Consistent Differences in Field Leaf Water-Use Efficiency among Soybean Cultivars
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Funding
Conflicts of Interest
References
- Condon, A.G.; Richards, R.A.; Rebetzke, G.L.; Farquhar, G.D. Breeding for High Water-Use Efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar] [CrossRef]
- Farquhar, G.D.; O’Leary, M.H.; Berry, J.A. On the Relationship between Carbon Isotope Discrimination and the Intercellular Carbon Dioxide Concentration in Leaves. Aust. J. Plant Physiol. 1982, 9, 121–137. [Google Scholar] [CrossRef]
- Condon, A.G.; Farquhar, G.D.; Richards, R.A. Genotypic Variation in Carbon Isotope Discrimination and Transpiration Efficiency in Wheat. Leaf Gas Exchange and Whole Plant Studies. Aust. J. Plant Physiol. 1990, 17, 9–22. [Google Scholar] [CrossRef]
- Wright, G.C.; Hubick, K.T.; Farquhar, G.D. Discrimination in Carbon Isotopes of Leaves Correlates with Water-Use Efficiency of Field-Grown Peanut Cultivars. Aust. J. Plant Physiol 1988, 15, 815–825. [Google Scholar] [CrossRef]
- Martin, B.; Thorstenson, Y.R. Stable Carbon Isotope Composition (δ13 C), Water Use Efficiency, and Biomass Productivity of Lycopersicon esculentum, Lycopersicon pennellii, and the F1 Hybrid. Plant Physiol. 1988, 88, 213–217. [Google Scholar] [CrossRef]
- Martin, B.; Tauer, C.G.; Lin, R.K. Carbon Isotope Discrimination as a Tool to Improve Water-Use Efficiency in Tomato. Crop Sci. 1999, 39, 1775–1783. [Google Scholar] [CrossRef]
- Ismail, A.M.; Hall, A.E. Correlation between Water-Use Efficiency and Carbon Isotope Discrimination in Diverse Cowpea Genotypes and Isogenic Lines. Crop Sci. 1992, 32, 7–12. [Google Scholar] [CrossRef]
- Saranga, Y.; Flash, I.; Yakir, D. Variation in Water-Use Efficiency and Its Relation to Carbon Isotope Ratio in Cotton. Crop Sci. 1998, 38, 782–787. [Google Scholar] [CrossRef]
- Anyia, A.O.; Slaski, J.J.; Nyachiro, J.M.; Archambault, D.J.; Juskiw, P. Relationship of Carbon Isotope Discriminatin to Water Use Efficiency and Productivity of Barley Under Field and Greenhouse Conditions. J. Agron. Crop Sci. 2007, 193, 313–323. [Google Scholar] [CrossRef]
- Rajabi, A.; Ober, E.S.; Griffiths, H. Genotypic Variation for Water Use Efficiency, Carbon Isotope Discrimination, and Potential Surrogate Measures in Sugar Beet. Field Crops Res. 2009, 112, 172–181. [Google Scholar] [CrossRef]
- Blum, A. Effective Use of Water (EUW) and not Water-Use Efficiency (WUE) is the Target of Crop Yield Improvement under Drought Stress. Field Crops Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Sinclair, T.R. Is Transpiration Efficiency a Viable Plant Trait in Breeding for Crop Improvement? Funct. Plant Biol. 2012, 39, 359–365. [Google Scholar] [CrossRef]
- Gioliani, R.; Koteyeva, N.; Voznesenskaya, E.; Evans, M.A.; Cousins, A.B.; Edwards, G.E. Coordination of Leaf Photosynthesis, Transpiration, and Structural Traits in Rice and Wild Relatives (Genus Oryza). Plant Physiol. 2013, 162, 1632–1651. [Google Scholar] [CrossRef]
- Zhao, B.; Kondo, M.; Maeda, M.; Ozaki, Y.; Zhang, J. Water-Use Efficiency and Carbon Isotope Discrimination in Two Cultivars of Upland Rice During Different Developmental Stages under Three Water Regimes. Plant Soil 2004, 261, 61–75. [Google Scholar] [CrossRef]
- Warren, C.R.; Adams, M.A. Internal Conductance does not Scale with Photosynthetic Capacity: Implications for Carbon Isotope Discrimination and the Economics of Water and Nitrogen Use in Photosynthesis. Plant Cell Physiol. 2006, 29, 192–201. [Google Scholar] [CrossRef]
- Barbour, M.M.; Warren, C.R.; Farquhar, G.D.; Forrester, G.; Brown, H. Variability in Mesophyll Conductance between Barley Genotypes, and Effects on Transpiration Efficiency and Carbon Isotype Discrimination. Plant Cell Environ. 2010, 33, 1176–1185. [Google Scholar]
- Seibt, U.; Rajabi, A.; Griffiths, H.; Berry, J.A. Carbon Isotopes and Water Use Efficiency: Sense and Sensitivity. Oecologia 2008, 155, 441–454. [Google Scholar] [CrossRef]
- Easlon, H.M.; Nemali, K.S.; Richards, J.H.; Hanson, D.T.; Juenger, T.E.; McKay, K. The Physiological Basis for Genetic Variation in Water Use Efficiency and Carbon Isotype Composition in Arabidopsis thaliana. Photosynth. Res. 2014, 119, 119–129. [Google Scholar] [CrossRef]
- Bernacchi, C.J.; Portis, A.R.; Nakano, H.; Von Caemmerer, S.; Long, S.P. Temperature Response of Mesophyll Conductance. Implications for the Determination of Rubisco Enzyme Kinetics and for Limitations to Photosynthesis in Vivo. Plant Physiol. 2002, 130, 1992–1998. [Google Scholar] [CrossRef]
- Von Caemmerer, S.; Evans, J.R. Temperature Responses of Mesophyll Conductance Differ Greatly between Species. Plant Cell Environ. 2015, 38, 629–637. [Google Scholar] [CrossRef]
- Hassiotou, F.; Ludwig, M.; Renton, M.; Venklaas, E.J.; Evans, J.R. Influence of Leaf Dry Mass Per Area, CO2, and Irradiance on Mesophyll Conductance in Sclerophylls. J. Exp. Bot. 2009, 60, 2303–2314. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Diaz-Espejo, A.; Galmes, J.; Kaldenhoff, R.; Medrano, H.; Ribas-Carbo, M. Rapid Variations of Mesophyll Conductance in Response to Changes in CO2 Concentration around Leaves. Plant Cell Environ. 2007, 30, 1284–1298. [Google Scholar] [CrossRef] [PubMed]
- Bunce, J. Variation among Soybean Cultivars in Mesophyll Conductance and Leaf Water Use Efficiency. Plants 2016, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Cowan, I.F.; Farquhar, G.D. Stomatal Conductance Correlates with Photosynthetic Capacity. Nature 1979, 282, 424–426. [Google Scholar] [CrossRef]
- Fletcher, A.L.; Sinclair, T.R.; Allen, L.H., Jr. Transpiration Responses to Vapor Pressure Deficit in Well-Watered “Slow-Wilting” and Commercial Soybean. Environ. Exp. Bot. 2007, 61, 145–151. [Google Scholar] [CrossRef]
- Sadok, W.; Sinclair, T.R. Genetic Variability of Transpiration Response to Vapor Pressure Deficit among Soybean Cultivars. Crop Sci. 2009, 49, 955–960. [Google Scholar] [CrossRef]
- Gilbert, M.E.; Holbrook, N.M.; Zwieniecki, M.A.; Sadok, W.; Sinclair, T.R. Field Confirmation of Genetic Variation in Soybean Transpiration Response to Vapor Pressure Deficit and Photosynthetic Compensation. Field Crops Res. 2011, 124, 85–92. [Google Scholar] [CrossRef]
- Medina, V.; Gilbert, M.E. Physiological Trade-Offs of Stomatal Closure under High Evaporative Gradients in Field Grown Soybean. Funct. Plant Biol. 2016, 43, 40–51. [Google Scholar] [CrossRef]
- Jahan, E.; Amthor, J.S.; Farquhar, G.D.; Trethowan, R.; Barbour, M.M. Variation in Mesophyll Conductance among Australian Wheat Genotypes. Funct. Plant Biol. 2014, 41, 568–580. [Google Scholar] [CrossRef]
- Pons, T.L.; Flexas, J.; Von Caemmeer, S.; Evans, J.R.; Genty, B.; Ribas-Carbo, M.; Brugnoli, E. Estimating Mesophyll Conductance to CO2: Methodology, Potential Errors, and Recommendations. J. Exp. Bot. 2009, 60, 2217–2234. [Google Scholar] [CrossRef]
- Tomeo, M.J.; Rosenthal, D.M. Variable Mesophyll Conductance among Soybean Cultivars Sets a Tradeoff between Photosynthesis and Water-Use-Efficiency. Plant Physiol. 2017, 174, 241–257. [Google Scholar] [CrossRef]
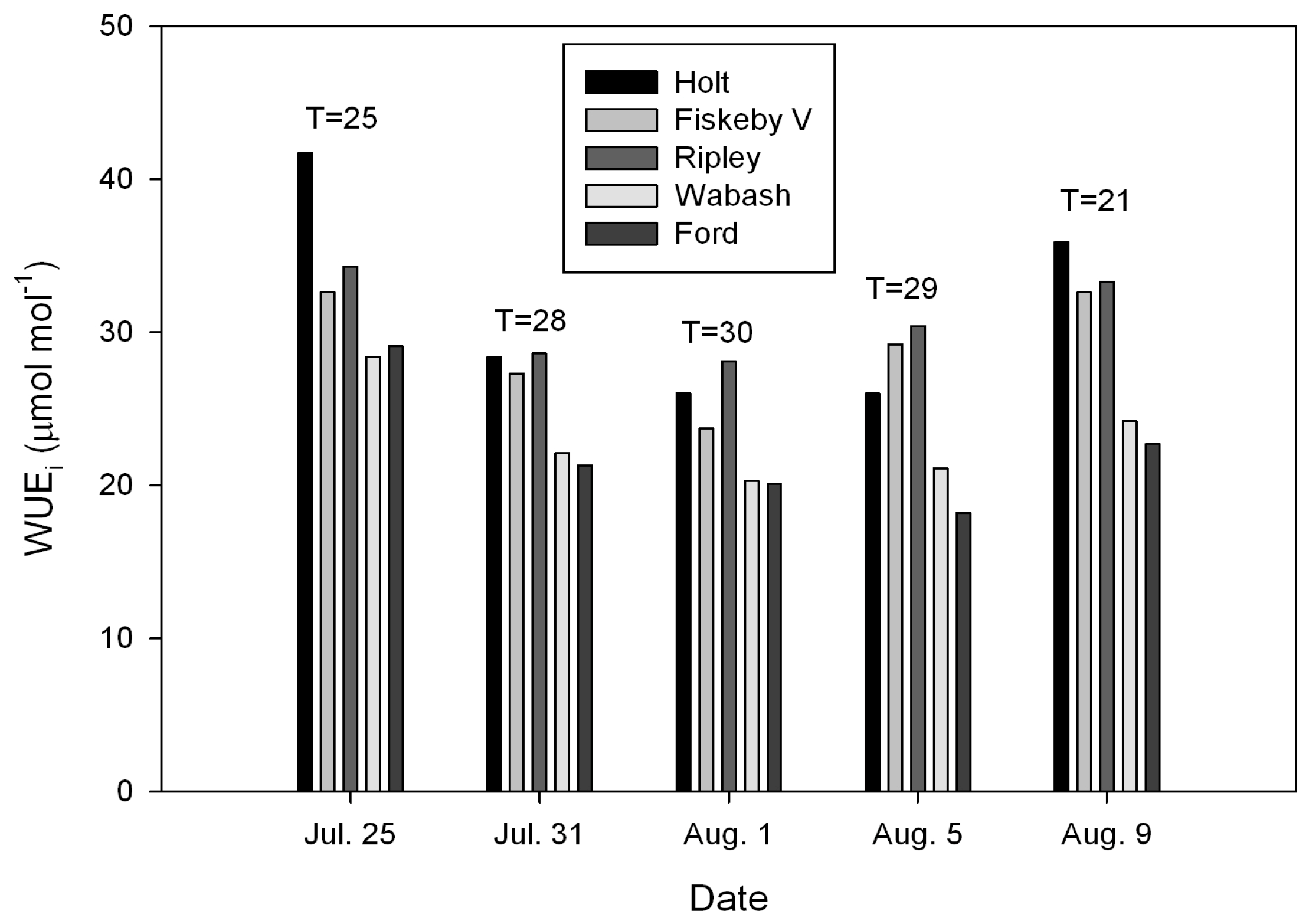

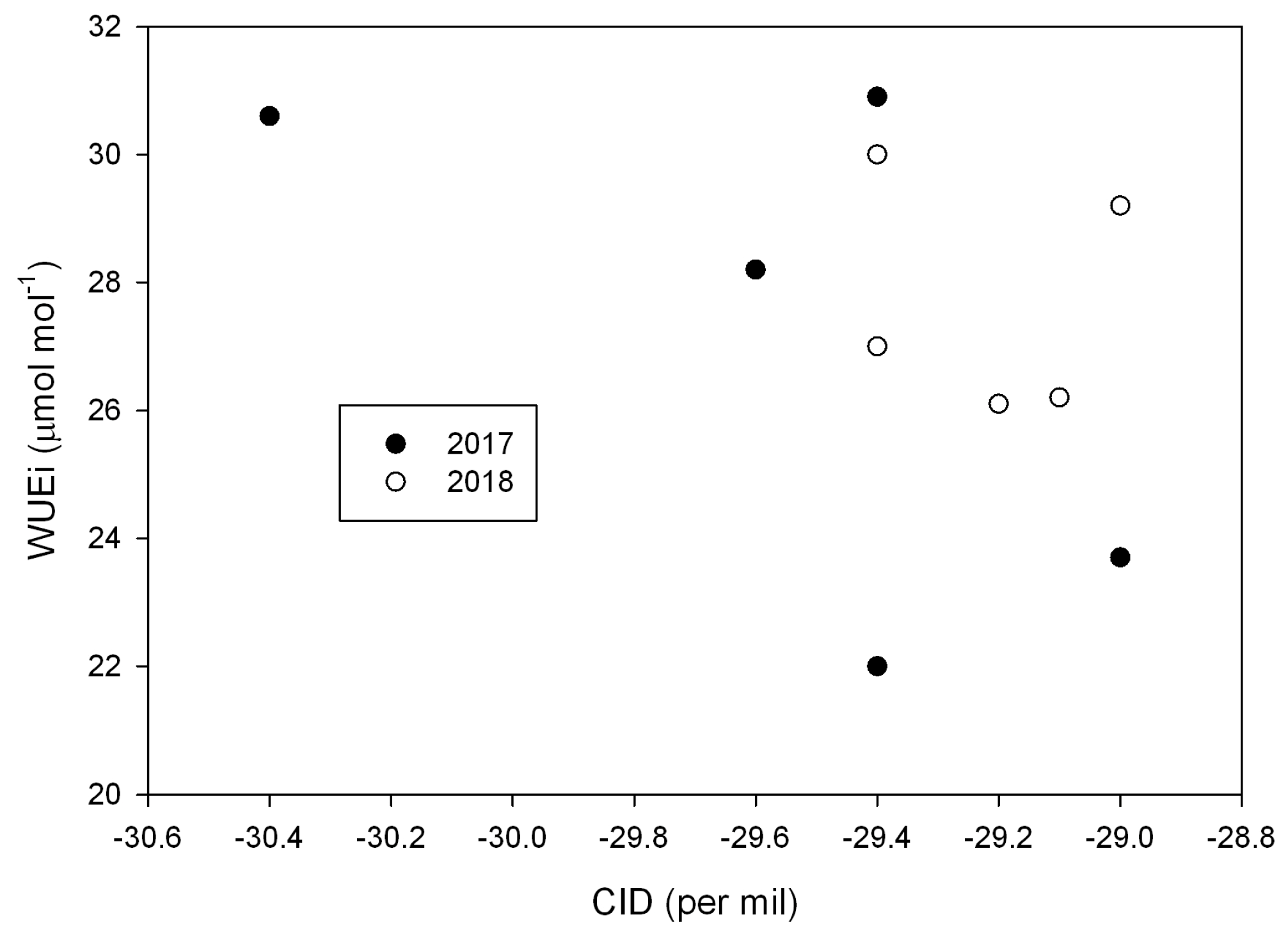
| Source | DF (Degrees of Freedom) | Sum of Squares | Mean Square | F-Value | P-Value |
|---|---|---|---|---|---|
| Cultivar | 4 | 1812 | 453 | 27.9 | <0.0001 |
| Date | 4 | 1796 | 449 | 27.7 | <0.0001 |
| Cultivar × Date | 16 | 525 | 32.8 | 2.03 | 0.0178 |
| Residual | 105 | 1701 | 16.2 |
| Source | DF | Sum of Squares | Mean Square | F-Value | P-Value |
|---|---|---|---|---|---|
| Cultivar | 4 | 335 | 83.8 | 7.04 | <0.0001 |
| Date | 3 | 2318 | 773 | 65 | <0.0001 |
| Cultivar × Date | 12 | 182 | 15.9 | 1.28 | 0.245 |
| Residual | 96 | 1141 | 11.9 |
| Cultivar | WUEi (µmol·mol−1) | A (µmol·m−2·s−1) | gs (mol·m−2·s−1) | CID (per·mil) |
|---|---|---|---|---|
| Fiskeby V | 28.2 b | 27.9 b | 0.990 b | −29.4 b |
| Ford | 22.0 c | 25.3 c | 1.149 a | −29.6 b |
| Holt | 30.6 a | 29.6 a | 0.967 b | −30.4 a |
| Ripley | 30.9 a | 28.9 ab | 0.936 b | −29.4 b |
| Wabash | 23.7 c | 24.7 c | 1.043 ab | −29.0 b |
| Cultivar | WUEi (µmol·mol−1) | A (µmol·m−2·s−1) | gs (mol·m−2·s−1) | CID (per·mil) |
|---|---|---|---|---|
| Fiskeby V | 29.2 a | 36.2 b | 1.24 bc | −29.0 b |
| Ford | 26.1 b | 31.6 c | 1.21 cd | −29.2 ab |
| Holt | 30.0 a | 39.6 a | 1.32 b | −29.4 a |
| Spencer | 27.0 b | 39.0 a | 1.44 a | −29.4 a |
| Wabash | 26.2 b | 29.8 c | 1.14 d | −29.1 b |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunce, J. Consistent Differences in Field Leaf Water-Use Efficiency among Soybean Cultivars. Plants 2019, 8, 123. https://doi.org/10.3390/plants8050123
Bunce J. Consistent Differences in Field Leaf Water-Use Efficiency among Soybean Cultivars. Plants. 2019; 8(5):123. https://doi.org/10.3390/plants8050123
Chicago/Turabian StyleBunce, James. 2019. "Consistent Differences in Field Leaf Water-Use Efficiency among Soybean Cultivars" Plants 8, no. 5: 123. https://doi.org/10.3390/plants8050123
APA StyleBunce, J. (2019). Consistent Differences in Field Leaf Water-Use Efficiency among Soybean Cultivars. Plants, 8(5), 123. https://doi.org/10.3390/plants8050123





