Virulent Rhodococcus fascians Produce Unique Methylated Cytokinins
Abstract
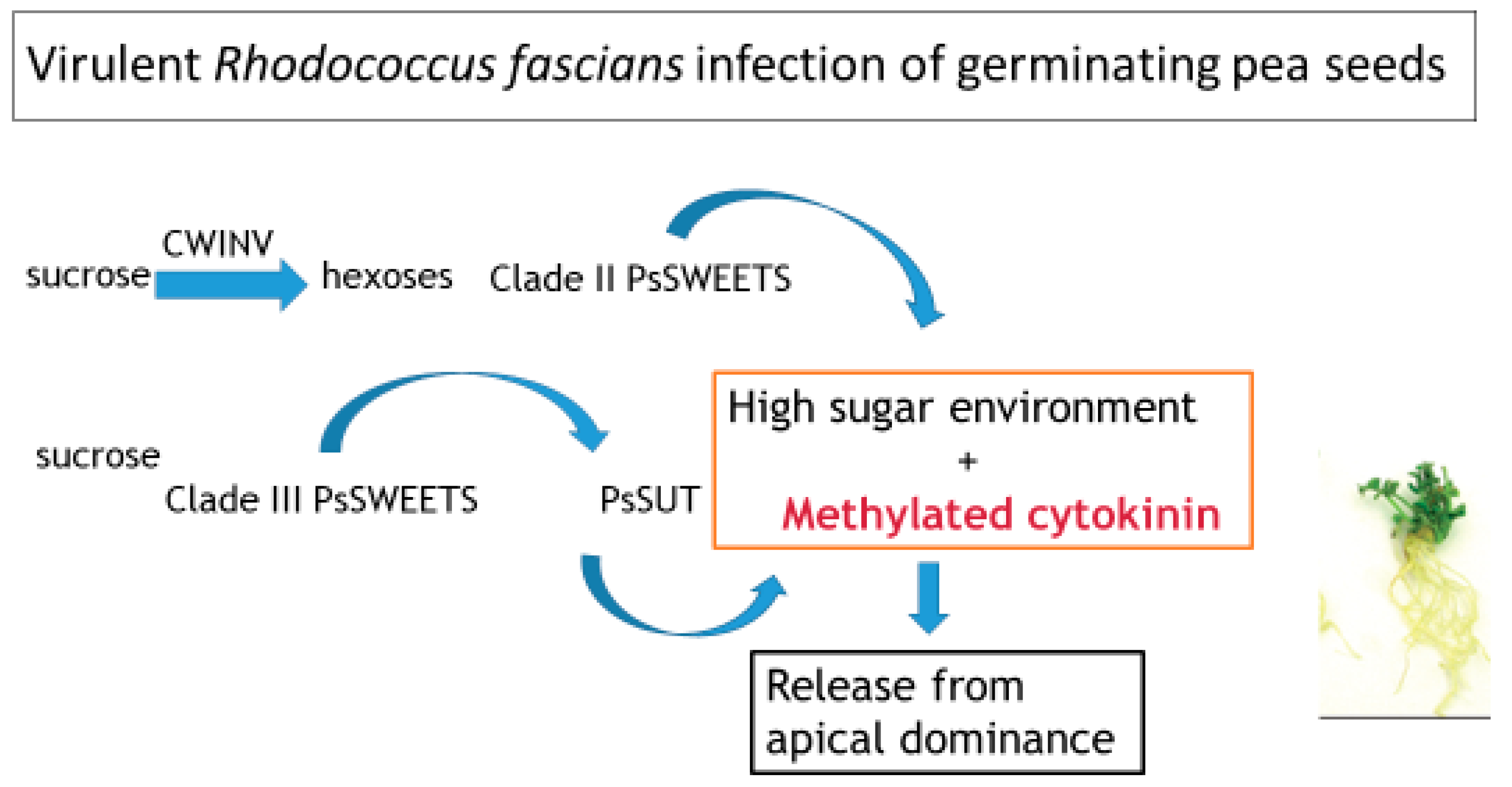
1. Introduction
Funding
Conflicts of Interest
References
- Thimann, K.V.; Sachs, T. The role of cytokinins in the “fasciation” disease caused by Corynebacterium fascians. Am. J. Bot. 1996, 53, 731–739. [Google Scholar] [CrossRef]
- Crespi, M.; Messens, E.; Caplan, A.B.; Van Montagu, M.; Desomer, J. Fasciation induction by the phytopathogen Rhodococcus fascians depends upon a linear plasmid encoding a cytokinin synthase gene. EMBO J. 1992, 11, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Stange, R.R.; Jeffares, D.; Young, C.; Scott, D.B.; Eason, J.R.; Jameson, P.E. PCR amplification of the fas-1 gene for the detection of virulent strains of Rhodococcus fascians. Plant Pathol. 1996, 45, 407–417. [Google Scholar] [CrossRef]
- Francis, I.; De Keyser, A.; De Backer, P.; Simon-Mateo, C.; Kalkus, J.; Pertry, I.; Ardiles-Diaz, W.; De Rycke, R.; Vandeputte, O.M.; El Jaziri, M.; et al. pFiD188, the linear virulence plasmid of Rhodococcus fascians D188. Mol. Plant Microbe Interact. 2012, 25, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Savory, E.A.; Fuller, S.L.; Weisberg, A.J.; Thomas, W.J.; Gordon, M.I.; Stevens, D.M.; Creason, A.L.; Belcher, M.S.; Serdani, M.; Wiseman, M.S.; et al. Evolutionary transitions between beneficial and phytopathogenic Rhodococcus challenge disease management. eLife 2017, 6, e30925. [Google Scholar] [CrossRef] [PubMed]
- Taller, B.J. Distribution, biosynthesis, and function of cytokinins in tRNA. In Cytokinins: Chemistry, Activity, and Function; Mok, D.W.S., Mok, M.C., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 101–112. [Google Scholar]
- Miyawaki, K.; Tarkowski, P.; Matsumoto-Kitano, M.; Kato, T.; Sato, S.; Tarkowska, D.; Tabata, S.; Sandberg, G.; Kakimoto, T. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 16598–16603. [Google Scholar] [CrossRef] [PubMed]
- Nishii, K.; Wright, F.; Chen, Y.Y.; Möller, M. Tangled history of a multigene family: The evolution of ISOPENTENYLTRANSFERASE genes. PLoS ONE 2018. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.J.; Scarbrough, E.; Skoog, F. Cytokinins in Corynebacterium fascians cultures: Isolation and identification of 6-(4-Hydroxy-3-methyl-cis-2-butenylamino)-2-methylthiopurine. Plant Physiol. 1976, 58, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Pertry, I.; Václavíková, K.; Gemrotová, M.; Spíchal, L.; Galuszka, P.; Depuydt, S.; Temmerman, W.; Stes, E.; De Keyser, A.; Riefler, M.; et al. Rhodococcus fascians impacts plant development through the dynamic fas-mediated production of a cytokinin mix. Mol. Plant Microbe Interact. 2010, 23, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Tarkowski, P.; Václavíkova, K.; Novák, O.; Pertry, I.; Hanuš, J.; Whenham, R.; Vereecke, D.; Šebela, M.; Strnad, M. Analysis of 2-methylthio-derivatives of isoprenoid cytokinins by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2010, 680, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Gális, I.; Bilyeu, K.; Wood, G.; Jameson, P.E. Rhodococcus fascians: Shoot proliferation without elevated cytokinins? Plant Growth Regul. 2005, 46, 109–115. [Google Scholar] [CrossRef]
- Dhandapani, P.; Song, J.; Novak, O.; Jameson, P.E. Infection by Rhodococcus fascians maintains cotyledons as a sink tissue for the pathogen. Ann. Bot. 2017, 119, 841–852. [Google Scholar] [PubMed]
- Dhandapani, P.; Song, J.; Novak, O.; Jameson, P.E. Both epiphytic and endophytic strains of Rhodococcus fascians influence transporter gene expression and cytokinins in infected Pisum sativum L. seedlings. Plant Growth Regul. 2018, 85, 231–242. [Google Scholar] [CrossRef]
- Jameson, P.E.; Dhandapani, P.; Song, J.; Zatloukal, M.; Strnad, M.; Remus-Emsermann, M.N.P.; Schlechter, R.O.; Novák, O. The cytokinin complex associated with Rhodococcus fascians: Which compounds are critical for virulence? Front. Plant Sci. 2019, 10, 674. [Google Scholar] [CrossRef] [PubMed]
- Pertry, I.; Václavíková, K.; Depuydt, S.; Galuszka, P.; Spíchal, L.; Temmerman, W.; Stes, E.; Schmülling, T.; Kakimoto, T.; Van Montagu, M.C.E.; et al. Identification of Rhodococcus fascians cytokinins and their modus operandi to reshape the plant. Proc. Natl. Acad. Sci. USA 2009, 10, 929–934. [Google Scholar] [CrossRef]
- Barbier, F.F.; Lunn, J.E.; Beveridge, C.A. Ready, steady, go! A sugar hit starts the race to shoot branching. Curr. Opin. Plant Biol. 2015, 25, 39–45. [Google Scholar] [CrossRef]
- Radhika, V.; Ueda, N.; Tsuboi, Y.; Kojima, M.; Kikuchi, J.; Kudo, K.; Sakakibara, H. Methylated cytokinins from the phytopathogen Rhodococcus fascians mimic plant hormone activity. Plant Physiol. 2015, 169, 1118–1126. [Google Scholar] [CrossRef]
- Pertry, I. How the Fas Locus Contributes to Rhodococcus Fascians Cytokinin Production: An In-Depth Molecular and Biochemical Analysis. Ph.D. Thesis, Ghent University, Gent, Belguim, 2009. Available online: http://hdl.handle.net/1854/LU-529624 (accessed on 25 April 2019).
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jameson, P.E. Virulent Rhodococcus fascians Produce Unique Methylated Cytokinins. Plants 2019, 8, 582. https://doi.org/10.3390/plants8120582
Jameson PE. Virulent Rhodococcus fascians Produce Unique Methylated Cytokinins. Plants. 2019; 8(12):582. https://doi.org/10.3390/plants8120582
Chicago/Turabian StyleJameson, Paula Elizabeth. 2019. "Virulent Rhodococcus fascians Produce Unique Methylated Cytokinins" Plants 8, no. 12: 582. https://doi.org/10.3390/plants8120582
APA StyleJameson, P. E. (2019). Virulent Rhodococcus fascians Produce Unique Methylated Cytokinins. Plants, 8(12), 582. https://doi.org/10.3390/plants8120582




