Responses of Tomato Plants under Saline Stress to Foliar Application of Copper Nanoparticles
Abstract
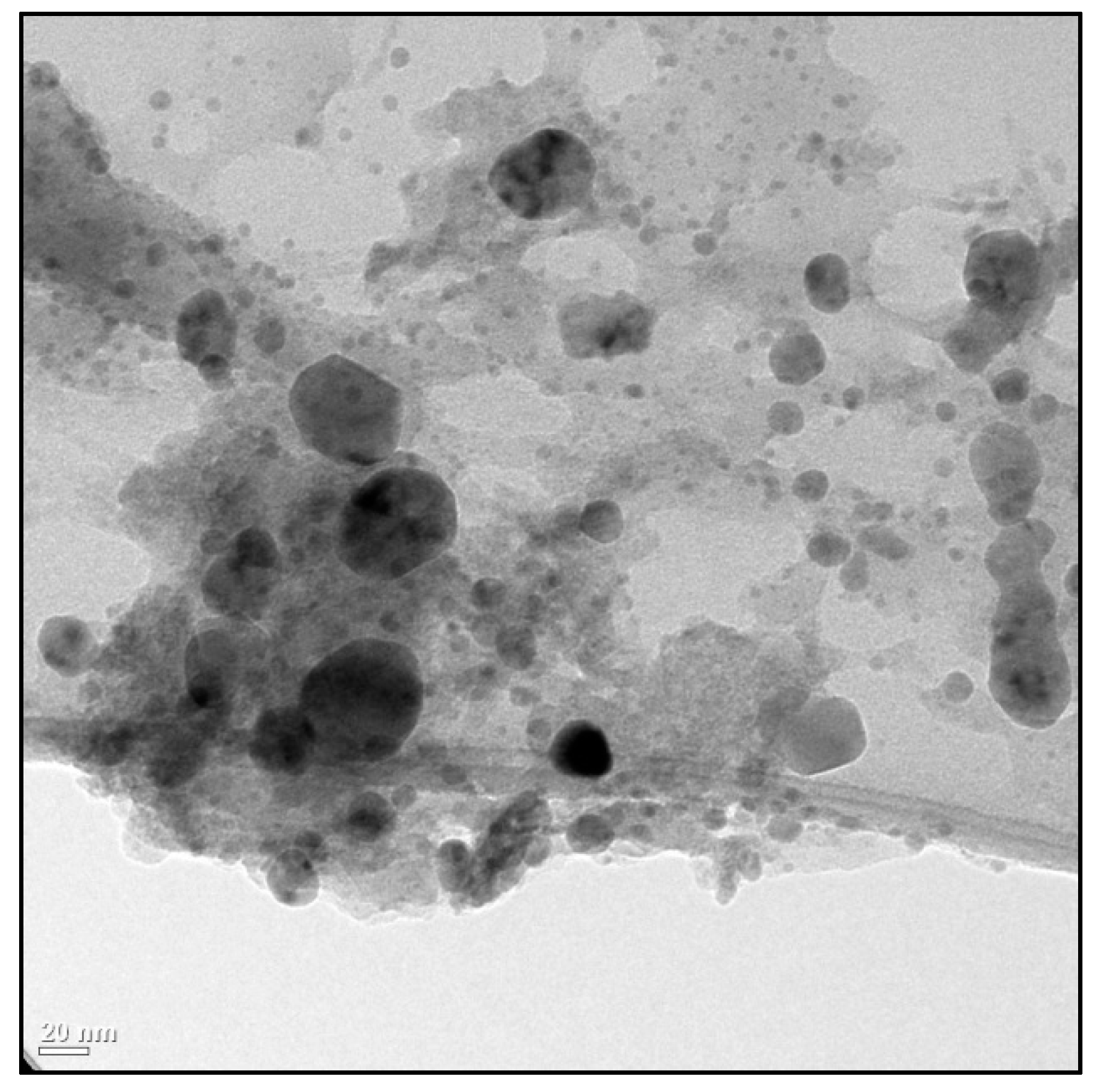
1. Introduction
2. Results and Discussion
2.1. Tomato Crop Growth
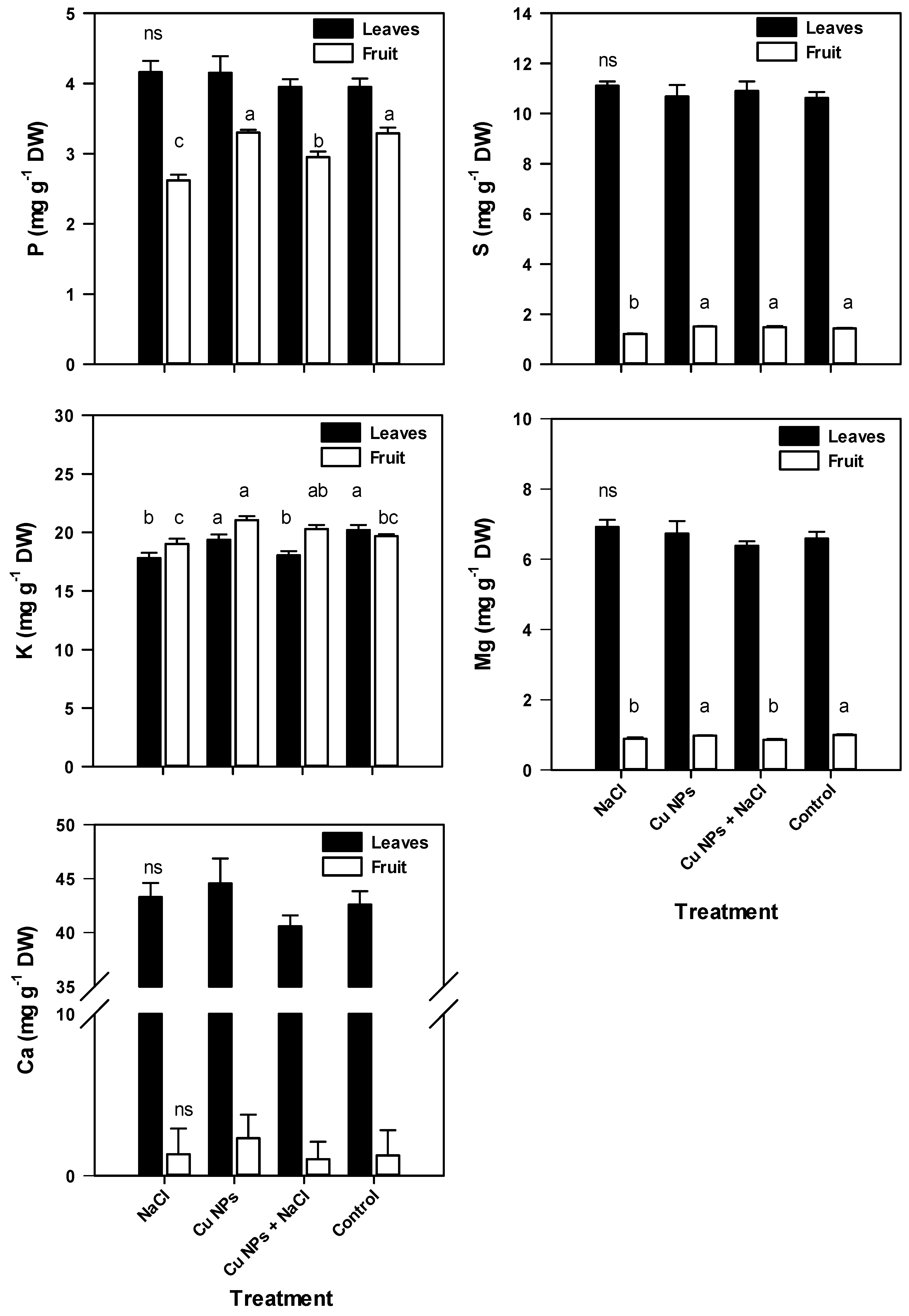
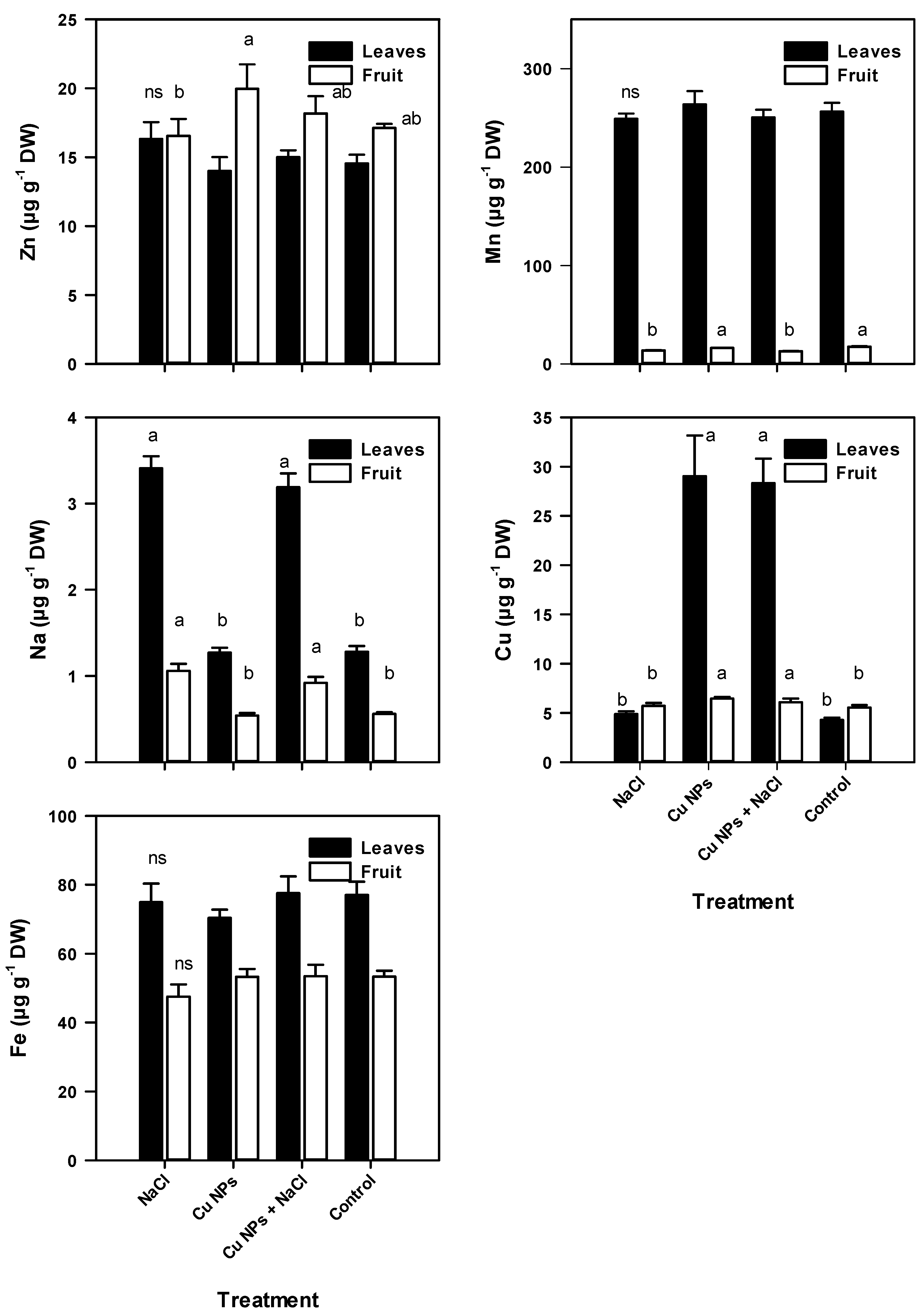
2.2. Mineral Content of Tomato Plants
2.3. Content of Photosynthetic Pigments
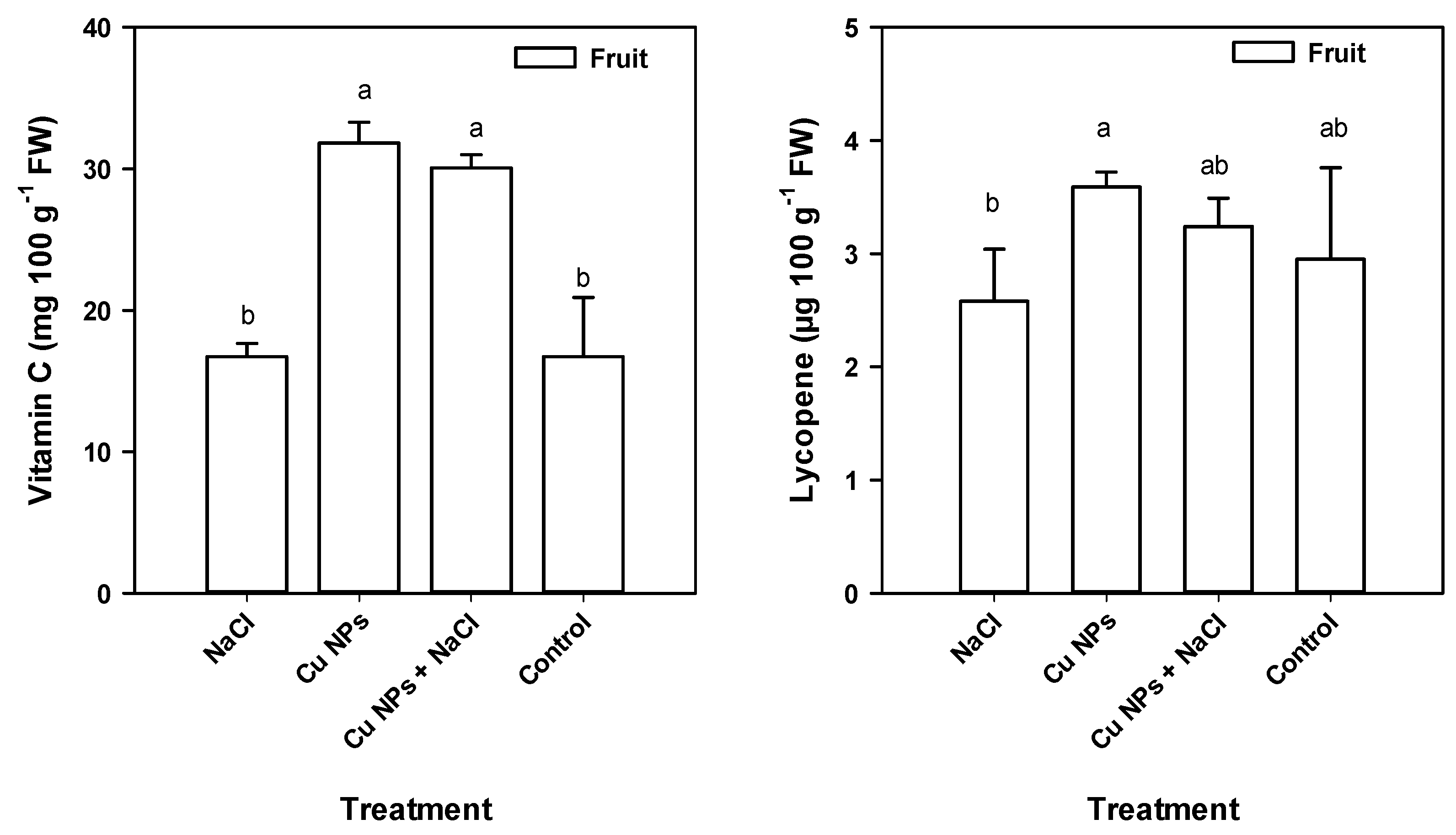
2.4. Tomato Fruit Quality
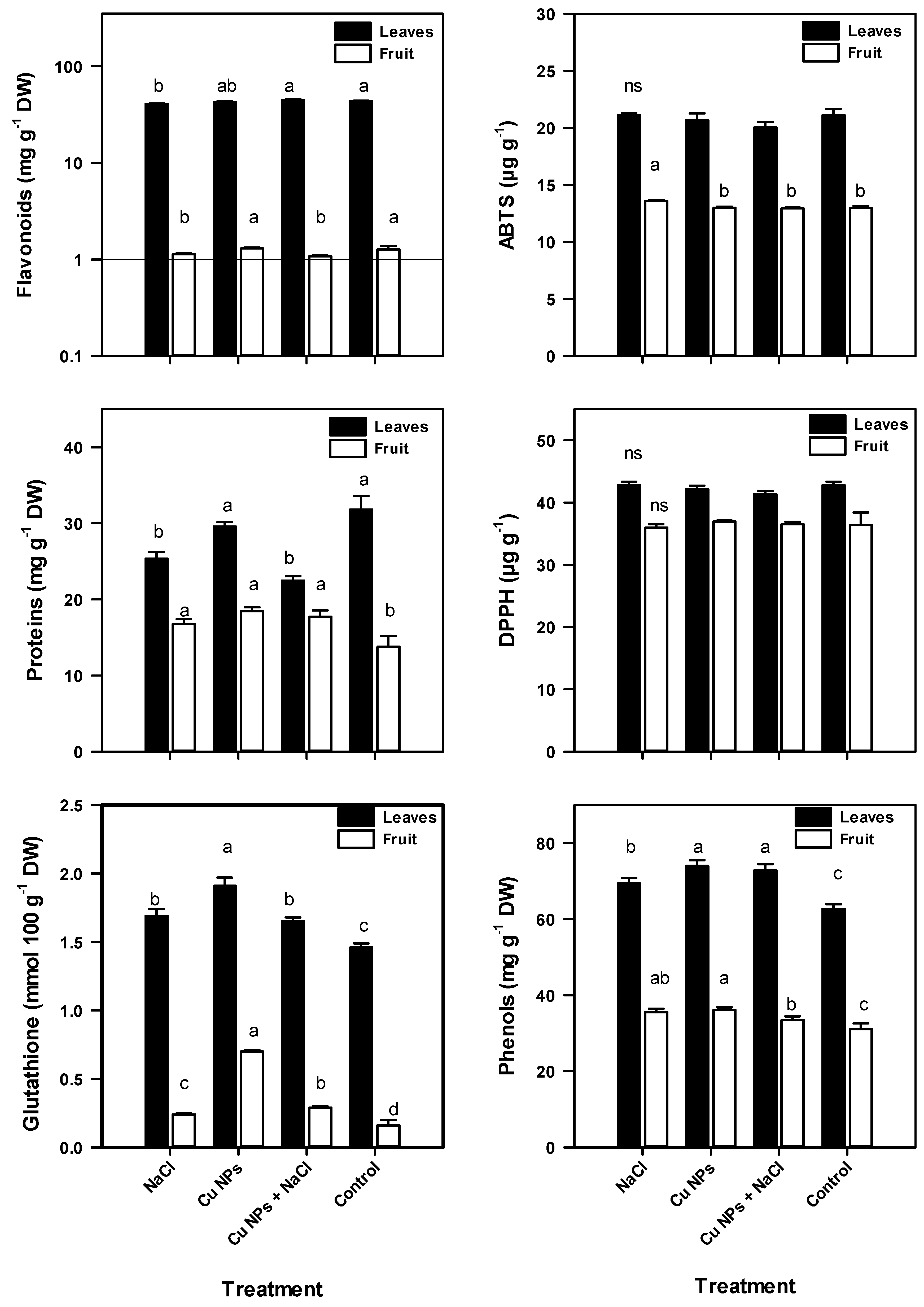
2.5. Antioxidant Compounds of Tomato Plants
2.6. Antioxidant Activity of Tomato Plants
3. Materials and Methods
3.1. Crop Growth
3.2. Treatments
3.3. Agronomical and Biochemical Variables
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Machado, R.; Serralheiro, R. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Kaushik, D.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Turkan, I. Emerging roles for ROS and RNS—Versatile molecules in plants. J. Exp. Bot. 2017, 69, 3313–3315. [Google Scholar] [CrossRef]
- Hossain, M.S.; Dietz, K.-J. Tuning of Redox Regulatory Mechanisms, Reactive Oxygen Species and Redox Homeostasis under Salinity Stress. Front. Plant Sci. 2016, 7, 548. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Hossain, M.S.; ElSayed, A.I.; Moore, M.; Dietz, K.J. Redox and reactive oxygen species network in acclimation for salinity tolerance in sugar beet. J. Exp. Bot. 2017, 68, 1283–1298. [Google Scholar] [CrossRef] [PubMed]
- Koleška, I.; Hasanagić, D.; Maksimović, I.; Bosančić, B.; Kukavica, B. The role of antioxidative metabolism of tomato leaves in long-term salt-stress response. Zeitschrift fur Pflanzenernahrung und Bodenkd 2017, 180, 105–112. [Google Scholar] [CrossRef]
- De Cássia Alves, R.; de Medeiros, A.S.; Nicolau, M.C.M.; Neto, A.P.; de Assis oliveira, F.; Lima, L.W.; Tezotto, T.; Gratão, P.L. The partial root-zone saline irrigation system and antioxidant responses in tomato plants. Plant Physiol. Biochem. 2018, 127, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Amador, B.; Reyes-Pérez, J.J.; Hernández-Montiel, L.G.; Rueda-Puente, E.O.; De Lucia, B.; Beltrán-Morales, F.A.; Ruiz-Espinoza, F.H. Physiological responses to salinity in Solanum lycopersicum L. varieties. Pak. J. Bot. 2017, 49, 809–818. [Google Scholar]
- Torabian, S.; Farhangi-Abriz, S.; Zahedi, M. Efficacy of FeSO4 nano formulations on osmolytes and antioxidative enzymes of sunflower under salt stress. Indian J. Plant Physiol. 2018, 23, 305–315. [Google Scholar] [CrossRef]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef] [PubMed]
- Abdel Latef, A.A.H.; Srivastava, A.K.; El-sadek, M.S.A.; Kordrostami, M.; Tran, L.-S.P. Titanium Dioxide Nanoparticles Improve Growth and Enhance Tolerance of Broad Bean Plants under Saline Soil Conditions. Land Degrad. Dev. 2017, 29, 1065–1073. [Google Scholar] [CrossRef]
- Karami Mehrian, S.; Heidari, R.; Rahmani, F. Effect of silver nanoparticles on free amino acids content and antioxidant defense system of tomato plants. Indian J. Plant Physiol. 2015, 20, 257–263. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Abu Alhmad, M.F.; Abdelfattah, K.E. The Possible Roles of Priming with ZnO Nanoparticles in Mitigation of Salinity Stress in Lupine (Lupinus termis) Plants. J. Plant Growth Regul. 2017, 36, 60–70. [Google Scholar] [CrossRef]
- Haghighi, M.; Pessarakli, M. Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage. Sci. Hortic. Amst. 2013, 161, 111–117. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Torabian, S. Nano-silicon alters antioxidant activities of soybean seedlings under salt toxicity. Protoplasma 2018, 255, 953–962. [Google Scholar] [CrossRef]
- El-Sharkawy, M.S.; El-Beshsbeshy, T.R.; Mahmoud, E.K.; Abdelkader, N.I.; Al-Shal, R.M.; Missaoui, A.M. Response of Alfalfa under Salt Stress to the Application of Potassium Sulfate Nanoparticles. Am. J. Plant Sci. 2017, 8, 1751–1773. [Google Scholar] [CrossRef]
- Hernández-Fuentes, A.D.; López-Vargas, E.R.; Pinedo-Espinoza, J.M.; Campos-Montiel, R.G.; Valdés-Reyna, J.; Juárez-Maldonado, A. Postharvest behavior of bioactive compounds in tomato fruits treated with Cu nanoparticles and NaCl stress. Appl. Sci. 2017, 7, 980. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.; González-Morales, S.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Effects of chitosan–PVA and Cu nanoparticles on the growth and antioxidant capacity of tomato under saline stress. Molecules 2018, 23, 178. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Aliasgharzadeh, N.; Laiegh, S.F.; Poschenrieder, C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 2010, 331, 313–327. [Google Scholar] [CrossRef]
- Shobha, G.; Moses, V.; Ananda, S. Biological synthesis of copper nanoparticles and its impact: A review. Int. J. Pharm. Sci. Invent. 2014, 3, 2319–6718. [Google Scholar]
- Pérez-de-Luque, A. Interaction of nanomaterials with plants: What do we need for real applications in agriculture? Front. Environ. Sci. 2017, 5, 12. [Google Scholar] [CrossRef]
- Fathi, A.; Zahedi, M.; Torabian, S.; Khoshgoftar, A. Response of wheat genotypes to foliar spray of ZnO and Fe2O3 nanoparticles under salt stress. J. Plant Nutr. 2017, 40, 1376–1385. [Google Scholar] [CrossRef]
- Blumwald, E. Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 2000, 12, 431–434. [Google Scholar] [CrossRef]
- Mosa, K.A.; El-Naggar, M.; Ramamoorthy, K.; Alawadhi, H.; Elnaggar, A.; Wartanian, S.; Ibrahim, E.; Hani, H. Copper Nanoparticles Induced Genotoxicty, Oxidative Stress, and Changes in Superoxide Dismutase (SOD) Gene Expression in Cucumber (Cucumis sativus) Plants. Front. Plant Sci. 2018, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- De Pilippis, L.; Pallagrani, C. Heavy Metal: Sources and Biological Effects. In Algae and Water Pollution; Rai, L.C., Gaur, J.P., Soeder, C.J., Eds.; Schweizerbart: Stuttgart, Germany, 1994; Volume 42, pp. 31–77. [Google Scholar]
- Chandra, R.; Kang, H. Mixed heavy metal stress on photosynthesis, transpiration rate, and chlorophyll content in poplar hybrids. For. Sci. Technol. 2016, 12, 55–61. [Google Scholar] [CrossRef]
- AlQuraidi, A.O.; Mosa, K.A.; Ramamoorthy, K. Phytotoxic and Genotoxic Effects of Copper Nanoparticles in Coriander (Coriandrum sativum—Apiaceae). Plants 2019, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Mestre, T.C.; Mittler, R.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.G.; Kobayashi, Y.; Sanuki, A.; Kondo, S.; Fukuda, N.; Ezura, H.; Sugaya, S.; Matsukura, C. Salinity induces carbohydrate accumulation and sugar-regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv. ’Micro-Tom’) fruits in an ABA-and osmotic stress-independent manner. J. Exp. Bot. 2010, 61, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Senge, M.; Dai, Y. Effects of Salinity Stress at Different Growth Stages on Tomato Growth, Yield, and Water-Use Efficiency. Commun. Soil Sci. Plant Anal. 2017, 48, 624–634. [Google Scholar] [CrossRef]
- Campos, C.A.B.; Fernandes, P.D.; Gheyi, H.R.; Blanco, F.F.; Gonçalves, C.B.; Campos, S.A.F. Yield and fruit quality of industrial tomato under saline irrigation. Sci. Agric. 2006, 63, 146–152. [Google Scholar] [CrossRef]
- Zhao, L.; Ortiz, C.; Adeleye, A.S.; Hu, Q.; Zhou, H.; Huang, Y.; Keller, A.A. Metabolomics to Detect Response of Lettuce (Lactuca sativa) to Cu(OH)2 Nanopesticides: Oxidative Stress Response and Detoxification Mechanisms. Environ. Sci. Technol. 2016, 50, 9697–9707. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, X.; Zhao, J.; Liu, X.; Feng, W.; White, J.C.; Xing, B. Xylem- and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environ. Sci. Technol. 2012, 46, 4434–4441. [Google Scholar] [CrossRef] [PubMed]
- López-Vargas, E.R.; Ortega-ortiz, H.; Cadenas-pliego, G.; De-Alba-Romenus, K.; Cabrera-De-La-Fuente, M.; Benavides-Mendoza, A.; Juarez-Maldonado, A. Foliar Application of Copper Nanoparticles Increases the Fruit Quality and the Content of Bioactive Compounds in Tomatoes. Appl. Sci. 2018, 8, 1020. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R. Reactive oxygen species and photosynthesis. In Oxidative Damage to Plants; Ahmad, P., Ed.; Elsevier: San Diego, CA, USA, 2014; pp. 1–63. [Google Scholar]
- Chen, Z.; Young, T.E.; Ling, J.; Chang, S.-C.; Gallie, D.R. Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc. Natl. Acad. Sci. USA 2003, 100, 3525–3530. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-García, B.; Queval, G.; Foyer, C. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From chemistry to biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Croft, K.D. The Chemistry and Biological Effects of Flavonoids and Phenolic Acidsa. Ann. N. Y. Acad. Sci. 1998, 854, 435–442. [Google Scholar] [CrossRef] [PubMed]
- D’Amelia, V.; Aversano, R.; Chiaiese, P.; Carputo, D. The antioxidant properties of plant flavonoids: Their exploitation by molecular plant breeding. Phytochem. Rev. 2018, 17, 611–625. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Pinedo-Guerrero, Z.H.; Delia Hernández-Fuentes, A.; Ortega-Ortiz, H.; Benavides-Mendoza, A.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Cu nanoparticles in hydrogels of chitosan-PVA affects the characteristics of post-harvest and bioactive compounds of jalapeño pepper. Molecules 2017, 22, 1–14. [Google Scholar] [CrossRef]
- Askary, M.; Talebi, S.M.; Amini, F.; Dousti, A.; Bangan, B. Effects of iron nanoparticles on Mentha piperita L. under salinity stress. Biologija 2017, 63, 65–75. [Google Scholar] [CrossRef]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q.; Chen, Z. Functional Analysis of the Arabidopsis PAL Gene Family in Plant Growth, Development, and Response to Environmental Stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef]
- Saibi, W.; Brini, F. Superoxide dismutase (SOD) and abiotic stress tolerance in plants: An overview. In Superoxide Dismutase: Structure, Synthesis and Applications; Magliozzi, S., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2018; pp. 101–142. [Google Scholar]
- Wu, H.; Shabala, L.; Shabala, S.; Giraldo, J.P. Hydroxyl radical scavenging by cerium oxide nanoparticles improves Arabidopsis salinity tolerance by enhancing leaf mesophyll potassium retention. Environ. Sci. Nano 2018, 5, 1567–1583. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Hwang, H.-M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Adeleye, A.S.; Zhao, L.; Minakova, A.S.; Anumol, T.; Keller, A.A. Antioxidant response of cucumber (Cucumis sativus) exposed to nano copper pesticide: Quantitative determination via LC-MS/MS. Food Chem. 2019, 270, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, M.V.J.; Sharma, P.K. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 2016, 54, 110–119. [Google Scholar] [CrossRef]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles Alter Secondary Metabolism in Plants via ROS Burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tito, N.; Giraldo, J.P. Anionic Cerium Oxide Nanoparticles Protect Plant Photosynthesis from Abiotic Stress by Scavenging Reactive Oxygen Species. ACS Nano 2017, 11, 11283–11297. [Google Scholar] [CrossRef] [PubMed]
- Schilmiller, A.L.; Last, R.L.; Pichersky, E. Harnessing plant trichome biochemistry for the production of useful compounds. Plant J. 2008, 54, 702–711. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef]
- Romero-Aranda, M.R.; Jurado, O.; Cuartero, J. Silicon alleviates the deleterious salt effect on tomato plant growth by improving plant water status. J. Plant Physiol. 2006, 163, 847–855. [Google Scholar] [CrossRef]
- Juárez-Maldonado, A.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; Valdés-Reyna, J.; Pinedo-Espinoza, J.; López-Palestina, C.; Hernández-Fuentes, A. Foliar Application of Cu Nanoparticles Modified the Content of Bioactive Compounds in Moringa oleifera Lam. Agronomy 2018, 8, 167. [Google Scholar] [CrossRef]
- Kurepa, J.; Paunesku, T.; Vogt, S.; Arora, H.; Rabatic, B.M.; Lu, J.; Wanzer, M.B.; Woloschak, G.E.; Smalle, J.A. Uptake and Distribution of Ultrasmall Anatase TiO2 Alizarin Red S Nanoconjugates in Arabidopsis thaliana. Nano Lett. 2010, 10, 2296–2302. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-N.; Tarafdar, J.C.; Biswas, P. Nanoparticle synthesis and delivery by an aerosol route for watermelon plant foliar uptake. J. Nanoparticle Res. 2013, 15, 1417. [Google Scholar] [CrossRef]
- Lv, J.; Christie, P.; Zhang, S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: Recent advances and methodological challenges. Environ. Sci. Nano 2019, 6, 41–59. [Google Scholar] [CrossRef]
- Keller, A.A.; Huang, Y.; Nelson, J. Detection of nanoparticles in edible plant tissues exposed to nano-copper using single-particle ICP-MS. J. Nanoparticle Res. 2018, 20, 101. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. J. Jpn. Soc. Food Sci. Technol. Shokuhin Kagaku Kogaku Kaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Sykłowska-Baranek, K.; Pietrosiuk, A.; Naliwajski, M.R.; Kawiak, A.; Jeziorek, M.; Wyderska, S.; Łojkowska, E.; Chinou, I. Effect of l-phenylalanine on PAL activity and production of naphthoquinone pigments in suspension cultures of Arnebia euchroma (Royle) Johnst. In Vitro Cell. Dev. Biol. Plant 2012, 48, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
| Treatment | Plant Height (cm) | Stem Diameter (mm) | Fresh Weight of Aerial Biomass (kg) | Dry Weight of Aerial Biomass (g) | Fruit Weight per Plant (kg) |
|---|---|---|---|---|---|
| NaCl | 245.78 b ± 1.66 | 12.07 ab ± 0.15 | 1.248 b ± 0.045 | 171.38 b ± 6.73 | 3.604 b ± 0.197 |
| Cu NPs | 263.33 a ± 3.04 | 12.53 a ± 0.42 | 1.500 a ± 0.0 52 | 213.29 a ± 8.58 | 5.950 a ± 0.121 |
| Cu NPs + NaCl | 242.94 b ± 9.25 | 12.49 a ± 0.19 | 1.226 b ± 0.040 | 176.73 b ± 5.38 | 3.857 b ± 0.132 |
| Control | 262.39 a ± 2.82 | 11.59 b ± 0.15 | 1.425 a ± 0.045 | 208.94 a ± 6.73 | 6.158 a ± 0.197 |
| Treatment | Chlorophyll a (mg 100 g−1 DW) | Chlorophyll b (mg 100 g−1 DW) | Total Chlorophyll (mg 100 g−1 DW) |
|---|---|---|---|
| NaCl | 46.90 a ± 1.94 | 24.13 a ± 1.41 | 71.03 a ± 2.97 |
| Cu NPs | 34.19 b ± 1.93 | 22.71 a ± 1.88 | 56.90 b ± 1.28 |
| Cu NPs + NaCl | 25.48 c ± 1.06 | 13.83 b ± 0.97 | 39.31 d ± 1.19 |
| Control | 46.90 a ± 1.94 | 24.13 a ± 1.41 | 71.03 a ± 2.97 |
| Treatment | Firmness (kg cm−2) | pH | EC (mS cm−1) | TSS (°Brix) | TA (% ac) | ORP (mV) |
|---|---|---|---|---|---|---|
| NaCl | 3.29 a ± 0.06 | 4.38 b ± 0.03 | 4.18 a ± 0.11 | 6.05 a ± 0.11 | 0.99 a ± 0.04 | 147.83 a ± 2.02 |
| Cu NPs | 3.46 a ± 0.11 | 4.48 a ± 0.02 | 3.66 ab ± 0.23 | 4.93 b ± 0.05 | 0.80 b ± 0.04 | 140.17 b ± 0.83 |
| Cu NPs + NaCl | 3.45 a ± 0.08 | 4.49 a ± 0.02 | 3.69 ab ± 0.23 | 6.07 a ± 0.14 | 0.87 ab ± 0.05 | 140.67 b ± 1.15 |
| Control | 2.85 b ± 0.15 | 4.37 b ± 0.02 | 3.33 b ± 0.23 | 5.15 b ± 0.14 | 0.94 a ± 0.05 | 140.00 b ± 1.15 |
| Treatment | PAL (U g−1 TP) | APX (U g−1 TP) | GPX (U g−1 TP) | SOD (U mL−1) | CAT (U g−1 TP) |
|---|---|---|---|---|---|
| Leaves | |||||
| NaCl | 4.72 a ± 0.14 | 186.29 b ± 1.44 | 115.32 ab ± 5.23 | 32.00 bc ± 0.51 | 675.14 b ± 21.8 |
| Cu NPs | 4.17 b ± 0.21 | 309.30 a ± 7.40 | 102.60 bc ± 3.63 | 38.09 a ± 0.51 | 669.75 b ± 28.1 |
| Cu NPs + NaCl | 4.00 b ± 0.08 | 321.69a ± 6.11 | 123.99 a ± 4.21 | 33.56 b ± 0.81 | 972.89 a ± 50.2 |
| Control | 1.96 c ± 0.08 | 134.14 c ± 4.94 | 98.39 c ± 4.15 | 30.95 c ± 1.38 | 505.13 c ± 39.8 |
| Fruit | |||||
| NaCl | 0.89 b ± 0.06 | 117.60 c ± 2.25 | 163.10 b ± 6.09 | 0.63 c ± 0.06 | 421.92 b ± 26.5 |
| Cu NPs | 1.81 a ± 0.07 | 137.97 b ± 3.83 | 157.33 b ± 6.07 | 4.62 b ± 0.56 | 691.18 a ± 20.9 |
| Cu NPs + NaCl | 1.76 a ± 0.09 | 181.68 a ± 11.42 | 194.92 a ± 12.74 | 3.55 bc ± 0.23 | 678.10 a ± 37.9 |
| Control | 0.87 b ± 0.05 | 140.03 b ± 4.47 | 215.48 a ± 8.98 | 65.65 a ± 2.13 | 268.80 c ± 21.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Labrada, F.; López-Vargas, E.R.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Responses of Tomato Plants under Saline Stress to Foliar Application of Copper Nanoparticles. Plants 2019, 8, 151. https://doi.org/10.3390/plants8060151
Pérez-Labrada F, López-Vargas ER, Ortega-Ortiz H, Cadenas-Pliego G, Benavides-Mendoza A, Juárez-Maldonado A. Responses of Tomato Plants under Saline Stress to Foliar Application of Copper Nanoparticles. Plants. 2019; 8(6):151. https://doi.org/10.3390/plants8060151
Chicago/Turabian StylePérez-Labrada, Fabián, Elsy Rubisela López-Vargas, Hortensia Ortega-Ortiz, Gregorio Cadenas-Pliego, Adalberto Benavides-Mendoza, and Antonio Juárez-Maldonado. 2019. "Responses of Tomato Plants under Saline Stress to Foliar Application of Copper Nanoparticles" Plants 8, no. 6: 151. https://doi.org/10.3390/plants8060151
APA StylePérez-Labrada, F., López-Vargas, E. R., Ortega-Ortiz, H., Cadenas-Pliego, G., Benavides-Mendoza, A., & Juárez-Maldonado, A. (2019). Responses of Tomato Plants under Saline Stress to Foliar Application of Copper Nanoparticles. Plants, 8(6), 151. https://doi.org/10.3390/plants8060151







