Allelopathic Potency and an Active Substance from Anredera cordifolia (Tenore) Steenis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction
2.3. Bioassay of the Crude Extract and Determination of I50
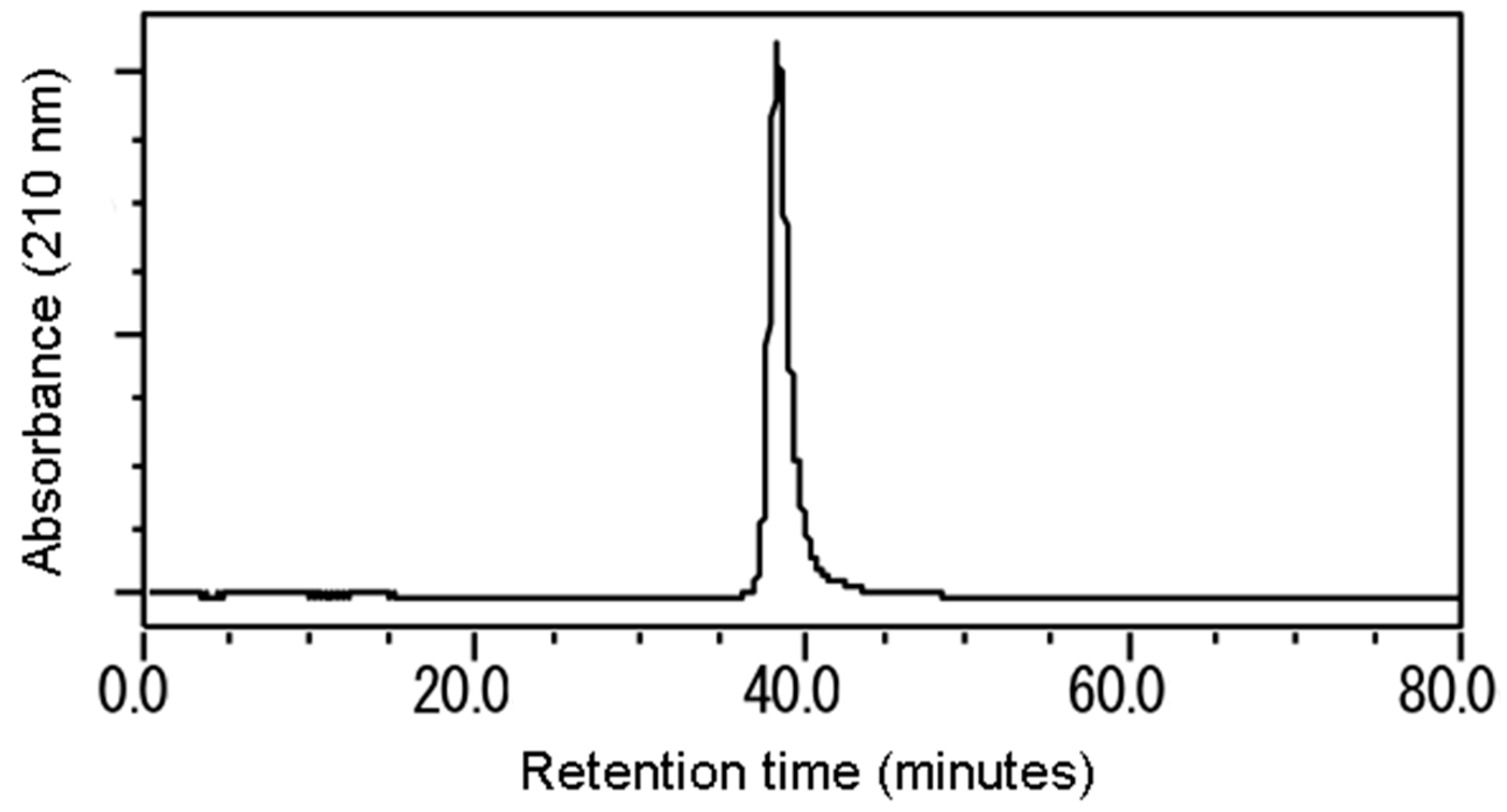
2.4. Isolation
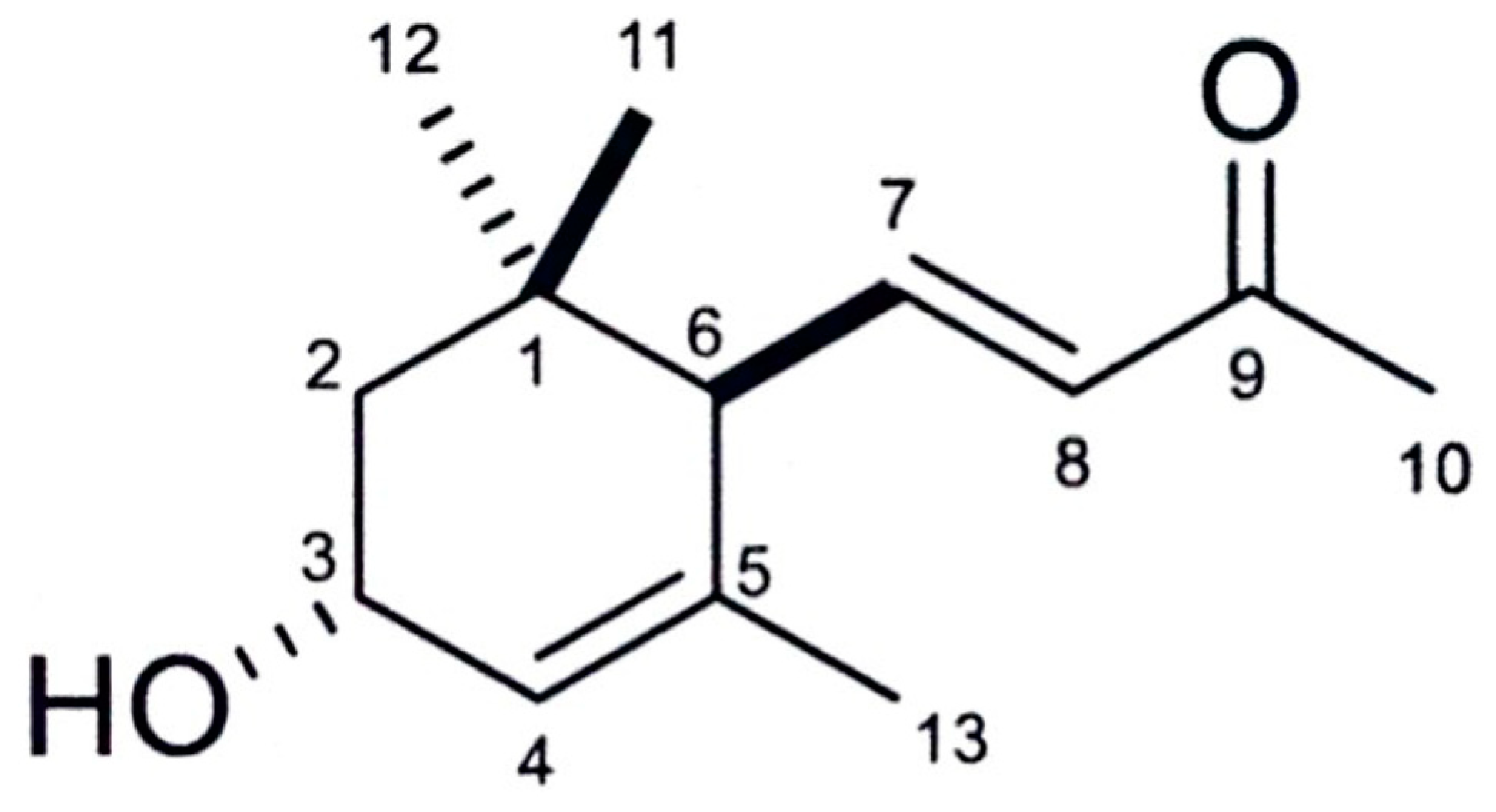
2.5. Identification of the Active Substance
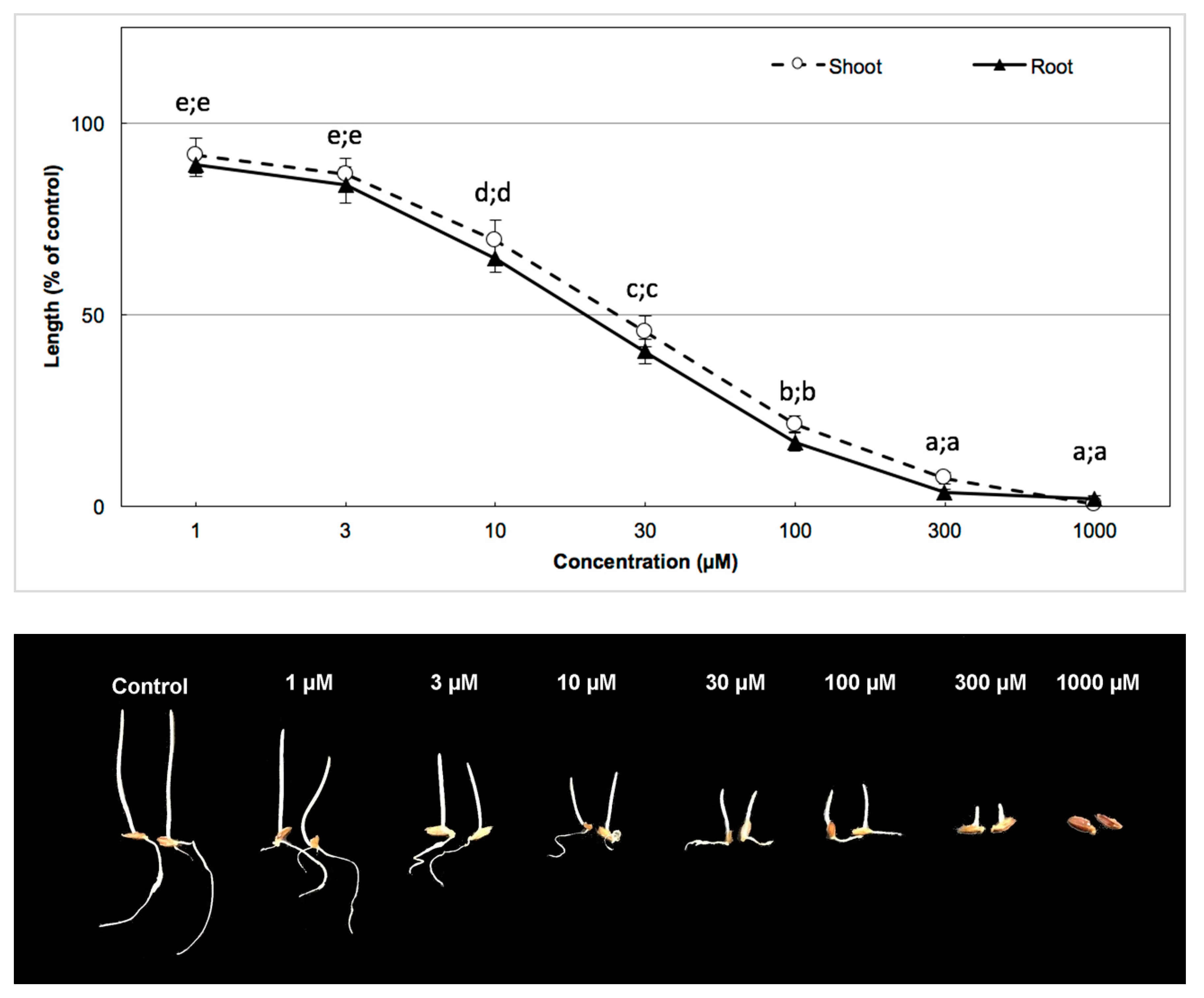
2.6. Bioassay of the Active Substance and Determination of I50
2.7. Analysis
3. Results
3.1. Biological Activity of the Crude Extract
3.2. Isolation and Identification of the Active Substance
3.3. Biological Activity of the Active Substance
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Purwasih, R.; Safitri, F.A.; Rahayu, W.E. The potency of Binahong leaves (Anredera cordifolia (Ten.) Steenis) to recovery process of wound in the livestock. Adv. Health Sci. Res. 2018, 5, 211–215. [Google Scholar]
- Johnson, K. Weed Management Guide: Anredera cordifolia (Madeira vine); Queensland Department of Agriculture, Fisheries and Forestry: Queensland, Australia, 2011; pp. 1–6. Available online: http://www.cabi.org/isc/datasheet/112290 (accessed on 17 August 2017).
- Zhou, J.; Xie, G.; Yan, X. Encyclopedia of Traditional Chinese Medicines-Molecular Structures, Pharmacological Activities, Natural Sources and Applications; Springer: Berlin/Heidelberg, Germany, 2016; Volume 6, 730p. [Google Scholar]
- Astuti, S.M.; Sakinah, A.M.M.; Andayani, B.M.R.; Risch, A. Determination of Saponin Compound from Anredera cordifolia (Ten) Steenis Plant (Binahong) to Potential Treatment for Several Diseases. J. Agric. Sci. 2011, 3, 224. [Google Scholar] [CrossRef]
- Djamil, R. Antioxidant Activity of Flavonoid from Anredera cordifolia (Ten) Steenis Leaves. Int. Res. J. Pharm. 2012, 3, 241–243. [Google Scholar]
- Lestari, D.; Sukandar, E.Y.; Fidrianny, I. Anredera cordifolia leaves extract as antihyperlipidemia and endothelial fat content reducer in male wistar rat. Int. J. Pharm. Clin. Res. 2015, 7, 435–439. [Google Scholar]
- Dwi, K.; Yulinah, E.; Fidrianny, I. Xanthine Oxidase Inhibitory and Antihyperuricemic Activities of Anredera Cordifolia (Ten) Steenis, Sonchus Arvensis, L., and Its Combination. Int. J. Pharm. Pharm. Sci. 2015, 7, 86–90. [Google Scholar]
- Howell, C. Consolidated List of Environmental Weeds in New Zealand; DOC Research & Development Series; Science & Technical Publishing Department of Conservation: Wellington, New Zealand, 2008; Volume 292. [Google Scholar]
- Wong, L.; Okada, C.L. Hawaii-Summaries of Exterior Quarantines; State of Hawaii, Department of Agriculture Plant: Honolulu, HI, USA, 2011.
- Van der Westhuizen, L. Initiation of a Biological Control Programme Against Madeira Vine, Anredera cordifolia (Ten.) Steenis (Basellaceae), in South Africa. Afr. Entomol. 2011, 19, 217–222. [Google Scholar] [CrossRef]
- Vivian-Smith, G.; Lawson, B.E.; Turnbull, I.; Downey, P.O. The biology of Australian weeds. 46. Anredera cordifolia (Ten.) Steenis. Plant Prot. Q. 2007, 22, 2–10. [Google Scholar]
- Harden, G.J.; Fox, M.D.; Fox, B.J. Monitoring and assessment of restoration of a rainforest remnant at Wingham Brush, NSW. Austral Ecol. 2004, 29, 489–507. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press Inc.: Orlando, FL, USA, 1984; 422p. [Google Scholar]
- Islam, M.S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Isolation and identification of two potential phytotoxic substances from the aquatic fern Marsilea crenata. J. Plant Biol. 2017, 60, 75–81. [Google Scholar] [CrossRef]
- Bari, I.N.; Kato-Noguchi, H. Phytotoxic effects of Cerbera manghas L. leaf extracts on seedling elongation of four monocot and four dicot test species. Acta Agrobot. 2017, 70, 1–7. [Google Scholar] [CrossRef]
- Bari, I.N.; Kato-Noguchi, H. Phytotoxic Effect of Filicium decipiens Leaf Extract. Am. J. Agric. Environ. Sci. 2017, 17, 288–292. [Google Scholar]
- Suwitchayanon, P.; Ohno, O.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic property of Piper retrofractum fruit extracts and compounds against the germination and seedling growth of weeds. Acta Physiol. Plant. 2019, 41, 33. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Suzuki, M.; Noguchi, K.; Ohno, O.; Suenaga, K.; Laosinwattana, C. A Potent Phytotoxic Substance in Aglaia odorata Lour. Chem. Biodivers. 2016, 13, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Alpha-Ionone|C13H20O. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/alpha-ionone (accessed on 8 May 2019).
- D’Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Monaco, P.; Oriano, P.; Temussi, F. Structure elucidation and phytotoxicity of C13 nor-isoprenoids from Cestrum parqui. Phytochemistry 2004, 65, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Salasanti, C.D.; Sukandar, E.Y.; Fidrianny, I. Acute and sub chronic toxicity study of ethanol extract of Anredera cordifolia (Ten.) v. steenis leaves. Int. J. Pharm. Pharm. Sci. 2014, 6, 348–352. [Google Scholar]
- Lutz-Wahl, S.; Fischer, P.; Schmidt-Dannert, C.; Wohlleben, W.; Hauer, B.; Schmid, R.D. Stereo- and regioselective hydroxylation of Alpha-ionone by Streptomyces strains. Appl. Environ. Microbiol. 1998, 64, 3878–3881. [Google Scholar] [PubMed]
- Pabst, A.; Barron, D.; Sémon, E.; Schreier, P. A 4-hydroxy-β-ionone disaccharide glycoside from raspberry fruits. Phytochemistry 1992, 31, 3105–3107. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Seki, T. Allelopathy of the moss Rhynchostegium pallidifolium and 3-hydroxy-β-ionone. Plant Signal Behav. 2010, 5, 702–704. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, M.; Hirose, T.; Sugata, Y.; Tokumasu, M.; Hiraga, Y.; Suga, T. 3-Hydroxy-5, 6-Epoxy-β-Ionone as Germination Inhibitory Active Constituent In Athyrium yokoscense. Nat. Prod. Lett. 1998, 12, 35–40. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research Progress on the use of Plant Allelopathy in Agriculture and the Physiological and Ecological Mechanisms of Allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
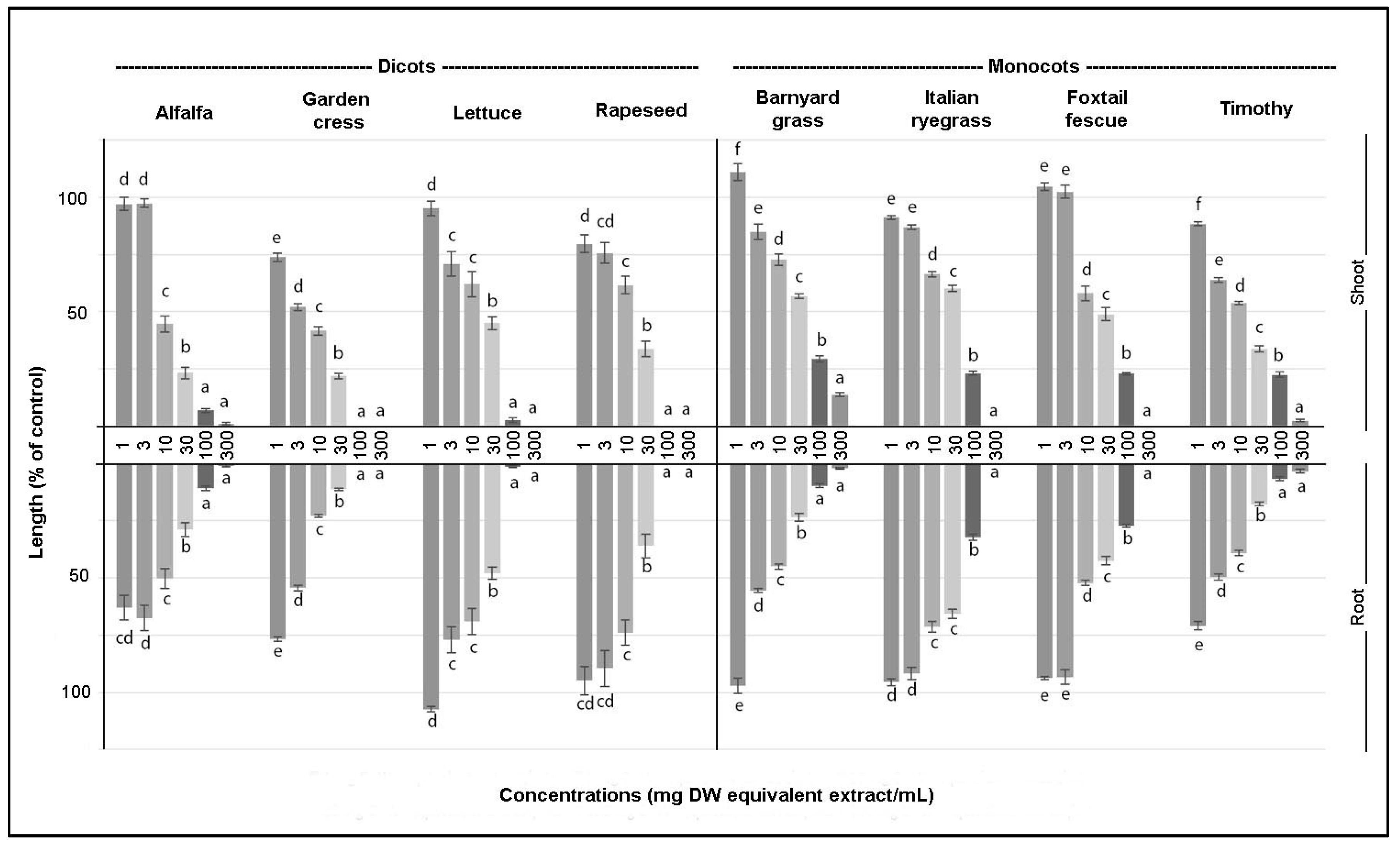

| Test Plants | Shoot | Root |
|---|---|---|
| Dicots: | ||
| Alfalfa | 11.00 | 6.43 |
| Garden cress | 4.61 | 3.45 |
| Lettuce | 16.26 | 21.17 |
| Rapeseed | 14.85 | 20.81 |
| Monocots: | ||
| Barnyard grass | 38.31 | 7.12 |
| Italian ryegrass | 32.58 | 48.19 |
| Foxtail fescue | 26.03 | 20.98 |
| Timothy | 11.19 | 23.70 |
| Test Plants | Shoot | Root |
|---|---|---|
| Garden cress | 35.60 µm | 38.03 µm |
| Barnyard grass | 41.00 µm | 53.24 µm |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bari, I.N.; Kato-Noguchi, H.; Iwasaki, A.; Suenaga, K. Allelopathic Potency and an Active Substance from Anredera cordifolia (Tenore) Steenis. Plants 2019, 8, 134. https://doi.org/10.3390/plants8050134
Bari IN, Kato-Noguchi H, Iwasaki A, Suenaga K. Allelopathic Potency and an Active Substance from Anredera cordifolia (Tenore) Steenis. Plants. 2019; 8(5):134. https://doi.org/10.3390/plants8050134
Chicago/Turabian StyleBari, Ichsan Nurul, Hisashi Kato-Noguchi, Arihiro Iwasaki, and Kiyotake Suenaga. 2019. "Allelopathic Potency and an Active Substance from Anredera cordifolia (Tenore) Steenis" Plants 8, no. 5: 134. https://doi.org/10.3390/plants8050134
APA StyleBari, I. N., Kato-Noguchi, H., Iwasaki, A., & Suenaga, K. (2019). Allelopathic Potency and an Active Substance from Anredera cordifolia (Tenore) Steenis. Plants, 8(5), 134. https://doi.org/10.3390/plants8050134





