed]
- Geitmann, A.; Emons, A.M.C. The cytoskeleton in plant and fungal cell tip growth. J. Microsc. 2000, 198, 218–245. [Google Scholar] [CrossRef] [PubMed]
- Franklin-Tong, V.E. Signaling and the modulation of pollen tube growth. Plant Cell. 1999, 11, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Vidali, L.; McKenna, S.T.; Hepler, P.K. Actin polymerization is essential for pollen tube growth. Mol. Biol. Cell. 2001, 12, 2534–2545. [Google Scholar] [CrossRef] [PubMed]
- Benkert, R.; Obermeyer, G.; Bentrup, F.-W. The turgor pressure of growing lily pollen tubes. Protoplasma. 1997, 198, 1–8. [Google Scholar] [CrossRef]
- Li, H.; Lin, Y.; Heath, R.M.; Zhu, M.X.; Yang, Z. Control of Pollen Tube Tip Growth by a Rop GTPase–Dependent Pathway That Leads to Tip-Localized Calcium Influx. Plant Cell 1999, 11, 1731–1742. [Google Scholar]
- Kaothien, P.; Ok, S.H.; Shuai, B.; Wengier, D.; Cotter, R.; Kelley, D.; Kiriakopolos, S.; Muschietti, J.; McCormick, S. Kinase partner protein interacts with the LePRK1 and LePRK2 receptor kinases and plays a role in polarized pollen tube growth. The Plant J. 2005, 42, 492–503. [Google Scholar] [CrossRef]
- Brewbaker, J.L.; Kwack, B.H. The essential role of calcium ion in pollen germination and pollen tube growth. Am. J. Bot. 1963, 50, 859–865. [Google Scholar] [CrossRef]
- Pierson, E.S.; Miller, D.D.; Callaham, D.A.; Shipley, A.M.; Rivers, B.A.; Cresti, M.; Hepler, P.K. Pollen tube growth 1s coupled to the extracellular calcium ion flux and the intracellular calcium gradient: Effect of BAPTA-type buffers and hypertonic media. Plant Cell 1994, 6, 1815–1828. [Google Scholar] [CrossRef] [PubMed]
- Pierson, E.S.; Miller, D.D.; Callaham, D.A.; van Aken, J.; Hackett, G.; Hepler, P.K. Tip-localized calcium entry fluctuates during pollen tube growth. Dev. Biol. 1996, 174, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Feijo, J.A.; Malho, R.; Obermeyer, G. Ion dynamics and its possible role during in vitro pollen germination and tube growth. Protoplasma. 1995, 187, 155–167. [Google Scholar] [CrossRef]
- Feijo, J.A.; Wudick, M.M. Calcium is life. J. Exp. Bot. 2018, 69, 4147–4150. [Google Scholar] [CrossRef]
- Dutta, R.; Robinson, K.R. Identification and characterization of stretch-activated ion channels in pollen protoplasts. Plant Physiol. 2004, 135, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Fan, L.-M.; Zhang, W.-Z.; Zhang, W.; Wu, W.-H. Ca2+-permeable channels in the plasma membrane of Arabidopsis pollen are regulated by actin microfilaments. Plant Physiol. 2004, 136, 3892–3904. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, X.; Li, S.; Liu, T.; Ma, L.; Shang, Z. Heterotrimeric G-protein participation in Arabidopsis pollen germination through modulation of a plasma membrane hyperpolarization-activated Ca2+-permeable channel. New Phytol. 2007, 176, 550–559. [Google Scholar] [CrossRef]
- Qu, H.-Y.; Shang, Z.-L.; Zhang, S.-L.; Liu, L.-M.; Wu, J.-Y. Identification of hyperpolarization-activated calcium channels in apical pollen tubes of Pyrus pyrifolia. New Phytol. 2007, 174, 524–536. [Google Scholar] [CrossRef]
- Shang, Z.-L.; Ma, L.-G.; Zhang, H.-L.; He, R.-R.; Wang, X.-C.; Cui, S.-J.; Sun, D.-Y. Ca2+ influx into lily pollen grains through a hyperpolarization-activated Ca2+-permeable channel which can be regulated by extracellular CaM. Plant Cell Physiol. 2005, 46, 598–608. [Google Scholar] [CrossRef]
- Chang, F.; Yan, A.; Zhao, L.-N.; Wu, W.-H.; Yang, Z. A putative calcium-permeable cyclic nucleotide-gated channel, CNGC18, regulates polarized pollen tube growth. J. Integr. Plant Biol. 2007, 49, 1261–1270. [Google Scholar] [CrossRef]
- Frietsch, S.; Wang, Y.-F.; Sladek, C.; Poulsen, L.R.; Romanowsky, S.M.; Schroeder, J.I.; Harper, J.F. A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc. Natl. Acad. Sci. USA 2007, 104, 14531–14536. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.-F.; Fei, C.-F.; Dong, J.-Y.; Gu, L.-L.; Wang, Y.-F. Arabidopsis CNGC18 is a Ca2+-permeable channel. Mol. Plant 2014, 7, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lan, W.; Jiang, Y.; Fang, W.; Luan, S. A calcium-dependent protein kinase interacts with and activates a calcium channel to regulate pollen tube growth. Mol. Plant 2014, 7, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.-F.; Gu, L.-L.; Wang, H.-Q.; Fei, C.-F.; Fang, X.; Hussain, J.; Sun, S.-J.; Dong, J.-Y.; Liu, H.; Wang, Y.-F. Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 3096–3101. [Google Scholar] [CrossRef] [PubMed]
- Tunc-Ozdemir, M.; Rato, C.; Brown, E.; Rogers, S.; Mooneyham, A.; Frietsch, S.; Myers, C.T.; Poulsen, L.R.; Malho, R.; Harper, J.F. Cyclic nucleotide gated channels 7 and 8 are essential for male reproductive fertility. PloS ONE 2013, 8, e55277. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chai, X.; Gao, Q.; Zhou, L.; Zhang, S.; Li, L.; Luan, S. Dynamic interactions of plant CNGC subunits and calmodulins drive oscillatory Ca2+ channel activities. Dev. Cell. 2019, 48, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pan, Y.; Tian, W.; Dong, M.; Zhu, H.; Luan, S.; Li, L. Arabidopsis CNGC14 mediates calcium influx required for tip growth in root hairs. Mol. Plant 2017, 10, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Michard, E.; Lima, P.T.; Borges, F.; Silva, A.C.; Portes, M.T.; Carvalho, J.E.; Gilliham, M.; Liu, L.-H.; Obermeyer, G.; Feijo, J.A. Glutamate Receptor–Like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-Serine. Science 2011, 332, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Wudick, M.M.; Portes, M.T.; Michard, E.; Rosas-Santiago, P.; Lizzio, M.A.; Nunes, C.O.; Campos, C.; Damineli, D.S.C.; Carvalho, J.C.; Lima, P.T.; et al. CORNICHON sorting and regulation of GLR channels underlie pollen tube Ca2+ homeostasis. Science 2018, 360, 533–536. [Google Scholar] [CrossRef]
- Basu, D.; Haswell, E.S. Plant mechanosensitive ion channels: An ocean of possibilities. Curr. Opin. Plant Biol. 2017, 40, 43–48. [Google Scholar] [CrossRef]
- Hamilton, E.S.; Jensen, G.S.; Maksaev, G.; Katims, A.; Sherp, A.M.; Haswell, E.S. Mechanosensitive channel MSL8 regulates osmotic forces during pollen hydration and germination. Science 2015, 350, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.S.; Haswell, E.S. The tension-sensitive ion transport activity of MSL8 is critical for its function in pollen hydration and germination. Plant Cell Physiol. 2017, 58, 1222–1237. [Google Scholar] [CrossRef] [PubMed]
- Baxter, I.; Tchieu, J.; Sussman, M.R.; Boutry, M.; Palmgren, M.G.; Gribskov, M.; Harper, J.F.; Axelsen, K.B. Genomic Comparison of P-Type ATPase Ion Pumps in Arabidopsis and Rice. Plant Physiol. 2003, 132, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Schiott, M.; Romanowsky, S.M.; Baekgaard, L.; Jakobsen, M.K.; Palmgren, M.G.; Harper, J.F. A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc. Natl. Acad. Sci. USA 2004, 101, 9502–9507. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, J.; Yang, Z.; Yang, D.-L. Plasma membrane-localized calcium pumps and copines coordinately regulate pollen germination and fertility in Arabidopsis. Int. J. Mol. Sci. 2018, 19, 1774. [Google Scholar] [CrossRef]
- Colaco, R.; Moreno, N.; Feijo, J.A. On the fast lane: Mitochondria structure, dynamics and function in growing pollen tubes. J. Microsc. 2012, 247, 106–118. [Google Scholar] [CrossRef]
- Selles, B.; Michaud, C.; Xiong, T.-C.; Leblanc, O.; Ingouff, M. Arabidopsis pollen tube germination and growth depend on the mitochondrial calcium uniporter complex. New Phytol. 2018, 219, 58–65. [Google Scholar] [CrossRef]
- Holdaway-Clarke, T.L.; Feijo, J.A.; Hackett, G.R.; Kunkel, J.G.; Hepler, P.K. Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell. 1999, 9, 1999–2010. [Google Scholar] [CrossRef]
- Polya, G.M.; Micucci, V.; Rae, A.L.; Harris, P.J.; Clarke, A.E. Ca2+-dependent protein phosphorylation in germinated pollen of Nicotiana alata, an ornamental tobacco. Physiol. Plant. 1986, 67, 151–157. [Google Scholar] [CrossRef]
- Estruch, J.J.; Kadwell, S.; Merlin, E.; Crossland, L. Cloning and characterization of a maize pollen-specific calcium-dependent calmodulin-independent protein kinase. Proc. Natl. Acad. Sci. USA 1994, 91, 8837–8841. [Google Scholar] [CrossRef]
- Harmon, A.C.; Gribskov, M.; Gubrium, E.; Harper, J.F. The CDPK superfamily of.protein kinases. New Phytol. 2001, 151, 175–183. [Google Scholar] [CrossRef]
- Yoon, G.M.; Dowd, P.E.; Gilroy, S.; McCubbin, A.G. Calcium-Dependent Protein Kinase Isoforms in Petunia Have Distinct Functions in Pollen Tube Growth, Including Regulating Polarity. Plant Cell. 2006, 18, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Deng, Y.; Chen, P.; Feng, F.; Chen, W.; Zhou, X.; Wang, Y. A calcium-dependent protein kinase, ZmCPK32, specifically expressed in maize pollen to regulate pollen tube growth. PLoS ONE 2018, 13, e0195787. [Google Scholar] [CrossRef] [PubMed]
- Hrabak, E.M.; Chan, C.W.M.; Gribskov, M.; Harper, J.F.; Choi, J.H.; Halford, N.; Kudla, J.; Luan, S.; Nimmo, H.G.; Sussman, M.R.; et al. The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol. 2003, 132, 666–680. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fu, Y.; Yang, Z. A Genome-wide functional characterization of Arabidopsis regulatory calcium sensors in pollen tubes. J. Integr. Plant Biol. 2009, 51, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.; Romanowsky, S.M.; Barron, Y.D.; Garg, S.; Azuse, C.L.; Curran, A.; Davis, R.M.; Hatton, J.; Harmon, A.C.; Harper, J.F. Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes. Plant J. 2009, 59, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-N.; Shen, L.-K.; Zhang, W.-Z.; Zhang, W.; Wang, Y.; Wu, W.-H. Ca2+-dependent protein kinase11 and 24 modulate the activity of the inward rectifying K+ channels in Arabidopsis pollen tubes. Plant Cell 2013, 25, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Gutermuth, T.; Lassig, R.; POrtes, M.-T.; Maierhofer, T.; Romeis, T.; Borst, J.-W.; Hedrich, R.; Feijo, J.A.; Konrad, K.R. Pollen tube growth regulation by free anions depends on the interaction between the anion channel SLAH3 and calcium-dependent protein kinases CPK2 and CPK20. Plant Cell 2013, 25, 4525–4543. [Google Scholar] [CrossRef]
- Zhou, L.; Lan, W.; Chen, B.; Fang, W.; Luan, S. A Calcium sensor-regulated protein kinase, CALCINEURIN B-LIKE PROTEIN-INTERACTING PROTEIN KINASE19, is required for pollen tube growth and polarity. Plant Physiol. 2015, 167, 1351–1360. [Google Scholar] [CrossRef]
- Steinhorst, L.; Mahs, A.; Ischebeck, T.; Zhang, C.; Zhang, X.; Arendt, S.; Schultke, S.; Heilmann, I.; Kudla, J. Vacuolar CBL-CIPK12 Ca2+-sensor-kinase complexes are required for polarized pollen tube growth. Curr. Biol. 2015, 25, 1475–1482. [Google Scholar] [CrossRef]
- Mahs, A.; Steinhorst, L.; Han, J.-P.; Shen, L.-K.; Wang, Y.; Kudla, J. The Calcineurin B-Like Ca2+ Sensors CBL1 and CBL9 function in pollen germination and pollen tube growth in Arabidopsis. Mol. Plant 2013, 6, 1149–1162. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, S.-S.; Wang, M.; Qiao, Z.; Bao, C.-C.; Zhang, W. Arabidopsis thaliana calmodulin-like protein CML24 regulates pollen tube growth by modulating the actin cytoskeleton and controlling the cytosolic Ca2+ concentration. Plant Mol. Biol. 2014, 86, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Diao, W.-Z.; Yang, X.; Qiao, Z.; Wang, M.; Acharya, B.R.; Zhang, W. Arabidopsis thaliana CML25 mediates the Ca2+ regulation of K+ transmembrane trafficking during pollen germination and tube elongation. Plant Cell Environ. 2015, 38, 2372–2386. [Google Scholar] [CrossRef] [PubMed]
- Suwinska, A.; Wasag, P.; Zakrzewski, P.; Lenartowska, M.; Lenartowski, R. Calreticulin is required for calcium homeostasis and proper pollen tube tip growth in Petunia. Planta 2017, 245, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Nardi, M.C.; Feron, R.; Navazio, L.; Mariani, P.; Pierson, E.; Wolters-Arts, M.; Knuiman, B.; Mariani, C.; Derksen, J. Expression and localization of calreticulin in tobacco anthers and pollen tubes. Planta 2006, 223, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Zhang, W.; Xing, Y.; Zhang, Q.; Yang, L.; Cao, Q.; Qin, L. Boron ‘Malus domestica pollen tube growth. Front. Plant Sci. 2016, 7, 208. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.F.; Du, B.S.; Zhang, Q.; Xing, Y.; Cao, Q.Q.; Qin, L. Boron deficiency alters cytosolic Ca2+ concentration and affects the cell wall components of pollen tubes in Malus domestica. Plant Biol. 2019, 21, 343–351. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, T.; Zhang, C.; Hao, H.; Liu, P.; Zheng, M.; Baluska, F.; Samaj, J.; Lin, J. Nitric oxide modulates the influx of extracellular Ca2+ and actin filament organization during cell wall construction in Pinus bungeana pollen tubes. New Phytol. 2009, 182, 851–862. [Google Scholar] [CrossRef]
- Kaya, H.; Nakajima, R.; Iwano, M.; Kanaoka, M.M.; Kimura, S.; Takeda, S.; Kawarazaki, T.; Senzaki, E.; Hamamura, Y.; Higashiyama, T.; et al. Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 2014, 26, 1069–1080. [Google Scholar] [CrossRef]
- Aloisi, I.; Cai, G.; Faleri, C.; Navazio, L.; Serafini-Fracassnini, D.; Del Duca, S. Spermine regulates pollen tube growth by modulating Ca2+-dependent actin organization and cell wall structure. Front. Plant Sci. 2017, 8, 1701. [Google Scholar] [CrossRef]
- Zheng, Z.-L.; Yang, Z. The Rop GTPase: An emerging signaling switch in plants. Plant Mol. Biol. 2000, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. Small GTPases: Versatile signaling switches in plants. Plant Cell. 2002, 14, s375–s388. [Google Scholar] [CrossRef] [PubMed]
- Klahre, U.; Becker, C.; Schmitt, A.C.; Kost, B. Nt-RhoGDI2 regulates Rac/Rop signaling and polar cell growth in tobacco pollen tubes. The Plant J. 2006, 46, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.-N.; Kang, H.; Song, S.-J.; Ge, F.-R.; Zhang, Y.-L.; Li, E.; Li, S.; Zhang, Y. Arabidopsis RhoGDIs are critical for cellular homeostasis of pollen tubes. Plant Physiol. 2016, 170, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Berken, A.; Thomas, C.; Wittinghofer, A. A new family of RhoGEFs activates the Rop molecular switch in plants. Nature 2005, 436, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, S.; Lord, E.M.; Yang, Z. Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase-dependent polar growth. Plant Cell 2006, 18, 366–381. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; McCormick, S. Two Arabidopsis AGC kinases are critical for the polarized growth of pollen tubes. Plant J. 2009, 58, 474–484. [Google Scholar] [CrossRef]
- Li, E.; Cui, Y.; Ge, F.-R.; Chai, S.; Zhang, W.-T.; Feng, Q.-N.; Jiang, L.; Li, S.; Zhang, Y. AGC1.5 kinase phosphorylates RopGEFs to control pollen tube growth. Mol. Plant 2018, 11, 1198–1209. [Google Scholar] [CrossRef]
- Bogre, L.; Okresz, L.; Henriques, R.; Anthony, R.G. Growth signalling pathways in Arabidopsis and the AGC protein kinases. Trends Plant Sci. 2003, 8, 424–431. [Google Scholar] [CrossRef]
- Yu, Y.; Song, J.; Tian, X.; Zhang, H.; Li, L.; Zhu, H. Arabidopsis PRK6 interacts specifically with AtRopGEF8/12 and induces depolarized growth of pollen tubes when overexpressed. Sci. China Life Sci. 2018, 61, 100–112. [Google Scholar] [CrossRef]
- Zhang, Y.; McCormick, S. A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 18830–18835. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Gu, Y.; Ma, H.; Yang, Z. AtPRK2 promotes ROP1 activation via RopGEFs in the control of polarized pollen tube growth. Mol. Plant 2013, 6, 1187–1201. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chu, L.-C.; Liang, Y.; Zhang, X.-Q.; Chen, L.-Q.; Ye, D. The Arabidopsis CrRLK1L protein kinases BUPS1 and BUPS2 are required for normal growth of pollen tubes in the pistil. Plant J. 2018, 95, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-U.; Vernoud, V.; Szumlanski, A.; Nielsen, E.; Yang, Z. A tip-localized Rho GTPase-activating protein controls cell polarity by globally inhibiting Rho GTPase at the cell apex. Curr. Biol. 2008, 18, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Luo, N.; Wang, W.; Liu, Z.; Chen, J.; Zhao, L.; Tan, L.; Wang, C.; Qin, Y.; Li, C.; et al. The REN4 rheostat dynamically coordinates the apical and lateral domains of Arabidopsis pollen tubes. Nat. Comm. 2018, 9, 2573. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gong, P.; Guo, J.; Li, H.; Li, R.; Xing, W.; Yang, Z.; Guan, Y. Glycolysis regulates pollen tube polarity via Rho GTPase signaling. PLoS Genet. 2018, 14, e1007373. [Google Scholar] [CrossRef]
- Rong, D.; Luo, N.; Mollet, J.C.; Liu, X.; Yang, Z. Salicylic acid regulates pollen tip growth through an NPR3/NPR4-independent pathway. Mol. Plant 2016, 9, 1478–1491. [Google Scholar] [CrossRef]
- Klahre, U.; Kost, B. Tobacco RhoGTPase ACTIVATING PROTEIN1 spatially restricts signaling of RAC/Rop to the apex of pollen tubes. Plant Cell 2006, 18, 3033–3046. [Google Scholar] [CrossRef]
- Luo, N.; Yan, A.; Liu, G.; Guo, J.; Rong, D.; Kanaoka, M.M.; Xiao, Z.; Xu, G.; Higashiyama, T.; Cui, X.; et al. Exocytosis-coordinated mechanisms for tip growth underlie pollen tube growth guidance. Nat. Comm. 2017, 8, 1687. [Google Scholar] [CrossRef]
- Takeuchi, H.; Higashiyama, T. Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 2016, 531, 245–260. [Google Scholar] [CrossRef]
- Berken, A. ROPs in the spotlight of plant signal transduction. Cell Mol. Life Sci. 2006, 63, 2446–2459. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Gu, Y.; Li, S.; Yang, Z. A Genome-wide analysis of Arabidopsis Rop-Interactive CRIB motif–containing proteins that act as Rop GTPase targets. Plant Cell 2001, 13, 2841–2856. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Szumlanski, A.; Nielsen, E.; Yang, Z. Rho-GTPase–dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J. Cell Biol. 2008, 181, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Fu, Y.; Dowd, P.; Li, S.; Vernoud, V.; Gilroy, S.; Yang, Z. A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J. Cell Biol. 2005, 169, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Shi, H.; Chen, B.; Zhang, R.; Huang, S.; Fu, Y. Arabidopsis RIC1 severs actin filaments at the apex to regulate pollen tube growth. Plant Cell 2015, 27, 1140–1161. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-W.; Wang, C.-S. Lily Cdc42/Rac-interactive binding motif-containing protein, a Rop target, involves calcium influx and phosphoproteins during pollen germination and tube growth. Plant Signal. Behav. 2010, 5, 1460–1463. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, S.; Gu, Y.; Yan, A.; Lord, E.; Yang, Z.-B. RIP1 (ROP Interactive Partner 1)/ICR1 marks pollen germination sites and may act in the ROP1 pathway in the control of polarized pollen growth. Mol. Plant 2008, 1, 1021–1035. [Google Scholar] [CrossRef]
- Lavy, M.; Bloch, D.; Hazak, O.; Gutman, I.; Poraty, L.; Sorek, N.; Sternberg, H.; Yalovsky, S. A novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr. Biol. 2007, 17, 947–952. [Google Scholar] [CrossRef]
- Masiero, L.; Lapidos, K.A.; Ambudkar, I.; Kohn, E.C. Regulation of the RhoA pathway in human endothelial cell spreading on type IV collagen: Role of calcium influx. J. Cell Sci. 1999, 112, 3205–3213. [Google Scholar]
- Gong, B.; Shen, W.; Xiao, W.; Meng, Y.; Meng, A.; Jia, S. The Sec14-like phosphatidylinositol transfer proteins Sec14l3/SEC14L2 act as GTPase proteins to mediate Wnt/Ca2+ signaling. eLife 2017, 6, e26362. [Google Scholar] [CrossRef]
- Stadler, S.; Nguyen, C.H.; Schachner, H.; Milovanovic, D.; Holzner, S.; Brenner, S.; Eichsteininger, J.; Stadler, M.; Senfter, D.; Krenn, L.; et al. Colon cancer cell-derived 12(S)-HETE induces the retraction of cancer-associated fibroblast via MLC2, RHO/ROCK and Ca2+ signalling. Cell. Mol. Life Sci. 2017, 74, 1907–1921. [Google Scholar] [CrossRef] [PubMed]
- Kelner, A.; Leitao, N.; Chabaud, M.; Charpentier, M.; de Carvalho-Niebel, F. Dual color sensors for simultaneous analysis of calcium signal dynamics in the nuclear and cytoplasmic compartments of plant cells. Front. Plant Sci. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-U.; Gu, Y.; Lee, Y.-J.; Yang, Z. Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol. Biol. Cell. 2005, 16, 5385–5399. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheible, N.; McCubbin, A. Signaling in Pollen Tube Growth: Beyond the Tip of the Polarity Iceberg. Plants 2019, 8, 156. https://doi.org/10.3390/plants8060156
Scheible N, McCubbin A. Signaling in Pollen Tube Growth: Beyond the Tip of the Polarity Iceberg. Plants. 2019; 8(6):156. https://doi.org/10.3390/plants8060156
Chicago/Turabian StyleScheible, Nolan, and Andrew McCubbin. 2019. "Signaling in Pollen Tube Growth: Beyond the Tip of the Polarity Iceberg" Plants 8, no. 6: 156. https://doi.org/10.3390/plants8060156
APA StyleScheible, N., & McCubbin, A. (2019). Signaling in Pollen Tube Growth: Beyond the Tip of the Polarity Iceberg. Plants, 8(6), 156. https://doi.org/10.3390/plants8060156



