The PIP Peptide of INFLORESCENCE DEFICIENT IN ABSCISSION Enhances Populus Leaf and Elaeis guineensis Fruit Abscission
Abstract
1. Introduction
2. Results
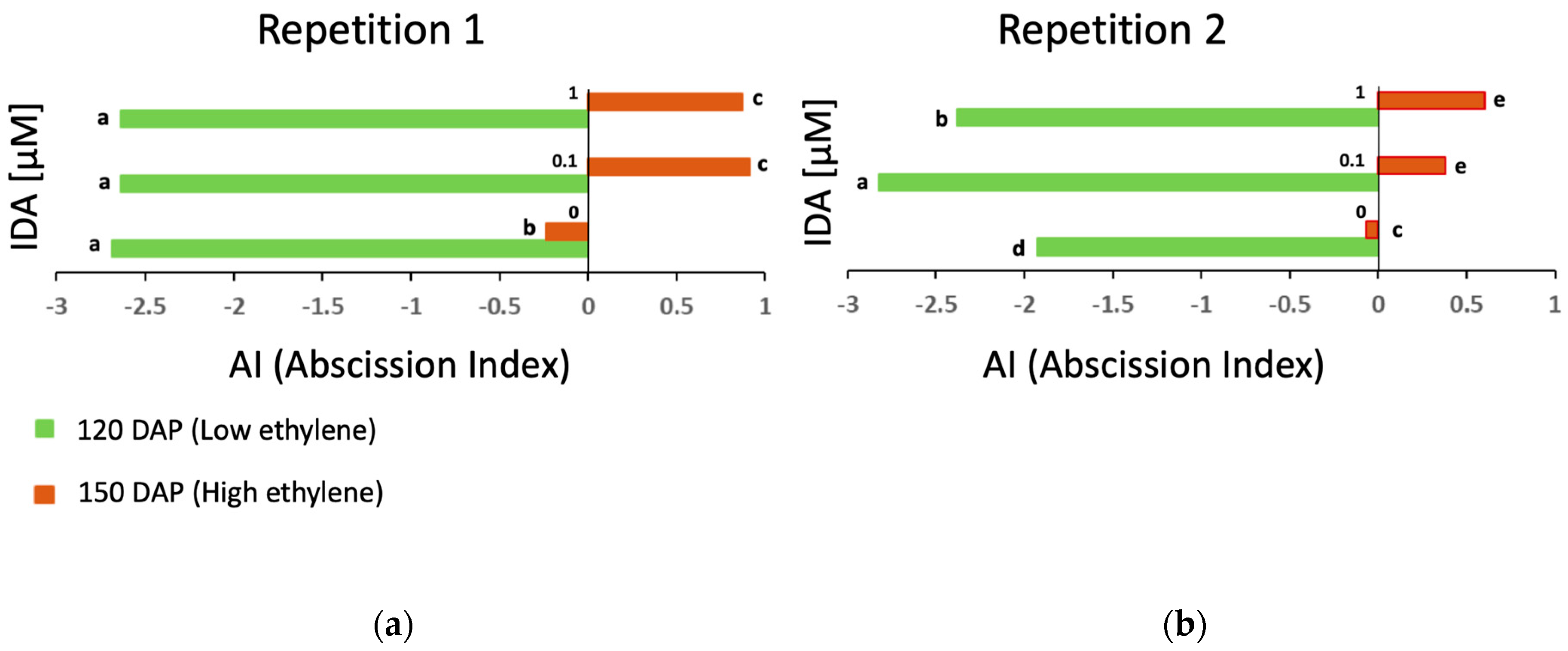
2.1. Exogenous Treatments of PtIDA and PtIDL1 Peptides and Their Effect on Poplar Leaf Abscission
2.2. Exogenous Treatments of the EgIDA Peptide and Effects on Cell Separation in the Oil Palm Fruit AZ
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. In Vitro Phenotype Tests of Oil Palm Fruit Abscission
4.3. Dark-Induced Abscission Experiments
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roberts, J.A.; Elliott, K.A.; Gonzalez-Carranza, Z.H. Abscission, dehiscence, and other cell separation processes. Annu. Rev. Plant Biol. 2002, 53, 131–158. [Google Scholar] [CrossRef] [PubMed]
- Estornell, L.H.; Agusti, J.; Merelo, P.; Talon, M.; Tadeo, F.R. Elucidating mechanisms underlying organ abscission. Plant Sci. 2013, 199–200, 48–60. [Google Scholar] [CrossRef]
- Addicott, F.T. Abscission; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 1982. [Google Scholar]
- Bleecker, A.B.; Patterson, S.E. Last exit: Senescence, abscission, and meristem arrest in Arabidopsis. Plant Cell 1997, 9, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.E. Cutting loose. Abscission and dehiscence in Arabidopsis. Plant Physiol. 2001, 126, 494–500. [Google Scholar] [CrossRef]
- Patterson, S.E.; Butenko, M.A.; Kim, J. Ethylene responses in abscission and other processes of cell separation in Arabidopsis. In Advances in Plant Ethylene Research; Ramina, A., Chang, C., Giovannoni, J., Klee, H., Perata, P., Woltering, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 271–278. [Google Scholar]
- Patharkar, O.R.; Walker, J.C. Core Mechanisms Regulating Developmentally Timed and Environmentally Triggered Abscission. Plant Physiol. 2016, 172, 510–520. [Google Scholar] [CrossRef]
- Patharkar, O.R.; Walker, J.C. Advances in abscission signaling. J. Exp. Bot. 2018, 69, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.E.; Bolivar-Medina, J.L.; Falbel, T.G.; Hedtcke, J.L.; Nevarez-McBride, D.; Maule, A.F.; Zalapa, J.E. Are We on the Right Track: Can Our Understanding of Abscission in Model Systems Promote or Derail Making Improvements in Less Studied Crops? Front. Plant Sci. 2016, 6, 1268. [Google Scholar] [CrossRef] [PubMed]
- Meir, S.; Philosoph-Hadas, S.; Riov, J.; Tucker, M.L.; Patterson, S.E.; Roberts, J.A. Re-evaluation of the ethylene-dependent and -independent pathways in the regulation of floral and organ abscission. J. Exp. Bot. 2019, 70, 1461–1467. [Google Scholar] [CrossRef]
- Butenko, M.A.; Patterson, S.E.; Grini, P.E.; Stenvik, G.E.; Amundsen, S.S.; Mandal, A.; Aalen, R.B. Inflorescence deficient in abscission controls floral organ abscission in Arabidopsis and identifies a novel family of putative ligands in plants. Plant Cell 2003, 15, 2296–2307. [Google Scholar] [CrossRef] [PubMed]
- Stenvik, G.E.; Tandstad, N.M.; Guo, Y.; Shi, C.L.; Kristiansen, W.; Holmgren, A.; Clark, S.E.; Aalen, R.B.; Butenko, M.A. The EPIP peptide of INFLORESCENCE DEFICIENT IN ABSCISSION is sufficient to induce abscission in arabidopsis through the receptor-like kinases HAESA and HAESA-LIKE2. Plant Cell 2008, 20, 1805–1817. [Google Scholar] [CrossRef]
- Butenko, M.A.; Wildhagen, M.; Albert, M.; Jehle, A.; Kalbacher, H.; Aalen, R.B.; Felix, G. Tools and Strategies to Match Peptide-Ligand Receptor Pairs. Plant Cell 2014, 26, 1838–1847. [Google Scholar] [CrossRef]
- Cho, S.K.; Larue, C.T.; Chevalier, D.; Wang, H.; Jinn, T.L.; Zhang, S.; Walker, J.C. Regulation of floral organ abscission in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2008, 105, 15629–15634. [Google Scholar] [CrossRef] [PubMed]
- Estornell, L.H.; Wildhagen, M.; Perez-Amador, M.A.; Talon, M.; Tadeo, F.R.; Butenko, M.A. The IDA Peptide Controls Abscission in Arabidopsis and Citrus. Front. Plant Sci. 2015, 6, 1003. [Google Scholar] [CrossRef] [PubMed]
- Ying, P.Y.; Li, C.Q.; Liu, X.C.; Xia, R.; Zhao, M.L.; Li, J.G. Identification and molecular characterization of an IDA-like gene from litchi, LcIDL1, whose ectopic expression promotes floral organ abscission in Arabidopsis. Sci. Rep.-UK 2016, 6, 37135. [Google Scholar] [CrossRef]
- Stø, I.M.; Orr, R.J.S.; Fooyontphanich, K.; Jin, X.; Knutsen, J.M.B.; Fischer, U.; Tranbarger, T.J.; Nordal, I.; Aalen, R.B. Conservation of the abscission signaling peptide IDA during Angiosperm evolution: Withstanding genome duplications and gain and loss of the receptors HAE/HSL2. Front. Plant Sci. 2015, 6, 931. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zimmermann, J.; Polle, A.; Fischer, U. Auxin is a long-range signal that acts independently of ethylene signaling on leaf abscission in Populus. Front. Plant Sci. 2015, 6, 634. [Google Scholar] [CrossRef]
- Henderson, J.; Osborne, D.J. Cell Separation and Anatomy of Abscission in the Oil Palm, Elaeis guineensis Jacq. J. Exp. Bot. 1990, 41, 203–210. [Google Scholar] [CrossRef]
- Osborne, D.J.; Henderson, J.; Corley, R.H.V. Controlling Fruit Shedding in the Oil Palm. Endeavour 1992, 16, 173–177. [Google Scholar] [CrossRef]
- Roongsattham, P.; Morcillo, F.; Jantasuriyarat, C.; Pizot, M.; Moussu, S.; Jayaweera, D.; Collin, M.; Gonzalez-Carranza, Z.; Amblard, P.; Tregear, J.W.; et al. Temporal and spatial expression of polygalacturonase gene family members reveals divergent regulation during fleshy fruit ripening and abscission in the monocot species oil palm. BMC Plant Biol. 2012, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Roongsattham, P.; Morcillo, F.; Fooyontphanich, K.; Jantasuriyarat, C.; Tragoonrung, S.; Amblard, P.; Collin, M.; Mouille, G.; Verdeil, J.L.; Tranbarger, T.J. Cellular and Pectin Dynamics during Abscission Zone Development and Ripe Fruit Abscission of the Monocot Oil Palm. Front. Plant Sci. 2016, 7, 540. [Google Scholar] [CrossRef] [PubMed]
- Fooyontphanich, K.; Morcillo, F.; Amblard, P.; Collin, M.; Jantasuriyarat, C.; Tangphatsornruang, S.; Verdeil, J.L.; Tranbarger, T.J. A phenotypic test for delay of abscission and non-abscission oil palm fruit and validation by abscission marker gene expression analysis. Acta Hortic. 2016, 1119, 97–104. [Google Scholar] [CrossRef]
- Tisné, S.; Denis, M.; Domonhédo, H.; Pallas, B.; Cazemajor, M.; Tranbarger, T.J.; Morcillo, F. Environmental and trophic determinisms of fruit abscission in African oil palm and climate change perspective. New Phytologist. submitted.
- Tranbarger, T.J.; Dussert, S.; Joet, T.; Argout, X.; Summo, M.; Champion, A.; Cros, D.; Omore, A.; Nouy, B.; Morcillo, F. Regulatory mechanisms underlying oil palm fruit mesocarp maturation, ripening, and functional specialization in lipid and carotenoid metabolism. Plant Physiol. 2011, 156, 564–584. [Google Scholar] [CrossRef] [PubMed]
- Jinn, T.L.; Stone, J.M.; Walker, J.C. HAESA, an Arabidopsis leucine-rich repeat receptor kinase, controls floral organ abscission. Genes Dev. 2000, 14, 108–117. [Google Scholar] [CrossRef]
- Santiago, J.; Brandt, B.; Wildhagen, M.; Hohmann, U.; Hothorn, L.A.; Butenko, M.A.; Hothorn, M. Mechanistic insight into a peptide hormone signaling complex mediating floral organ abscission. Elife 2016, 5. [Google Scholar] [CrossRef]
- Stenvik, G.E.; Butenko, M.A.; Urbanowicz, B.R.; Rose, J.K.; Aalen, R.B. Overexpression of INFLORESCENCE DEFICIENT IN ABSCISSION activates cell separation in vestigial abscission zones in Arabidopsis. Plant Cell 2006, 18, 1467–1476. [Google Scholar] [CrossRef]
- Wilmowicz, E.; Kućko, A.; Ostrowski, M.; Panek, K. INFLORESCENCE DEFICIENT IN ABSCISSION-like is an abscission-associated and phytohormone-regulated gene in flower separation of Lupinus luteus. Plant Growth Regul. 2018, 85, 91–100. [Google Scholar] [CrossRef]
- Henderson, J.; Osborne, D.J. Intertissue Signaling during the 2-Phase Abscission in Oil Palm Fruit. J. Exp. Bot. 1994, 45, 943–951. [Google Scholar] [CrossRef]
- Newman, D. The distribution of range in samples from a normal population, expressed in terms of an independent estimate of standard deviation. Biometrika 1939, 31, 20–30. [Google Scholar] [CrossRef]
- Keuls, M. The use of the “studentized range” in connection with an analysis of variance. Euphytica 1952, 1, 112–122. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tranbarger, T.J.; Domonhédo, H.; Cazemajor, M.; Dubreuil, C.; Fischer, U.; Morcillo, F. The PIP Peptide of INFLORESCENCE DEFICIENT IN ABSCISSION Enhances Populus Leaf and Elaeis guineensis Fruit Abscission. Plants 2019, 8, 143. https://doi.org/10.3390/plants8060143
Tranbarger TJ, Domonhédo H, Cazemajor M, Dubreuil C, Fischer U, Morcillo F. The PIP Peptide of INFLORESCENCE DEFICIENT IN ABSCISSION Enhances Populus Leaf and Elaeis guineensis Fruit Abscission. Plants. 2019; 8(6):143. https://doi.org/10.3390/plants8060143
Chicago/Turabian StyleTranbarger, Timothy John, Hubert Domonhédo, Michel Cazemajor, Carole Dubreuil, Urs Fischer, and Fabienne Morcillo. 2019. "The PIP Peptide of INFLORESCENCE DEFICIENT IN ABSCISSION Enhances Populus Leaf and Elaeis guineensis Fruit Abscission" Plants 8, no. 6: 143. https://doi.org/10.3390/plants8060143
APA StyleTranbarger, T. J., Domonhédo, H., Cazemajor, M., Dubreuil, C., Fischer, U., & Morcillo, F. (2019). The PIP Peptide of INFLORESCENCE DEFICIENT IN ABSCISSION Enhances Populus Leaf and Elaeis guineensis Fruit Abscission. Plants, 8(6), 143. https://doi.org/10.3390/plants8060143





