Combination of Plant Growth Regulators, Maltose, and Partial Desiccation Treatment Enhance Somatic Embryogenesis in Selected Malaysian Rice Cultivar
Abstract
1. Introduction
2. Results and Discussion
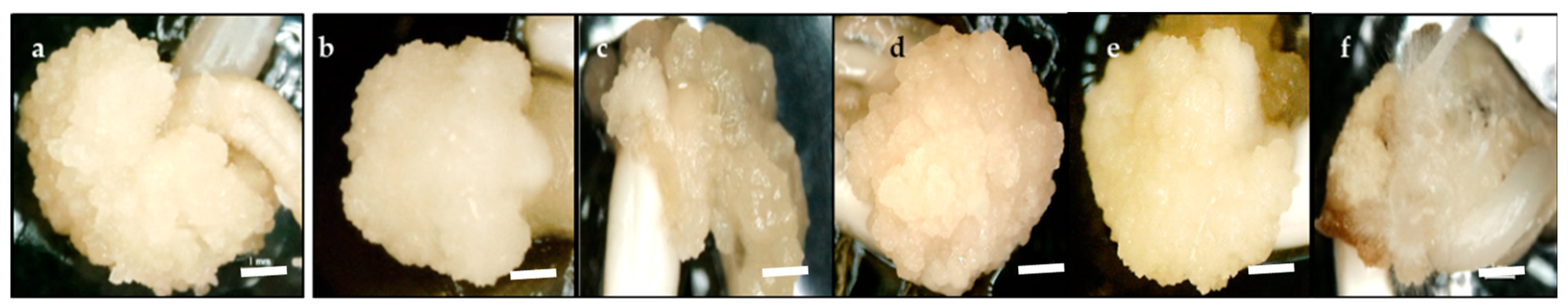
2.1. Effect of Basal Medium Type and 2, 4-D Concentration on Callus Induction Percentage
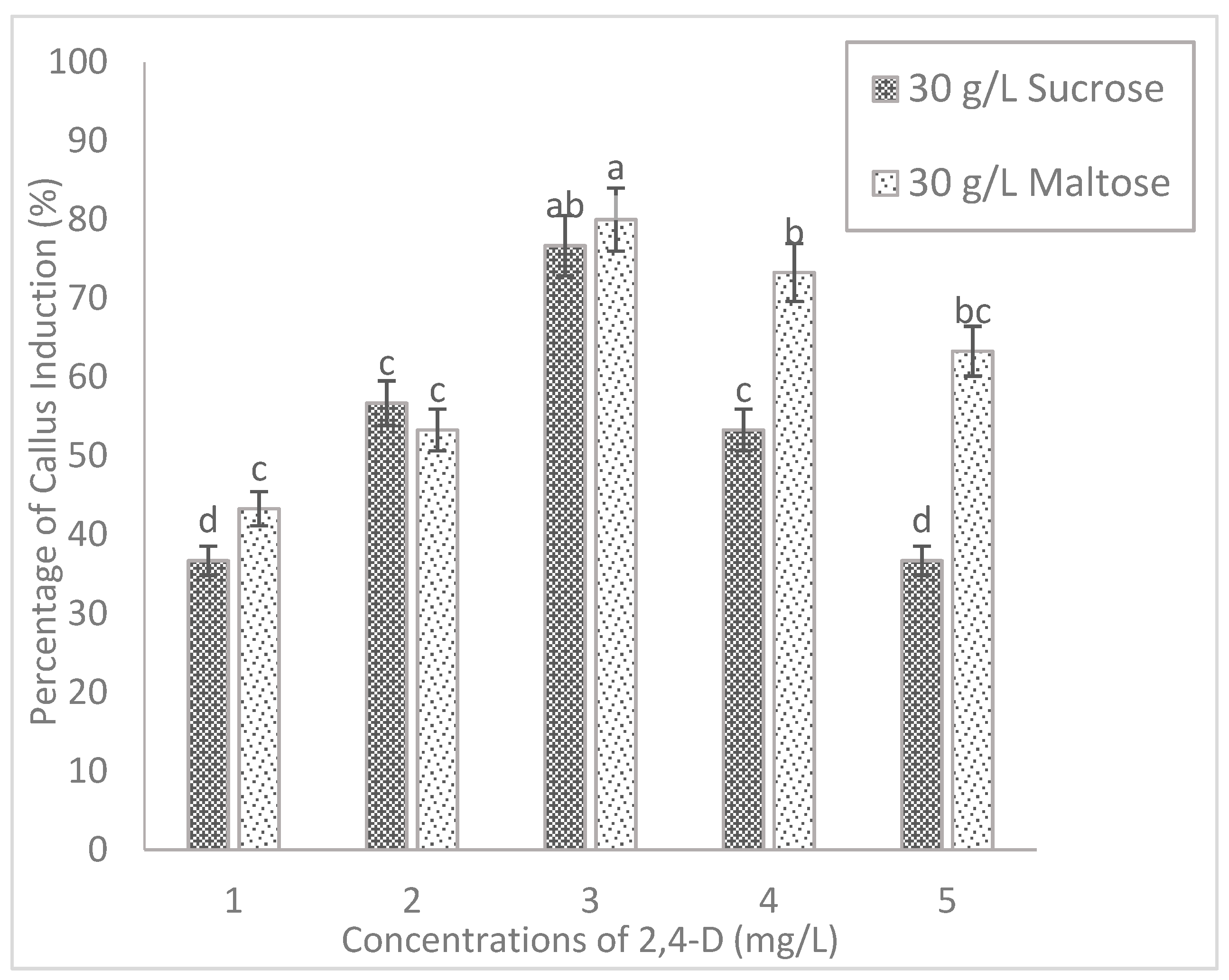
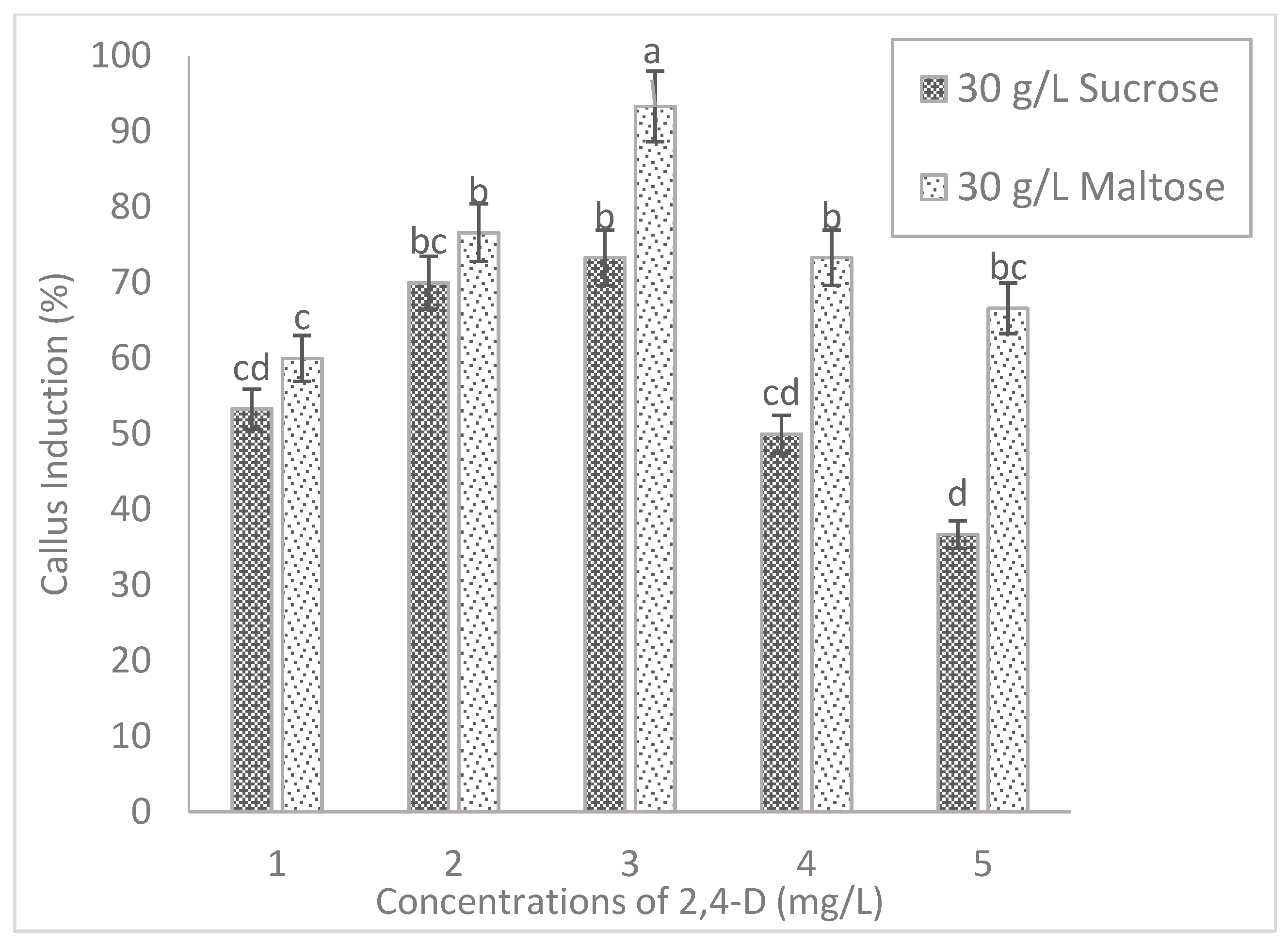
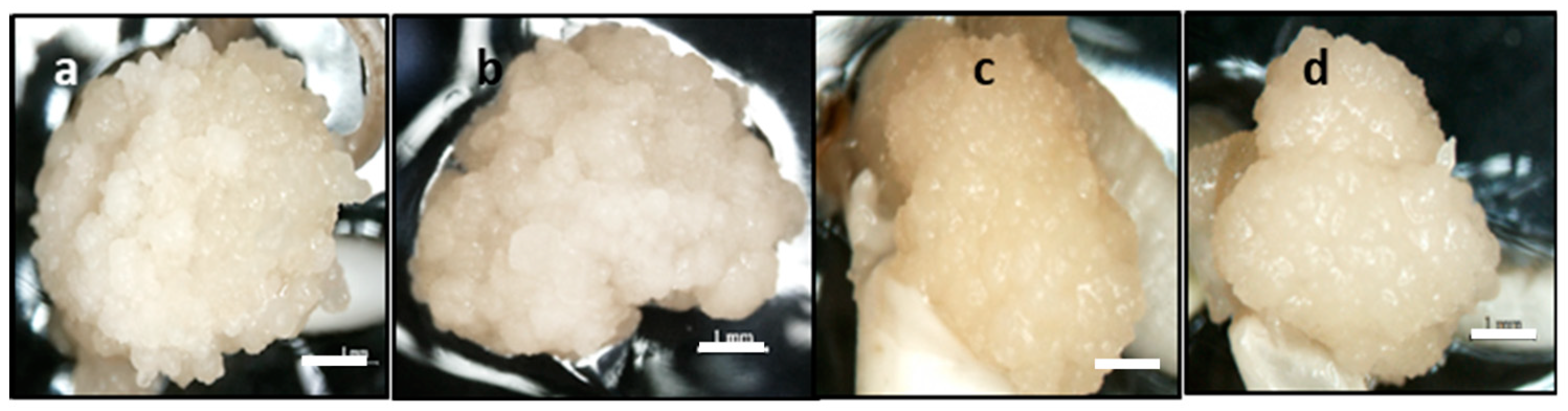
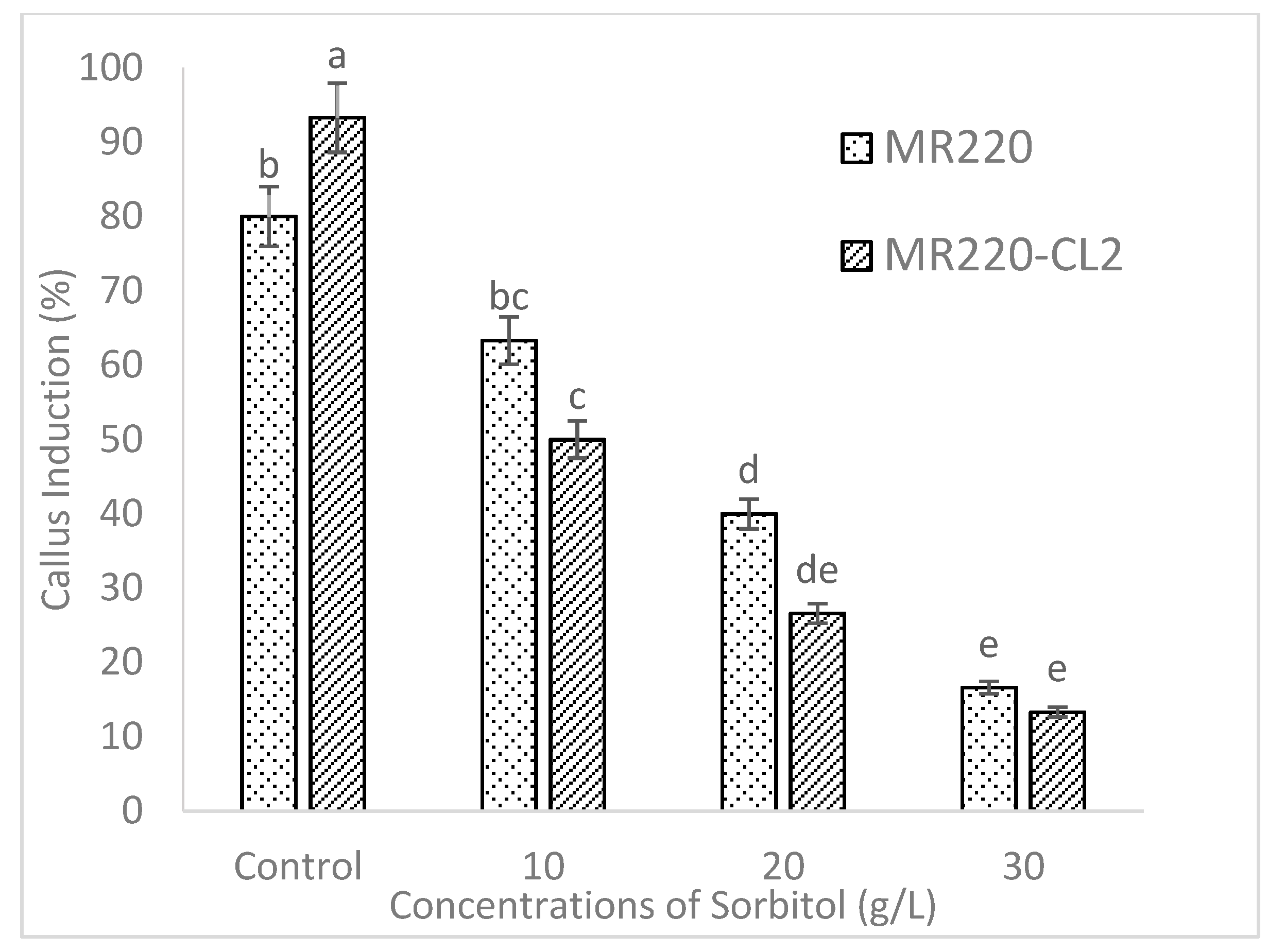
2.2. Effect of Carbohydrate Sources
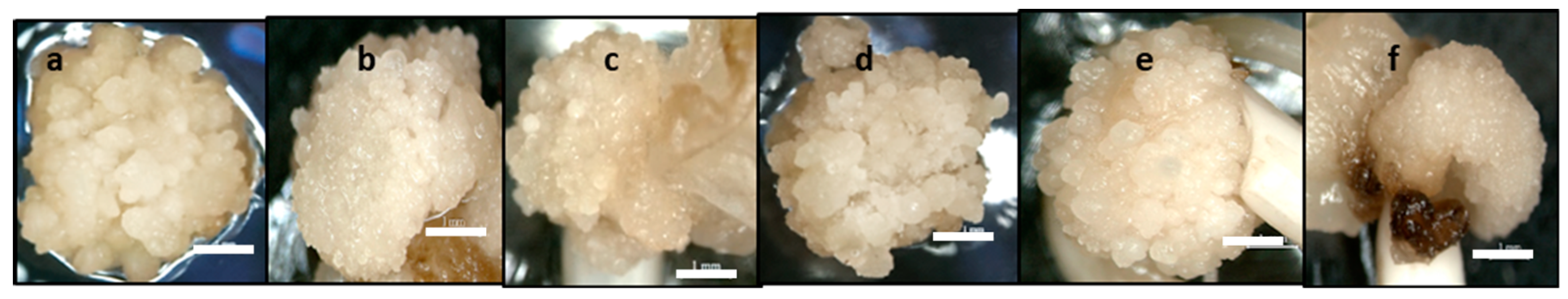
2.3. Scanning Electron Microscopy (SEM) and Histology
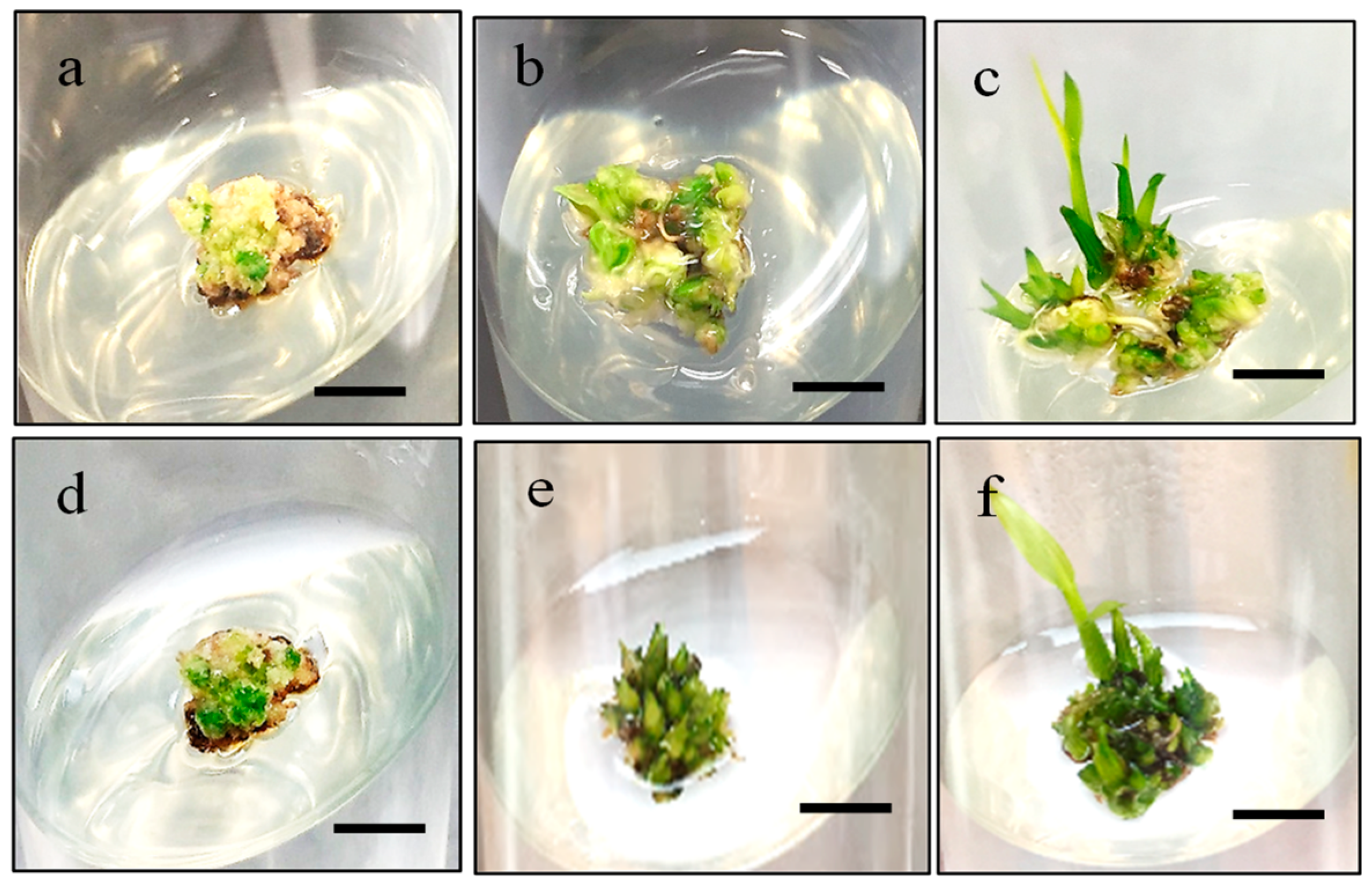
2.4. Effect of Cytokinin on Regeneration
2.5. Effect of Desiccation Treatment on Regeneration percentage
3. Discussions
4. Materials and Methods
4.1. Plant Materials and Culture Conditions
4.2. Effect of Different Media Types and 2, 4-D on Callus Percentage
4.3. Effect of Carbohydrates on Callus Percentage
4.4. Scanning Electron Microscopy (SEM) and Histological Analysis
4.5. Effect of Cytokinin on Plant Regeneration
4.6. Effect of Desiccation Treatment on Plant Regeneration
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abiri, R.; Maziah, M.; Shaharuddin, N.A.; Yusof, Z.N.B.; Atabaki, N.; Hanafi, M.M.; Sahebi, M.; Azizi, P.; Kalhori, N.; Valdiani, A. Enhancing somatic embryogenesis of Malaysian rice cultivar MR219 using adjuvant materials in a high-efficiency protocol. Int. J. Environ. Sci. Technol. 2017, 14, 1091–1108. [Google Scholar] [CrossRef]
- Binte Mostafiz, S.; Wagiran, A. Efficient callus induction and regeneration in selected Indica rice. Agronomy 2018, 8, 77. [Google Scholar] [CrossRef]
- Binte Mostafiz, S.; Wagiran, A.; Syafiqoh Johan, N.; Saliha Abdullah Zulkifli, N.; Ming Ng, J. The effects of temperature on callus induction and regeneration in selected Malaysian rice cultivar indica. Sains Malays. 2018, 47, 2647–2655. [Google Scholar] [CrossRef]
- Abiri, R.; Valdiani, A.; Maziah, M.; Shaharuddin, N.A.; Sahebi, M.; Yusof, Z.N.B.; Atabaki, N.; Talei, D. A Critical Review of the Concept of Transgenic Plants: Insights into Pharmaceutical Biotechnology and Molecular Farming. Curr. Issues Mol. Biol. 2016, 18, 21–42. [Google Scholar] [PubMed]
- Ikram-Ul-Haq, N.; Chang-Xing, Z.; Mukhtar, Z.; Jaleel, C.A.; Azooz, M.M. Effect of physical desiccation on plant regeneration efficiency in rice (Oryza sativa L.) variety super basmati. J. Plant Physiol. 2009, 166, 1568–1575. [Google Scholar] [CrossRef]
- Mohd Din, A.R.J.; Iliyas Ahmad, F.; Wagiran, A.; Abd Samad, A.; Rahmat, Z.; Sarmidi, M.R. Improvement of efficient in vitro regeneration potential of mature callus induced from Malaysian upland rice seed (Oryza sativa cv. Panderas). Saudi J. Biol. Sci. 2016, 23, S69–S77. [Google Scholar] [CrossRef]
- Narciso, J.O.; Hattori, K. Genotypic differences in morphology and ultrastructures of callus derived from selected rice varieties. Philipp. Sci. Lett. 2010, 3, 59–65. [Google Scholar]
- Bevitori, R.; Popielarska-Konieczna, M.; dos Santos, E.M.; Grossi-de-Sá, M.F.; Petrofeza, S. Morpho-anatomical characterization of mature embryo-derived callus of rice (Oryza sativa L.) suitable for transformation. Protoplasma 2014, 251, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.S. Enhancing Awareness on Weedy Rice Management in Iloilo Province, Philippines, 1st ed.; MARDI Publications: Selangor, Malaysia, 2008. [Google Scholar]
- Azizi, P.; Rafii, M.Y.; Mahmood, M.; Abdullah, S.N.A.; Hanafi, M.M.; Nejat, N.; Latif, M.A.; Sahebi, M. Differential gene expression reflects morphological characteristics and physiological processes in rice immunity against blast pathogen Magnaporthe Oryzae. PLoS ONE 2015, 10, e0126188. [Google Scholar] [CrossRef] [PubMed]
- Zuraida, A.R.; Naziah, B.; Zamri, Z.; Sreeramanan, S. Efficient plant regeneration of Malaysian indica rice MR 219 and 232 via somatic embryogenesis system. Acta Physiol. Plant. 2011, 33, 1913–1921. [Google Scholar] [CrossRef]
- Hoque, M.E.; Mansfield, J.W. Effect of genotype and explant age on callus induction and subsequent plant regeneration from root-derived callus of indica rice genotypes. Plant Cell Tissue Organ Cult. 2004, 78, 217–223. [Google Scholar] [CrossRef]
- Visarada, K.B.R.S.; Sailaja, M.; Sarma, N.P. Effect of callus induction media on morphology of embryogenic calli in rice genotypes. Biol. Plant. 2002, 45, 495–502. [Google Scholar] [CrossRef]
- Baker Siddique, A.; Ara, I.; Islam, S.M.; Tuteja, N. Effect of air desiccation and salt stress factors on in vitro regeneration of rice (Oryza sativa L.). Plant Signal. Behav. 2014, 9, 1–10. [Google Scholar]
- Tian, W.; Rance, I.; Sivamani, E.; Fauquet, C.; Beachy, N.R. Improvement of plant regeneration frequency in vitro in indica rice. Chin. J. Genet. 1994, 21, 215–221. [Google Scholar]
- Krishna Repalli, S.; Geda, C.; NSN, P.; Gjn, R. Regeneration Enhancement in tissue culture of indica rice’s through partial desiccation and chemical supplements. J. Plant Biochem. Physiol. 2017, 5, 196. [Google Scholar]
- Vega, R.; Vásquez, N.; M Espinoza, A.; Gatica-Arias, A.; Valdez, M. Histology of somatic embryogenesis in rice (Oryza sativa cv. 5272). Rev. Biol. Trop. 2009, 57, 141–150. [Google Scholar]
- Pawar, B.; Kale, P.; Bahurupe, J.; Jadhav, A.; Kale, A.; Pawar, S. Proline and glutamine improve in vitro callus induction and subsequent shooting in rice. Rice Sci. 2015, 22, 283–289. [Google Scholar] [CrossRef]
- Rancé, I.M.; Tian, W.; Mathews, H.; de Kochko, A.; Beachy, R.N.; Fauquet, C. Partial desiccation of mature embryo-derived calli, a simple treatment that dramatically enhances the regeneration ability of indica rice. Plant Cell Rep. 1994, 13, 647–651. [Google Scholar] [CrossRef]
- Chand, S.; Sahrawat, A.K. Stimulatory effect of partial desiccation on plant regeneration in indica rice (Oryza sativa L.). J. Plant Biochem. Biot. 2012, 10, 43–47. [Google Scholar] [CrossRef]
- Abdul Rahman, Z.; Roowi, S.; Wan Sembok, W.Z.; Subramaniam, S. Regeneration of Malaysian indica rice (Oryza sativa) variety MR232 via optimised somatic embryogenesis system. J. Phytol. 2010, 2, 30–38. [Google Scholar]
- Vennapusa, A.; S V, R.; Reddy, R.; Chandrashekar, B.; Kiranmai, K.; Nareshkumar, A.; Sudhakar, C. An efficient callus induction and regeneration protocol for a drought tolerant rice Indica genotype AC39020. J. Plant Sci. 2015, 3, 248–254. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 3, 473–497. [Google Scholar] [CrossRef]
- Chu, C.C.; Wang, C.C.; Sun, C.S.; Hsu, C.; Yin, K.C.; Chu, C.Y.; Pi, F.Y. Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Sci. Sin. 1975, 5, 659–668. [Google Scholar]



| 2, 4-D (mg/L) | MR220 | MR220-CL2 | ||
|---|---|---|---|---|
| MS | N6 | MS | N6 | |
| 1 | 36.7 ± 3 a | 33.3 ± 8 a | 53.3 ± 6 ab | 33.3 ± 8 a |
| 2 | 56.7 ± 3 b | 40.0 ± 0 a | 70.0 ± 0 bc | 40.0 ± 5 a |
| 3 | 76.7 ± 3 c | 53.3 ± 3 a | 73.3 ± 3 c | 56.7 ± 3 a |
| 4 | 53.3 ± 3 b | 43.3 ± 3 a | 50.0 ± 0 a | 46.7 ± 6 a |
| 5 | 36.7 ± 3 a | 46.7 ± 6 a | 36.7 ± 3 a | 43.3 ± 3 a |
| Treatment | PGR (mg/L) | Plantlet Regeneration (%) | |||
|---|---|---|---|---|---|
| NAA | KIN | BAP | MR220 | MR220-CL2 | |
| T1 | 0.5 | 1 | 0 | 20.0 ± 5.77 a | 20.0 ± 5.77 ab |
| T2 | 0.5 | 2 | 0 | 30.0 ± 5.77 ab | 40.0 ± 5.77 b |
| T3 | 0.5 | 3 | 0 | 16.6 ± 3.33 a | 26.6 ± 3.33 ab |
| T4 | 0.5 | 4 | 0 | 16.6 ± 3.33 a | 16.6 ± 3.33 a |
| T5 | 0.5 | 5 | 0 | 13.3 ± 3.33 a | 13.3 ± 3.33 a |
| T6 | 0.5 | 2 | 1 | 40 ± 5.77 b | 70.0 ± 5.77 c |
| T7 | 0.5 | 2 | 2 | 23.3 ± 8.81 ab | 46.6 ± 8.81 bc |
| T8 | 0.5 | 2 | 3 | 16.6 ± 6.67 a | 20.0 ± 5.57 ab |
| T9 | 0.5 | 2 | 4 | 13.3 ± 3.33 a | 20.0 ± 5.57 ab |
| T10 | 0.5 | 2 | 5 | 10.0 ± 0 a | 16.6 ± 3.33 a |
| Treatment | PGR (mg/L) | Green Somatic Embryo (%) | |||
|---|---|---|---|---|---|
| NAA | Kinetin | BAP | MR220 | MR220-CL2 | |
| T5 | 0.5 | 0 | 0 | 0 | 0 |
| T6 | 0.5 | 2 | 1 | 60.0 ± 8.16 c | 72.5 ± 9.57 c |
| T7 | 0.5 | 2 | 2 | 55.0 ± 5.77 bc | 57.5 ± 5 bc |
| T8 | 0.5 | 2 | 3 | 40.0 ± 8.16 ab | 45.0 ± 5.77 ab |
| T9 | 0.5 | 2 | 4 | 35.0 ± 10 a | 35.0 ± 5.77 a |
| T10 | 0.5 | 2 | 5 | 27.5 ± 5 a | 30.0 ± 8.16 a |
| Desiccation Treatment (h) | Green Somatic Embryo (%) | Number of Plantlets Regenerated (%) | ||
|---|---|---|---|---|
| MR220 | MR220-CL2 | MR220 | MR220-CL2 | |
| 0 (control) | 63.3 ± 3.33 | 70.0 ± 5.77 | 43.3 ± 3.11 a | 66.6 ± 3.11 ab |
| 24 | 66.6 ± 3.33 | 63.3 ± 3.33 | 50.0 ± 3.11 ab | 70.0 ± 3.11 b |
| 48 | 70.0 ± 5.77 | 66.6 ± 3.33 | 60.0 ± 3.11 ab | 73.3 ± 3.11 b |
| 72 | 53.3 ± 3.33 | 53.3 ± 3.33 | 40.0 ± 3.11 a | 46.3 ± 3.11 a |
| Carbohydrate (g/L) | MS/N6 Media | MS Media | MS |
|---|---|---|---|
| Sucrose (control) | 30 | 0 | 0 |
| Maltose | 0 | 30 | 30 |
| Sorbitol | 0 | 0 | 10, 20, 30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ming, N.J.; Binte Mostafiz, S.; Johon, N.S.; Abdullah Zulkifli, N.S.; Wagiran, A. Combination of Plant Growth Regulators, Maltose, and Partial Desiccation Treatment Enhance Somatic Embryogenesis in Selected Malaysian Rice Cultivar. Plants 2019, 8, 144. https://doi.org/10.3390/plants8060144
Ming NJ, Binte Mostafiz S, Johon NS, Abdullah Zulkifli NS, Wagiran A. Combination of Plant Growth Regulators, Maltose, and Partial Desiccation Treatment Enhance Somatic Embryogenesis in Selected Malaysian Rice Cultivar. Plants. 2019; 8(6):144. https://doi.org/10.3390/plants8060144
Chicago/Turabian StyleMing, NG Ja, Suraiya Binte Mostafiz, Nur Syafiqoh Johon, Nur Saliha Abdullah Zulkifli, and Alina Wagiran. 2019. "Combination of Plant Growth Regulators, Maltose, and Partial Desiccation Treatment Enhance Somatic Embryogenesis in Selected Malaysian Rice Cultivar" Plants 8, no. 6: 144. https://doi.org/10.3390/plants8060144
APA StyleMing, N. J., Binte Mostafiz, S., Johon, N. S., Abdullah Zulkifli, N. S., & Wagiran, A. (2019). Combination of Plant Growth Regulators, Maltose, and Partial Desiccation Treatment Enhance Somatic Embryogenesis in Selected Malaysian Rice Cultivar. Plants, 8(6), 144. https://doi.org/10.3390/plants8060144






