Selenium Biofortification Differentially Affects Sulfur Metabolism and Accumulation of Phytochemicals in Two Rocket Species (Eruca Sativa Mill. and Diplotaxis Tenuifolia) Grown in Hydroponics
Abstract
1. Introduction
2. Results
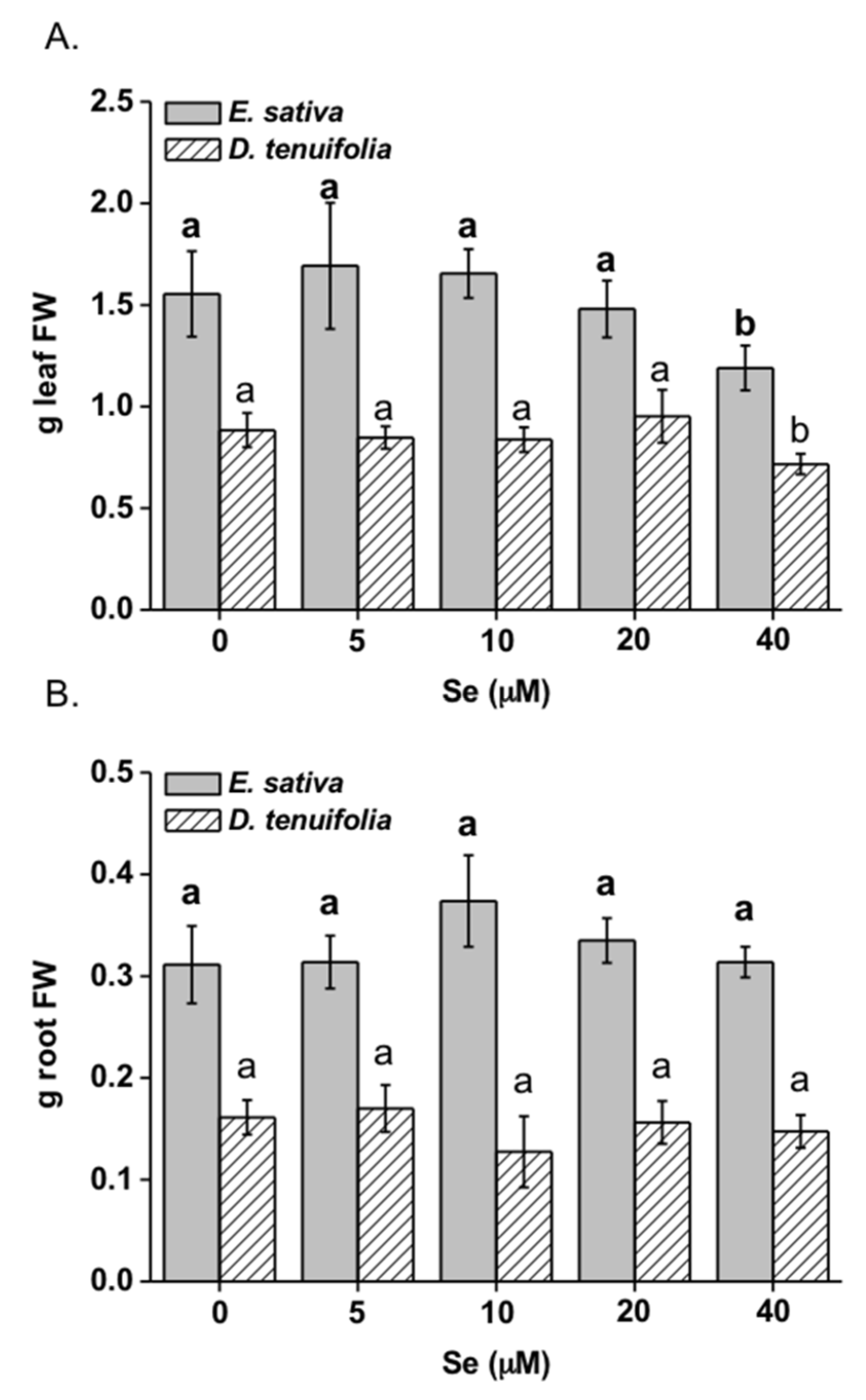
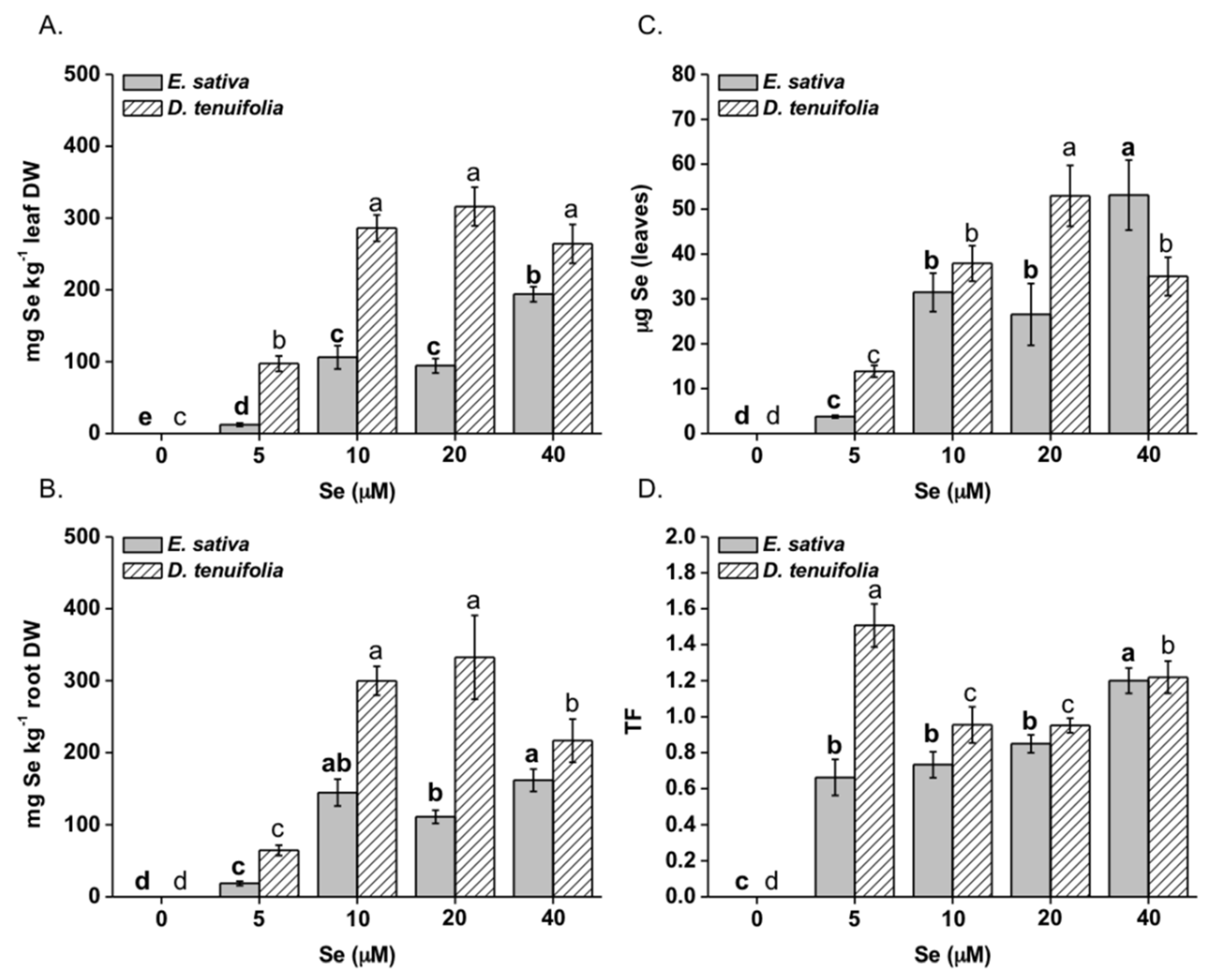
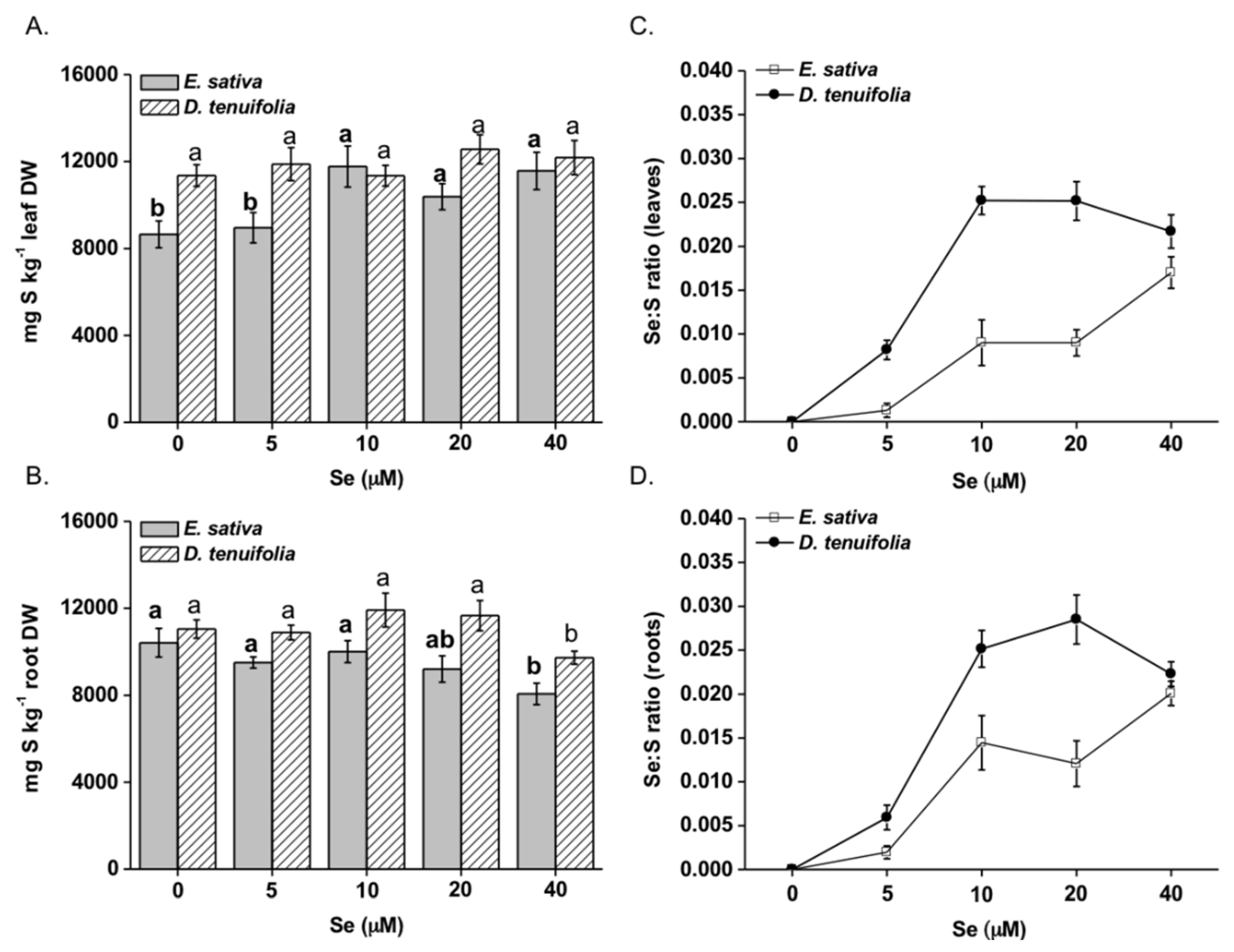
2.1. Effect of Selenium on Plant Growth, Se and S Accumulation
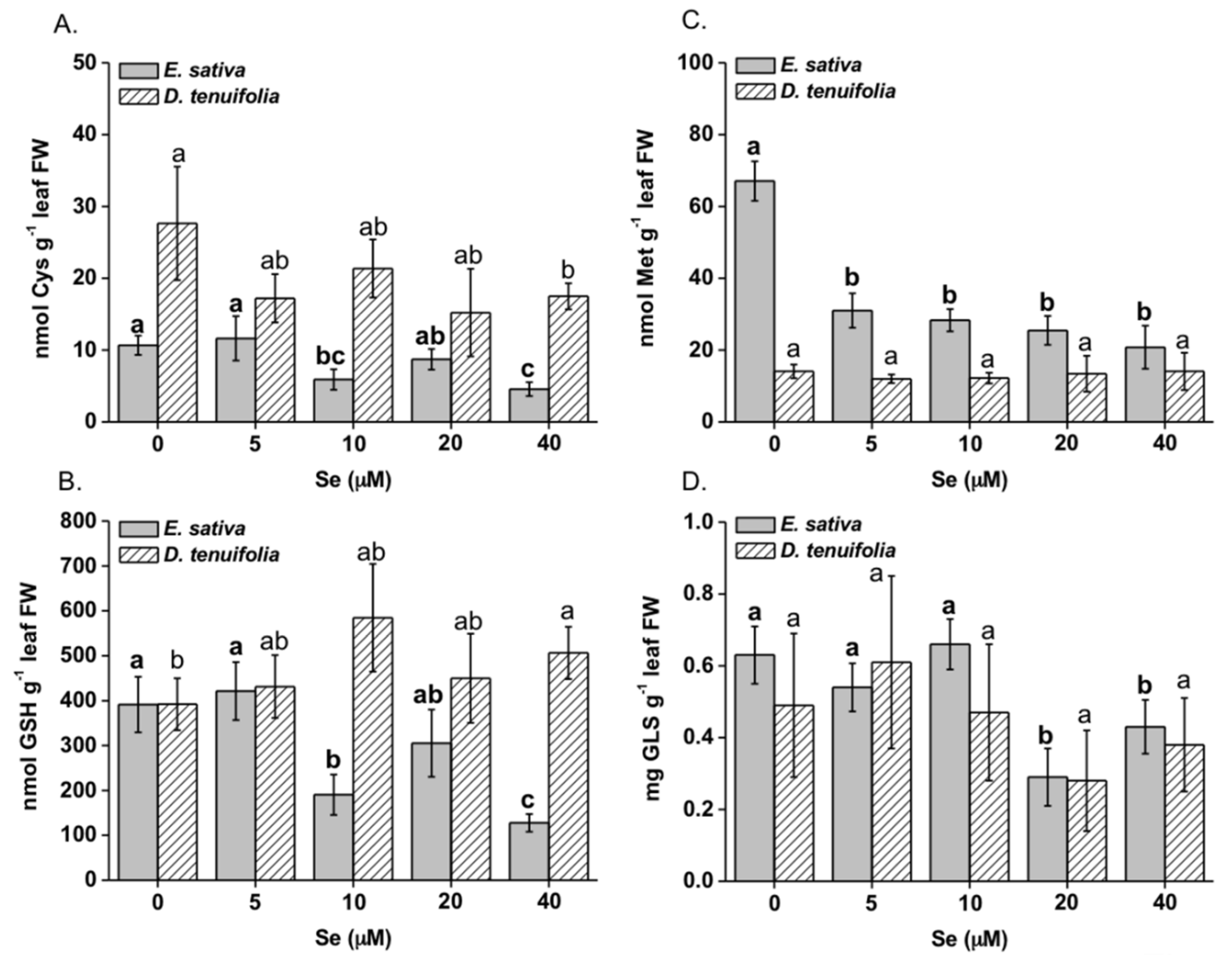
2.2. Effect of Se on Plant S Compounds
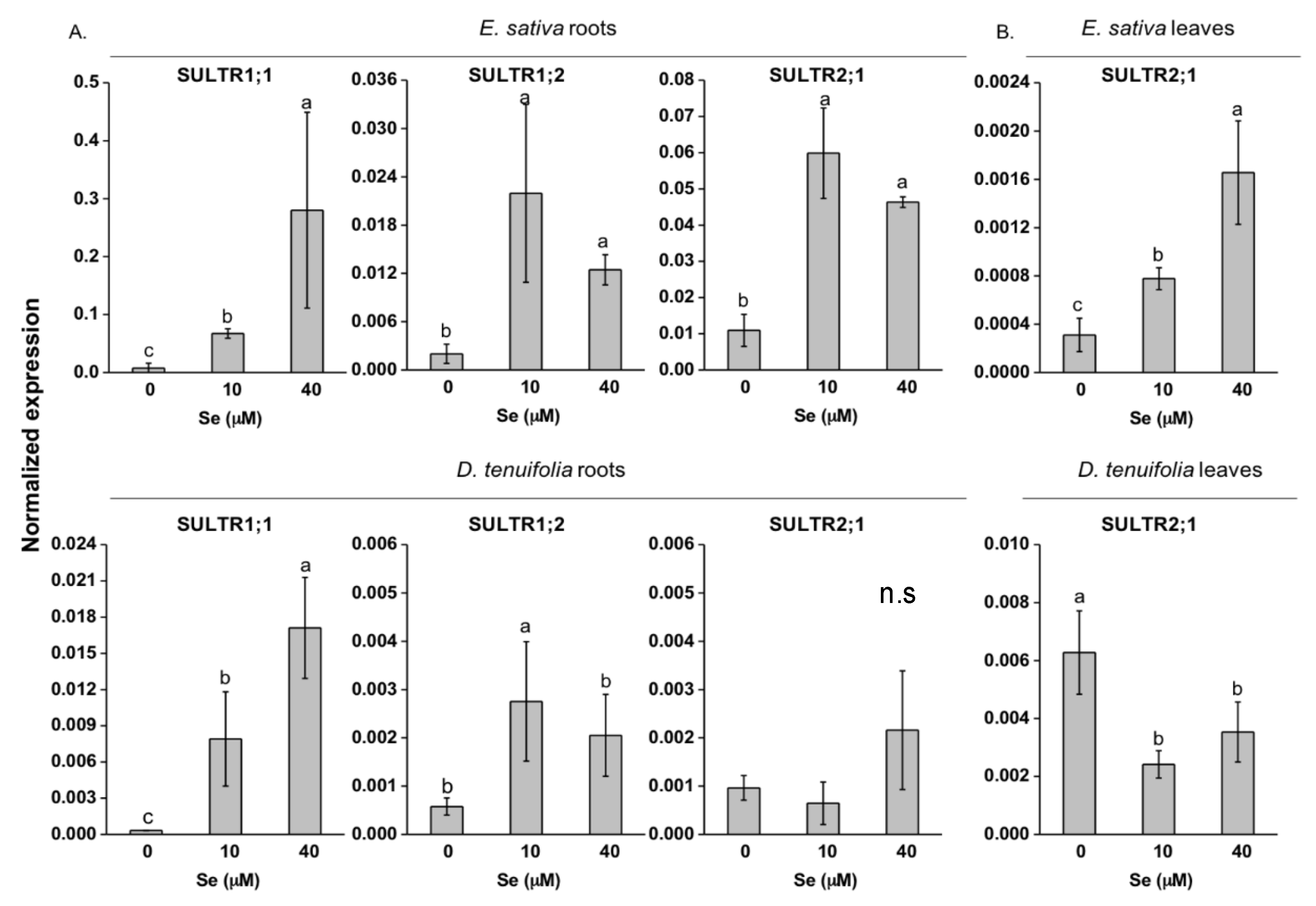
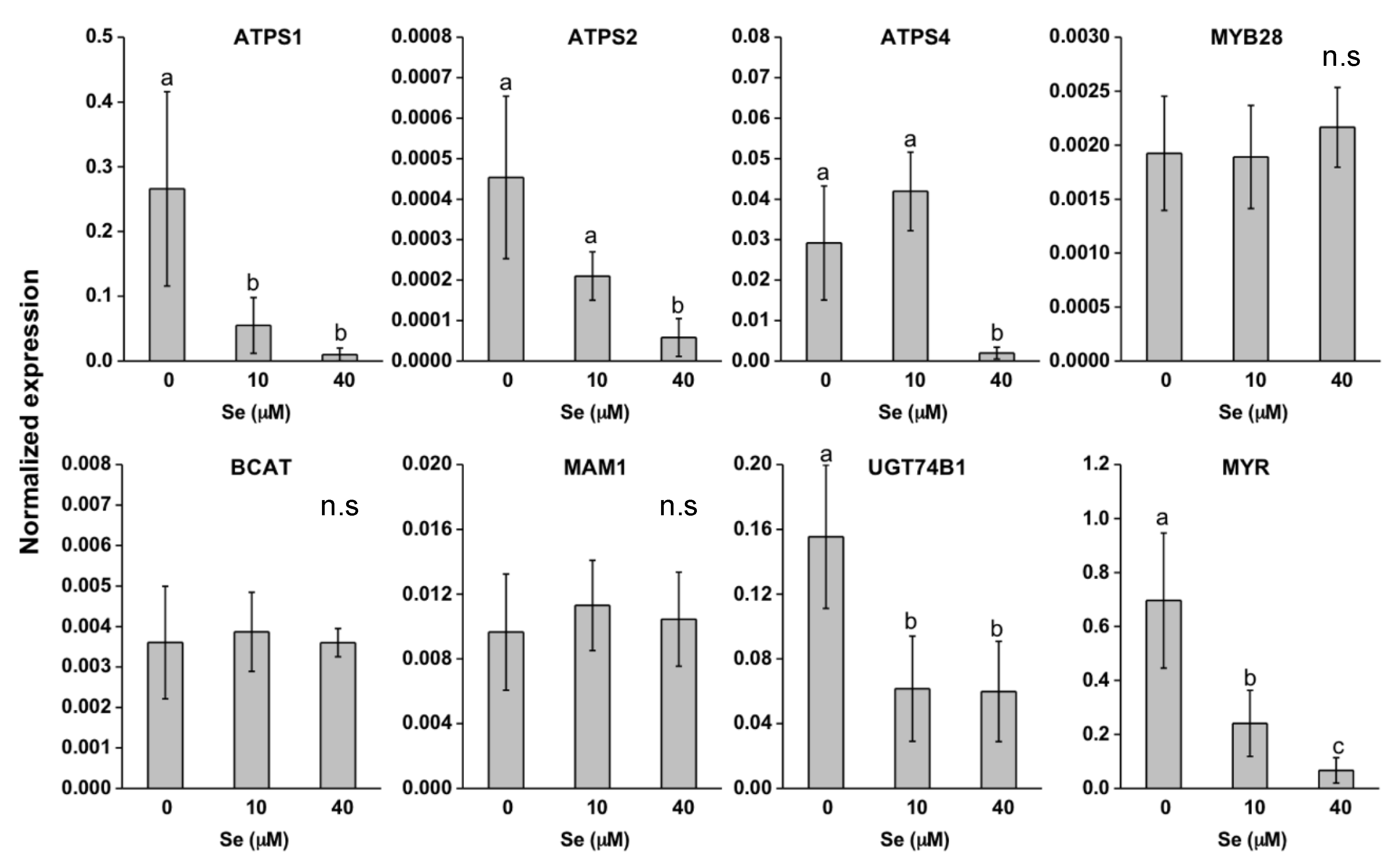
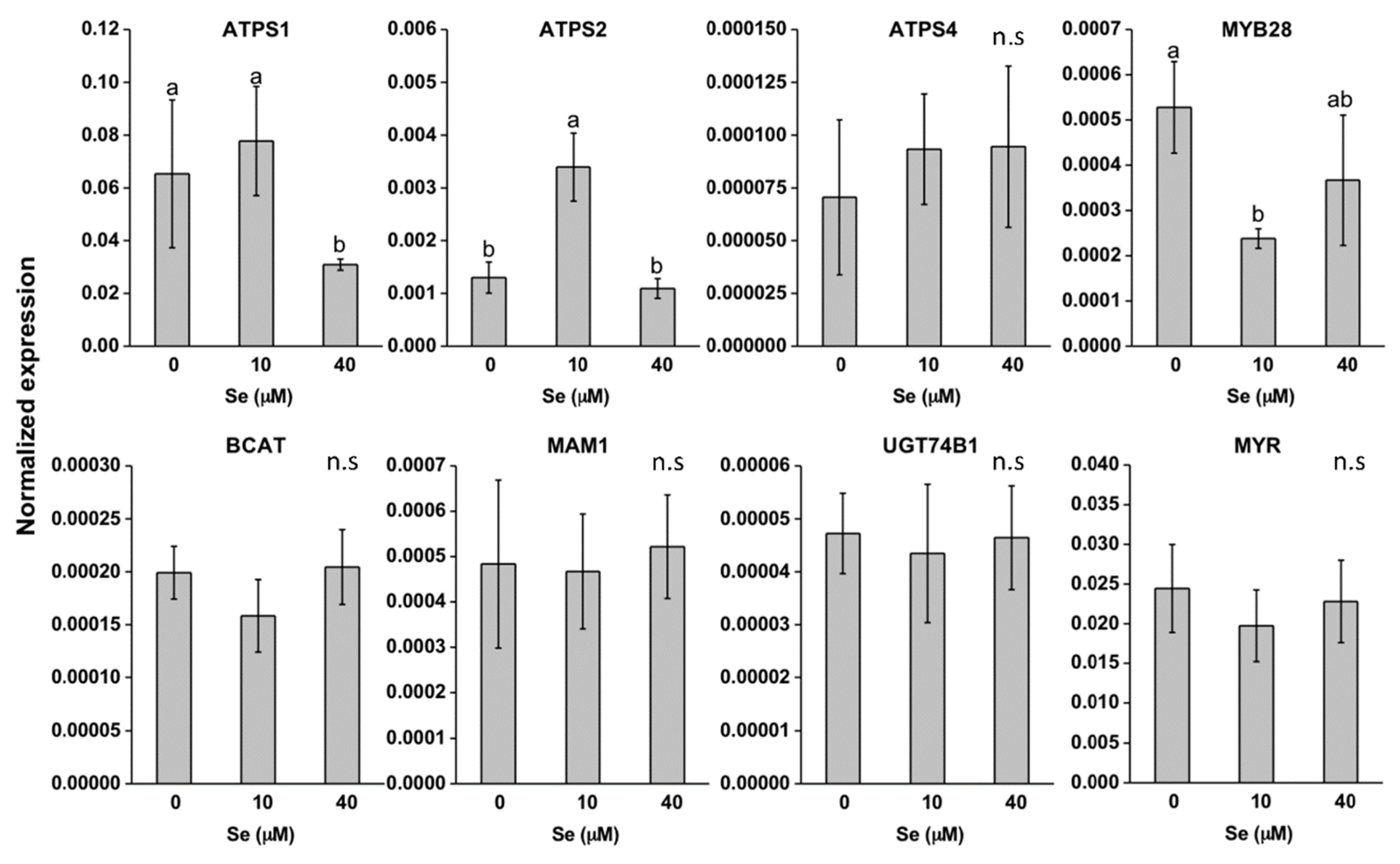
2.3. Effect of Se on S Transport and Assimilation Genes
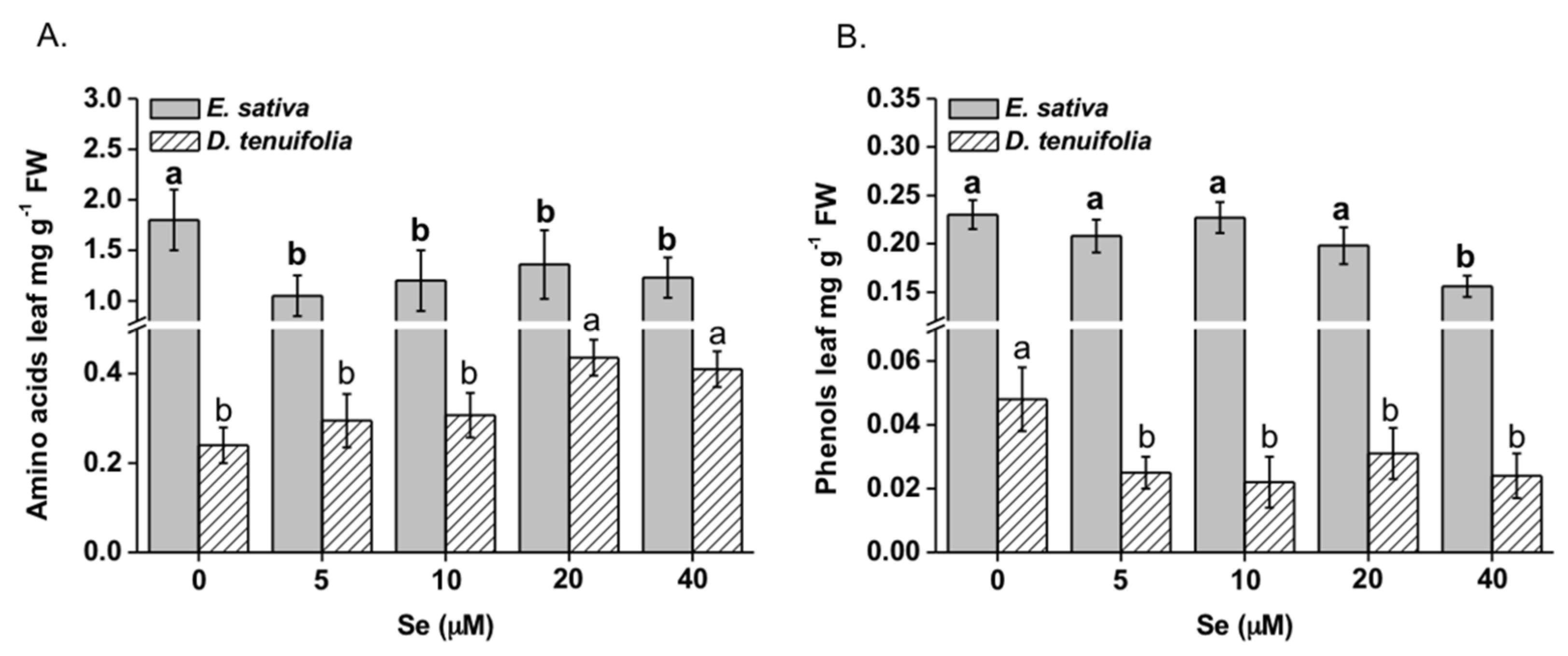
2.4. Effect of Se on Amino Acids and Phenolic Compounds
3. Discussion
4. Materials and Methods
4.1. Experimental Set Up
4.2. Determination of Total Se and S
4.3. Identification and Quantification of Glucosinolates
4.4. Identification and Quantification of Polyphenols
4.5. Free Amino Acids
4.6. Determination of Low Molecular Weight Thiol Compounds
4.7. Gene Expression via qRT-PCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent to Participate
References
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Stadtman, T.C. Selenium biochemistry. Annu. Rev. Biochem. 1990, 59, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Pilon-Smits, E.A.H. The fascinating facets of plant selenium accumulation biochemistry, physiology, evolution and ecology. New Phytol. 2017, 213, 1582–1596. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Ertani, A.; Parrasia, S.; Dalla Vecchia, F.D. Selenium accumulation and metabolism in algae. Aquat. Toxicol. 2017, 189, 1–8. [Google Scholar] [CrossRef] [PubMed]
- USDA. National Nutrient Database for Standard Reference, Release 25; Nutrient Data Laboratory Home Page; U.S. Department of Agriculture, Agricultural Research Service: Beltsville, MD, USA, 2012.
- Winkel, L.H.E.; Johnson, C.A.; Lenz, M.; Grundl, T.; Leupin, O.X.; Amini, M.; Charlet, L. Environmental selenium research: From microscopic processes to global understanding. Environ. Sci. Technol. 2011, 46, 571–579. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.; Zhang, J.; Jiang, C.; Deng, Y.; Özten, N.; Bosland, M.C. Cancer chemoprevention research with selenium in the post-SELECT era: Promises and challenges. Nutr. Cancer 2016, 68, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Macfarquhar, J.K.; Broussard, D.L.; Melstrom, P.; Hutchinson, R.; Wolkin, A.; Martin, C.; Burk, R.F.; Dunn, J.R.; Green, A.L.; Hammond, R.; et al. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010, 170, 256–261. [Google Scholar] [CrossRef]
- Morris, J.S.; Crane, S.B. Selenium toxicity from a misformulated dietary supplement, adverse health effects, and the temporal response in the nail biologic monitor. Nutrients 2013, 5, 1024–1057. [Google Scholar] [CrossRef]
- Combs, G.F., Jr. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H. Selenium deficiency risk predicted to increase under future climate change. PNAS 2017, 114, 2848–2853. [Google Scholar] [CrossRef]
- Wang, D.; Dinh, Q.T.; Thu, T.T.A.; Zhou, F.; Yang, W.; Wang, M.; Song, W.; Liang, D. Effect of selenium-enriched organic material amendment on selenium fraction transformation and bioavailability in soil. Chemosphere 2018, 199, 417–426. [Google Scholar] [CrossRef]
- Kaur, N.; Sharma, S.; Kaur, S. Selenium in agriculture: A nutrient or contaminant for crops. Arch. Agron. Soil Sci. 2014, 60, 1593–1624. [Google Scholar] [CrossRef]
- Bañuelos, G.S.; Arroyo, I.; Pickering, I.J.; Yang, S.I.; Freeman, J.L. Selenium biofortification of broccoli and carrots grown in soil amended with Se-enriched hyperaccumulator Stanleya pinnata. Food Chem. 2015, 166, 603–608. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Quinn, C.F.; Tapken, W.; Malagoli, M.; Schiavon, M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009, 12, 267–274. [Google Scholar] [CrossRef]
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2016, 117, 217–235. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smits, E.A.H. Selenium biofortification and phytoremediation phytotechnologies: A review. J. Environ. Qual. 2017, 46, 10–19. [Google Scholar] [CrossRef]
- Hartikainen, H.; Xue, T.; Piironen, V. Selenium as an antioxidant and pro-oxidant in ryegrass. Plant Soil 2000, 225, 193–200. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef]
- Ip, C.; Thompson, H.J.; Zhu, Z.; Ganther, H.E. In vitro and in vivo studies of methylseleninic acid: Evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Cancer Res. 2000, 60, 2882–2886. [Google Scholar]
- Dong, Y.; Lisk, D.; Block, E.; Ip, C. Characterization of the biological activity of γ-glutamyl-Se-methylselenocysteine: A novel, naturally occurring anticancer agent from garlic. Cancer Res. 2001, 61, 2923–2928. [Google Scholar]
- Malagoli, M.; Schiavon, M.; Dall’Acqua, S.; Pilon-Smits, E.A. Effects of selenium biofortification on crop nutritional quality. Front. Plant Sci. 2015, 6, 280. [Google Scholar] [CrossRef]
- White, P.J.; Bowen, H.C.; Parmaguru, P.; Fritz, M.; Spracklen, W.P.; Spiby, R.E.; Meacham, M.C.; Mead, A.; Harriman, M.; Trueman, L.J.; et al. Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J. Exp. Bot. 2004, 55, 1927–1937. [Google Scholar] [CrossRef]
- El Kassis, E.; Cathala, N.; Rouached, H.; Fourcroy, P.; Berthomieu, P.; Terry, N.; Davidian, J.C. Characterization of a selenate-resistant Arabidopsis mutant. Root growth as a potential target for selenate toxicity. Plant Physiol. 2007, 143, 1231–1241. [Google Scholar] [CrossRef]
- El Mehdawi, A.F.; Jiang, Y.; Guignardi, Z.S.; Esmat, A.; Pilon, M.; Pilon-Smits, E.A.H.; Schiavon, M. Influence of sulfate supply on selenium uptake dynamics and expression of sulfate/selenate transporters in selenium hyperaccumulator and nonhyperaccumulator Brassicaceae. New Phytol. 2018, 217, 194–205. [Google Scholar] [CrossRef]
- Sors, T.G.; Ellis, D.R.; Salt, D.E. Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth. Res. 2005, 86, 373–389. [Google Scholar] [CrossRef]
- Van Hoewyk, D.; Takahashi, H.; Inoue, E.; Hess, A.; Tamaoki, M.; Pilon-Smits, E.A.H. Transcriptome analyses give insight into selenium-stress responses and selenium tolerance mechanisms in Arabidopsis. Physiol. Plant 2008, 132, 236–253. [Google Scholar] [CrossRef]
- Van Hoewyk, D. A tale of two toxicities: Malformed selenoproteins and oxidative stress both contribute to selenium stress in plants. Ann. Bot. 2013, 112, 965–972. [Google Scholar] [CrossRef]
- Agerbirk, N.; Olsen, C.E. Glucosinolate structures in evolution. Phytochemistry 2012, 77, 16–45. [Google Scholar] [CrossRef]
- Jørgensen, M.E.; Nour-Eldin, H.H.; Halkier, B.A. Transport of defense compounds from source to sink: Lessons learned from glucosinolates. Trends Plant Sci. 2015, 20, 508–514. [Google Scholar] [CrossRef]
- Robbins, R.J.; Keck, A.-S.; Bañuelos, G.; Finley, J.W. Cultivation conditions and selenium fertilization alter the phenolic profile, glucosinolate, and sulforaphane content of broccoli. J. Med. Food 2005, 8, 204–214. [Google Scholar] [CrossRef]
- Barickman, T.C.; Kopsell, D.A.; Sams, C.E. Selenium influences glucosinolate and isothiocyanates and increases sulfur uptake in Arabidopsis thaliana and rapid-cycling Brassica oleracea. J. Agric. Food Chem. 2013, 61, 202–209. [Google Scholar] [CrossRef]
- Sepúlveda, I.; Barrientos, H.; Mahn, A.; Moenne, A. Changes in SeMSC, glucosinolates and sulforaphane levels, and in proteome profile in broccoli (Brassica oleracea var. Italica) fertilized with sodium selenate. Molecules 2013, 18, 5221–5234. [Google Scholar] [CrossRef]
- Tian, M.; Xu, X.; Liu, Y.; Xie, L.; Pan, S. Effect of Se treatment on glucosinolate metabolism and health-promoting compounds in the broccoli sprouts of three cultivars. Food Chem. 2016, 190, 374–380. [Google Scholar] [CrossRef]
- Schiavon, M.; Berto, C.; Malagoli, M.; Trentin, A.; Sambo, P.; Dall’Acqua, S.; Pilon-Smits, E.A.H. Selenium biofortification in radish enhances nutritional quality via accumulation of methyl-selenocysteine and promotion of transcripts and metabolites related to glucosinolates, phenolics amino acids. Front. Plant Sci. 2016, 7, 1371. [Google Scholar] [CrossRef]
- Yin, H.; Qi, Z.; Li, M.; Ahammed, G.J.; Chu, X.; Zhou, J. Selenium forms and methods of application differentially modulate plant growth, photosynthesis, stress tolerance, selenium content and speciation in Oryza sativa L. Ecotoxicol. Environ. Saf. 2019, 169, 911–917. [Google Scholar] [CrossRef]
- Schiavon, M.; Dall’Acqua, S.; Mietto, A.; Pilon-Smits, E.A.H.; Sambo, P.; Masi, A.; Malagoli, M. Selenium fertilization alters the chemical composition and antioxidant constituents of tomato (Solanum lycopersicon L.). J. Agric. Food Chem. 2013, 61, 10542–10554. [Google Scholar] [CrossRef]
- Schiavon, M.; Lima, L.W.; Jiang, Y.; Hawkesford, M.J. Effects of selenium on plant metabolism and implications for crops and consumers. In Selenium in Plants, Plant Ecophysiology; Pilon-Smits, E.A.H., Winkel, L.H.E., Lin, Z.-Q., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; Volume 11, pp. 257–271. [Google Scholar]
- Pasini, F.; Verardo, V.; Caboni, M.F.; D’Antuono, L.F. Determination of glucosinolates and phenolic compounds in rocket salad by HPLC-DAD–MS: Evaluation of Eruca sativa Mill. and Diplotaxis tenuifolia L. genetic resources. Food Chem. 2012, 133, 1025–1033. [Google Scholar] [CrossRef]
- Griffiths, D.W.; Deighton, N.; Birch, A.N.; Patrian, B.; Baur, R.; Städler, E. Identification of glucosinolates on the leaf surface of plants from the Cruciferae and other closely related species. Phytochemistry 2001, 57, 693–700. [Google Scholar] [CrossRef]
- Matthäus, B.; Luftmann, H. Glucosinolates in members of the family Brassicaceae: Separation and identification by LC/ESI-MS-MS. J Agric. Food Chem. 2000, 48, 2234–2239. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Iori, R.; Piekarska, A.; Namieśnik, J.; Bartoszek, A. Convenient identification of desulfoglucosinolates on the basis of mass spectra obtained during liquid chromatography-diode array electro spray ionisation mass spectrometry analysis: Method verification for sprouts of different Brassicaceae species extracts. J. Chromatogr. A 2013, 1278, 108–115. [Google Scholar]
- Matich, A.J.; McKenzie, M.J.; Lill, R.E.; McGhie, T.K.; Chen, R.K.Y.; Rowan, D.D. Distribution of selenoglucosinolates and their metabolites in Brassica treated with sodium selenate. J. Agric. Food Chem. 2015, 63, 1896–1905. [Google Scholar] [CrossRef]
- Bennet, R.N.; Rosa, E.A.S.; Mellon, F.A.; Kroon, P.A. Ontogenic profiling of glucosinolates, flavonoids and other secondary metabolites in Eruca sativa (salad rocket), Diplotaxis erucoides (wall rocket), Diplotaxis tenuifolia (wild rocket), and Bunias orientalis (turkish rocket). J. Agric. Food Chem. 2006, 54, 4005–4015. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Llorach, R.; Gil, M.I.; Ferreres, F. Identification of new flavonoid glycosides and flavonoid profiles to characterize rocket leafy salads (Eruca vesicaria and Diplotaxis tenuifolia). J. Agric. Food Chem. 2007, 55, 1356–1363. [Google Scholar] [CrossRef]
- Tian, M.; Yang, Y.; Ávila, F.W.; Fish, T.; Yuan, H.; Hui, M.; Pan, S.; Thannhauser, T.W.; Li, L. Effects of selenium supplementation on glucosinolate biosynthesis in Broccoli. J. Agric. Food Chem. 2018, 66, 8036–8044. [Google Scholar] [CrossRef]
- Zhang, L.-H.; Abdel-Ghany, S.E.; Freeman, J.L.; Ackley, A.R.; Schiavon, M.; Pilon-Smits, E.A.H. Investigation of selenium tolerance mechanisms in Arabidopsis thaliana. Physiol. Plant. 2006, 128, 212–223. [Google Scholar] [CrossRef]
- Hawkesford, M.J. Transporter gene families in plants: The sulphate transporter gene family-redundancy or specialization? Physiol. Plant. 2003, 117, 155–163. [Google Scholar] [CrossRef]
- Kataoka, T.; Hayashi, N.; Yamaya, T.; Takahashi, H. Root-to-shoot transport of sulfate in Arabidopsis. Evidence for the role of SULTR3;5 as a component of low-affinity sulfate transport system in the root vasculature. Plant Physiol. 2004, 136, 4198–4204. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.; Hwang, S.; Mel, L.C.; Zhu, Y.; Tai, J.C.; Bravo, R.C.; Chen, Y.; Leustek, T.; Terry, N. Overexpression of ATP sulfurylase in indian mustard leads to increased selenate uptake, reduction, and tolerance. Plant Physiol. 1999, 119, 123–132. [Google Scholar] [CrossRef]
- Hsu, F.C.; Wirtz, M.; Heppel, S.; Bogs, J.; Kramer, U.; Khan, M.S.; Bub, A.; Hell, R.; Rausch, T. Generation of Se-fortified broccoli as functional food: Impact of Se fertilization on S metabolism. Plant Cell Environ. 2011, 34, 192–207. [Google Scholar] [CrossRef]
- Ávila, F.W.; Yang, Y.; Faquin, V.; Ramos, S.J.; Guilherme, L.R.; Thannhauser, T.W.; Li, L. Impact of selenium supply on Se-methylselenocysteine and glucosinolate accumulation in selenium-biofortified Brassica sprouts. Food Chem. 2014, 15, 578–586. [Google Scholar] [CrossRef]
- McKenzie, M.J.; Chen, R.K.Y.; Leung, S.; Joshi, S.; Rippon, P.E.; Joyce, N.I.; McManus, M.T. Selenium treatment differentially affects sulfur metabolism in high and low glucosinolate producing cultivars of broccoli (Brassica oleracea L.). Plant Physiol. Biochem. 2017, 121, 176–186. [Google Scholar] [CrossRef]
- Mahn, A. Modelling of the effect of selenium fertilization on the content of bioactive compounds in broccoli heads. Food Chem. 2017, 233, 492–499. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Devi, D.D.; Shanker, A.K.; Sheeba, A.; Bangarusamy, U. Selenium-an antioxidative protectant in soybean during senescence. Plant Soil 2005, 272, 77–86. [Google Scholar] [CrossRef]
- D’Amato, R.; Fontanella, M.C.; Falcinelli, B.; Beone, G.M.; Bravi, E.; Marconi, O.; Benincasa, P.; Businelli, D. Selenium Biofortification in Rice (Oryza sativa L.) Sprouting: Effects on Se yield and nutritional traits with focus on phenolic acid profile. J. Agric. Food Chem. 2018, 66, 4082–4090. [Google Scholar] [CrossRef]
- Bell, L.; Oruna-Concha, M.J.; Wagstaff, C. Identification and quantification of glucosinolate and flavonol compounds in rocket salad (Eruca sativa, Eruca vesicaria and Diplotaxis tenuifolia) by LC-MS: Highlighting the potential for improving nutritional value of rocket crops. Food Chem. 2015, 172, 852–861. [Google Scholar] [CrossRef]
- Bell, L.; Wagstaff, C. Glucosinolates, myrosinase hydrolysis products, and flavonols found in rocket (Eruca sativa and Diplotaxis tenuifolia). J. Agric. Food Chem. 2014, 62, 4481–4492. [Google Scholar] [CrossRef]
- Toledo-Martín, E.M.; Font, R.; Obregón-Cano, S.; De Haro-Bailón, A.; Villatoro-Pulido, M.; Del Río-Celestino, M. Rapid and cost-effective quantification of glucosinolates and total phenolic content in rocket leaves by visible/near-infrared spectroscopy. Molecules 2017, 22, 851. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures; Wiley: New York, NY, USA, 1962. [Google Scholar]
- Zarcinas, B.A.; Cartwright, B.; Spouncer, L.R. Nitric acid digestion and multi-element analysis of plant material by inductively coupled plasma spectrometry. Commun. Soil Sci. Plan. 1987, 18, 131–146. [Google Scholar] [CrossRef]
- Fassel, V.A. Quantitative elemental analyses by plasma emission spectroscopy. Science 1978, 202, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Argentieri, M.P.; Accogli, R.; Fanizzi, F.P.; Avato, P. Glucosinolates profile of “mugnolo”, a variety of Brassica oleracea L. native to southern Italy (Salento). Planta Med. 2011, 77, 287–292. [Google Scholar] [CrossRef]
- Masi, A.; Ghisi, R.; Ferretti, M. Measuring low-molecular-weight thiols by detecting the fluorescence of fheir SBD-derivatives: Application to studies of diurnal and UV-B induced changes in Zea Mays L. J. Plant Physiol. 2002, 159, 499–507. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Muller, P.Y.; Janovjak, H.; Miserez, A.R.; Dobbie, Z. Processing of gene expression data generated by quantitative real- time RT–PCR. Biotechniques 2002, 32, 1372–1379. [Google Scholar]
| Glucosinolate | Se (μM) | ||||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 20 | 40 | |
| E. Sativa | |||||
| Glucoraphanin | 62.23 ± 25.04a | 94.66 ± 21.98a | 76.91 ± 21.61a | 26.88 ± 7.71b | 38.06 ± 0.15b |
| Glucocheirolin | 3.77 ± 2.31a | 3.35 ± 2.25a | 2.15 ± 0.83a | 2.48 ± 1.70a | 2.59 ± 1.00a |
| Glucoerucin | 13.48 ± 3.77a | 14.19 ± 4.76a | 10.33 ± 2.15a | 5.98 ± 2.47b | 8.90 ± 1.59ab |
| DMB-GLS | 64.50 ± 7.44a | 68.69 ± 6.18a | 47.67 ± 7.62b | 42.55 ± 3.54b | 54.73 ± 0.80b |
| Glucosativin | 2.84 ± 1.23ab | 2.98 ± 1.34ab | 1.93 ± 0.67b | 2.17 ± 0.36ab | 2.98 ± 0.09a |
| Neoglucobrassicin | 1.38 ± 0.37a | 1.92 ± 0.88a | 1.49 ± 0.29a | 0.78 ± 0.24a | 2.71 ± 1.57a |
| D. Tenuifolia | |||||
| Glucoraphanin | 20.75 ± 6.91ab | 31.56 ± 13.51a | 25.64 ± 9.54ab | 8.96 ± 6.98b | 12.68 ± 5.22b |
| Glucocheirolin | 1.26 ± 0.41a | 1.12 ± 0.37a | 0.72 ± 0.24a | 0.82 ± 0.27a | 0.86 ± 0.28a |
| Glucoerucin | 4.49 ± 1.49a | 4.73 ± 1.57a | 3.44 ± 1.14a | 1.99 ± 1.66a | 2.97 ± 0.98a |
| DMB-GLS | 21.50 ± 9.16a | 22.89 ± 10.63a | 15.89 ± 8.29a | 14.18 ± 10.72a | 18.24 ± 6.08a |
| Glucosativin | 0.95 ± 0.31a | 0.99 ± 0.33a | 0.64 ± 0.21a | 0.72 ± 0.24a | 0.99 ± 0.33a |
| Neoglucobrassicin | 0.46 ± 0.15a | 0.64 ± 0.21a | 0.49 ± 0.16ab | 0.26 ± 0.08b | 0.90 ± 0.30a |
| Amino Acid (mg/100g FW) | Se (μM) | ||||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 20 | 40 | |
| E. Sativa | |||||
| Phenylalanine | 7.29 ± 0.97a | 6.15 ± 0.98ab | 4.58 ± 0.85b | 4.05 ± 1.37b | 4.92 ± 1.21b |
| Isoleucine | 2.59 ± 0.22a | 3.07 ± 0.23a | 2.06 ± 0.47a | 2.36 ± 0.81a | 2.57 ± 0.88a |
| Leucine | 0.24 ± 0.13b | 0.27 ± 0.09b | 0.39 ± 0.13b | 0.25 ± 0.11b | 0.65 ± 0.18a |
| Histidine | 13.56 ± 0.11a | 7.63 ± 0.15b | 8.92 ± 0.06b | 11.78 ± 1.15a | 12.63 ± 1.08a |
| Tyrosine | 0.91 ± 0.16a | 0.59 ± 0.25b | 0.50 ± 0.13b | 0.52 ± 0.27b | 0.56 ± 0.14b |
| Tryptophan | 1.25 ± 0.10b | 1.13 ± 0.31ab | 1.10 ± 0.30ab | 1.18 ± 0.30ab | 1.56 ± 0.18a |
| Arginine | 0.10 ± 0.03a | 0.04 ± 0.02b | 0.05 ± 0.01b | 0.06 ± 0.03ab | 0.07 ± 0.01b |
| Glutamine | 72.39 ± 6.84a | 30.32 ± 7.04b | 32.22 ± 12.53b | 42.09 ± 15.49b | 29.63 ± 2.61b |
| Valine | 5.82 ± 0.68a | 2.70 ± 0.47c | 3.32 ± 0.43bc | 3.96 ± 0.68b | 4.20 ± 0.91ab |
| Proline | 83.08 ± 17.18a | 47.56 ± 8.61b | 55.44 ± 16.58ab | 65.52 ± 15.82ab | 56.16 ± 8.44b |
| Se-cysteine | - | 0.21 ± 0.11a | 0.32 ± 0.23a | 0.34 ± 0.92a | 0.31 ± 0.06a |
| D. Tenuifolia | |||||
| Phenylalanine | 1.11 ± 0.21b | 0.93 ± 0.20b | 1.53 ± 0.69ab | 1.19 ± 0.12b | 1.88 ± 0.54a |
| Isoleucine | 0.73 ± 0.15a | 0.33 ± 0.15b | 0.36 ± 0.06b | 0.41 ± 0.19b | 0.58 ± 0.12ab |
| Leucine | 0.39 ± 0.24ab | 0.36 ± 0.13b | 0.32 ± 0.11b | 0.49 ± 0.21ab | 0.84 ± 0.38a |
| Histidine | 4.51 ± 2.20a | 3.82 ± 1.14a | 1.27 ± 0.27b | 1.26 ± 0.48b | 1.87 ± 0.80b |
| Alanine | 0.87 ± 0.56ab | 0.91 ± 0.38ab | 1.26 ± 0.49a | 0.64 ± 0.18b | 0.63 ± 0.07b |
| Tyrosine | 0.34 ± 0.09b | 0.25 ± 0.09b | 0.23 ± 0.05b | 0.24 ± 0.11b | 0.56 ± 0.03a |
| Tryptophan | 0.53 ± 0.20a | 0.95 ± 0.18a | 0.74 ± 0.38a | 1.11 ± 0.53a | 0.66 ± 0.10a |
| Arginine | 0.18 ± 0.03ab | 0.22 ± 0.02a | 0.16 ± 0.03b | 0.15 ± 0.02b | 0.16 ± 0.06ab |
| Glutamine | 6.37 ± 0.63b | 5.55 ± 0.45b | 6.20 ± 1.26b | 7.06 ± 0.59ab | 8.46 ± 1.25a |
| Glutamic acid | 3.60 ± 1.11a | 3.82 ± 1.13a | 3.36 ± 0.78a | 3.97 ± 1.35a | 3.56 ± 0.53a |
| Valine | 3.71 ± 0.83a | 2.86 ± 0.27a | 2.89 ± 0.61a | 3.46 ± 1.07a | 3.00 ± 0.48a |
| Lysine | 0.58 ± 0.12a | 0.63 ± 0.13a | 0.64 ± 0.13a | 0.51 ± 0.11a | 0.65 ± 0.09a |
| Proline | 0.91 ± 0.15b | 1.94 ± 0.55b | 3.20 ± 0.95b | 15.48 ± 10.09a | 10.78 ± 8.39ab |
| Methionine | 0.21 ± 0.03a | 0.18 ± 0.03a | 0.18 ± 0.01a | 0.20 ± 0.08a | 0.21 ± 0.08a |
| Se-cysteine | - | 0.69 ± 0.05a | 0.87 ± 0.09a | 0.76 ± 0.26a | 0.73 ± 0.19a |
| Phenol Compound (mg/100g FW) | Se (μM) | ||||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 20 | 40 | |
| E. Sativa | |||||
| K-3-sinapoil-triglucoside-7-glicoside | 0.46 ± 0.15a | 0.20 ± 0.10ab | 0.19 ± 0.05b | 0.00 ± 0.00d | 0.06 ± 0.01c |
| K-3-diglucoside-7-glicoside | 0.24 ± 0.07a | 0.16 ± 0.09a | 0.16 ± 0.06a | 0.23 ± 0.07a | 0.20 ± 0.07a |
| Q-3-glucoside | 0.27 ± 0.04b | 0.28 ± 0.03b | 0.45 ± 0.04a | 0.38 ± 0.03a | 0.26 ± 0.04b |
| Q-3,4′-diglucoside | 1.49 ± 0.08a | 1.30 ± 0.18a | 2.88 ± 1.17a | 2.41 ± 0.22a | 1.24 ± 0.20a |
| K-3,4′-diglucoside | 13.07 ± 0.32a | 11.94 ± 1.03ab | 12.67 ± 1.32ab | 12.19 ± 1.05ab | 10.38 ± 0.27b |
| I-3,4′-diglucoside | 1.25 ± 0.12a | 0.83 ± 0.21a | 2.08 ± 1.03a | 1.54 ±0.39a | 0.89 ± 0.19a |
| K-3-O-feruloildiglucoside-7-O-glucoside | 0.34 ± 0.06a | 0.34 ± 0.06a | 0.32 ± 0.12a | 0.32 ± 0.08a | 0.31 ± 0.06a |
| K-3-glucoside | 1.90 ± 0.12a | 1.87 ± 0.10a | 1.32 ± 0.63ab | 1.89 ± 0.20a | 0.92 ± 0.08b |
| I-3-glucoside | 0.28 ± 0.03a | 0.28 ± 0.02a | 0.54 ± 0.29a | 0.45 ± 0.20a | 0.22 ± 0.10a |
| Q-3-glucoside 3′ (6-sinapoilglucoside) | 0.44 ± 0.08a | 0.42 ± 0.04a | 0.22 ± 0.04b | 0.00 ± 0.00c | 0.20 ± 0.05b |
| K-3-(2-sinapoil-glucoside)-4′-glucoside | 2.01 ± 0.08a | 2.38 ± 0.15a | 0.65 ± 0.10b | 0.19 ± 0.10c | 0.49 ± 0.12b |
| K-3-O-feruloil glucoside-7-O-glucoside | 0.56 ± 0.04a | 0.58 ± 0.05a | 0.47 ± 0.07a | 0.24 ± 0.02b | 0.58 ± 0.04a |
| D. Tenuifolia | |||||
| Q-3-glucoside | 0.27 ± 0.07a | 0.20 ± 0.05a | 0.14 ± 0.08a | 0.22 ± 0.04a | 0.21 ± 0.04a |
| Q-3,4′-diglucoside | 1.04 ± 0.03a | 0.54 ± 0.10b | 0.48 ± 0.14b | 0.74 ± 0.12b | 0.52 ± 0.14b |
| Q-3,3′,4′-triglucoside | 1.11 ± 0.04a | 0.51 ± 0.13bc | 0.46 ± 0.05c | 0.73 ± 0.16b | 0.48 ± 0.6c |
| K-3,4′-diglucoside | 0.71 ± 0.04a | 0.30 ± 0.06b | 0.41 ± 0.05b | 0.37 ± 0.04b | 0.34 ± 0.03b |
| I-3,4′-diglucoside | 0.60 ± 0.24a | 0.44 ± 0.14a | 0.35 ± 0.12a | 0.56 ± 0.12a | 0.33 ± 0.10a |
| Q-3,4′-diglucoside 3′ (6-sinapoilglucoside) | 0.48 ± 0.04 | 0.17 ± 0.04 | 0.07 ± 0.04 | 0.39 ± 0.04 | 0.21 ± 0.04 |
| Q-3,4′-diglucoside 3′ (6-feruloilglucoside) | 0.16 ± 0.06ab | 0.15 ± 0.06ab | 0.06 ± 0.04b | 0.31 ± 0.12a | 0.22 ± 0.10ab |
| I 3-glucoside | 0.26 ± 0.05a | 0.27 ±0.04a | 0.27 ± 0.03a | 0.22 ± 0.03a | 0.30 ± 0.04a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dall’Acqua, S.; Ertani, A.; Pilon-Smits, E.A.H.; Fabrega-Prats, M.; Schiavon, M. Selenium Biofortification Differentially Affects Sulfur Metabolism and Accumulation of Phytochemicals in Two Rocket Species (Eruca Sativa Mill. and Diplotaxis Tenuifolia) Grown in Hydroponics. Plants 2019, 8, 68. https://doi.org/10.3390/plants8030068
Dall’Acqua S, Ertani A, Pilon-Smits EAH, Fabrega-Prats M, Schiavon M. Selenium Biofortification Differentially Affects Sulfur Metabolism and Accumulation of Phytochemicals in Two Rocket Species (Eruca Sativa Mill. and Diplotaxis Tenuifolia) Grown in Hydroponics. Plants. 2019; 8(3):68. https://doi.org/10.3390/plants8030068
Chicago/Turabian StyleDall’Acqua, Stefano, Andrea Ertani, Elizabeth A.H. Pilon-Smits, Marta Fabrega-Prats, and Michela Schiavon. 2019. "Selenium Biofortification Differentially Affects Sulfur Metabolism and Accumulation of Phytochemicals in Two Rocket Species (Eruca Sativa Mill. and Diplotaxis Tenuifolia) Grown in Hydroponics" Plants 8, no. 3: 68. https://doi.org/10.3390/plants8030068
APA StyleDall’Acqua, S., Ertani, A., Pilon-Smits, E. A. H., Fabrega-Prats, M., & Schiavon, M. (2019). Selenium Biofortification Differentially Affects Sulfur Metabolism and Accumulation of Phytochemicals in Two Rocket Species (Eruca Sativa Mill. and Diplotaxis Tenuifolia) Grown in Hydroponics. Plants, 8(3), 68. https://doi.org/10.3390/plants8030068








